Abstract
Flavonoids are polyphenolic compounds that are ubiquitous in plants and have biological effects on cancer cells and other cell types. In particular, apigenin (API) has been shown to bind to estrogen receptors, which affect the development, maturation, function, and plasticity of the nervous system. The aim of this study was to investigate the effects of 4′,5,7-trihydroxyflavone (API) upon the neural differentiation of human pluripotent stem cells. Treatment of both human embryonic stem cells and human induced pluripotent stem cells with API increased the number of nestin (NES+) neural progenitor cells compared to untreated controls. API also induced the expression of neuronal markers, such as β-tubulin-III (TUBB3), microtubule-associated protein 2 (MAP2), polysialylated-neural cell adhesion molecule (PSA-NCAM), synapsin 1 (SYN1), neurofilament (NEF), choline acetyltransferase (CHAT), glutamate decarboxylase (GAD1), and parvalbumin (PVALB) proteins. Antagonists of estrogen receptors (ESR1 and ESR2) suppressed the effects of API. API-induced differentiation was followed by increased expression of retinoic acid (RA) receptors (RARA and RARB) and retinoic X receptor (RXR) G, but not RARG1 or RXRB. Neural differentiation induced by API was drastically reduced by the inhibition of RARs. In addition, API also increased synaptogenesis in RA-differentiated neurons. These findings suggest that API induces neural differentiation of human pluripotent stem cells through estrogen receptor and RAR signaling and improves their functional differentiation into neurons.
Flavonoids are polyphenolic compounds present in plants, which also have biological effects on animal cells. Plants containing these bioactive compounds have been used for centuries to promote cardiovascular health, reduce inflammation, and prevent cancer (Citation1). Understanding the effect of flavonoids on human neural differentiation and maturation is particularly interesting since these compounds may offer a natural and accessible way to prevent or treat neurological disorders. Incorporating flavonoids into stem cell cultures may also generate more robust and reliable models of neurons in a dish.
It has been previously shown that the flavonoid agathisflavone (FAB), a product of the oxidative coupling of an apigenin (API) dimer, enhances neurogenesis in murine pluripotent stem cells by increasing the expression of all trans retinoic acid (RA) receptors (RAR) (Citation2). As a single molecule, API also exhibits neuroprotective effects in mice models (Citation3–Citation5).
API has also been described as pro-estrogenic, acting as a phyto-estrogen and/or as an estrogen receptor (ER) modulator (Citation6–Citation8). Estrogen is known to affect the development, maturation, function, and plasticity of the nervous system, including synaptogenesis (Citation9).
It is known that human and rodent neural progenitors express ERs, including the ESR1 and ESR2 forms (Citation10–Citation12), and that ER and RAR signaling pathways interact during cell division and differentiation (Citation13, Citation14). Therefore, in human pluripotent stem cells and neural derivatives, ERs could represent a target for API to affect proliferation and differentiation (Citation15, Citation16), not necessarily having the same expected effects as estrogens (Citation17). Nevertheless, the potential of API to modulate the proliferation and differentiation of neural progenitor cells (NPCs) or neurons through ER and RAR pathways has never been explored.
Here we showed that API promotes neuronal conversion of human pluripotent stem cells as well as neuronal maturation through ER and RAR signaling.
Materials and methods
Methods are provided in the Supplementary file.
Results
API enhances expression of neural markers in human pluripotent stem cells
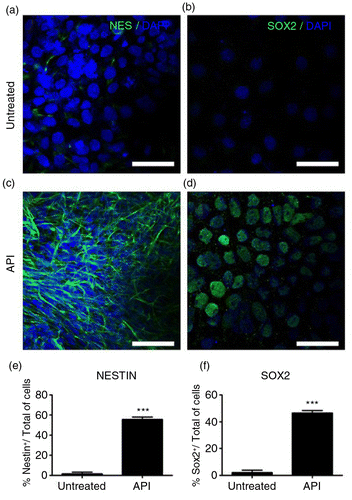
The role of the pro-estrogenic flavonoid API as a mediator of neurogenesis in human pluripotent stem cells was investigated by measuring the expression of neural markers (Citation18, Citation19) such as nestin (NES+; 5.1±1.7% control cells; 59.0±2.4% API-treated cells; a, c, and e; p=0.00103) and SOX2 (6.0±2.1% control cells; 46.1±1.8% API-treated cells; b, d, and f; p=0.00421), both upregulated in treated cells. API was used at a concentration of 10 µM (Supplementary Fig. 1a). Treatments with less than 10 µM of API (1–10 µM) to induce neurogenesis produced inconsistent results in our model. Nonlinear results at lower doses of API have also been described in other cells (Citation20, Citation21).
Fig. 1 Apigenin (API) enhances the expression of human neural precursor cell markers. Representative immunofluorescence images for detection of neural precursor cell markers nestin (NES; green, a and c) and SOX2 (green, b and d) counterstained with DAPI (blue). Human embryonic stem (hES) cells treated with 10 µM API for the six initial days of culture showed intense immunostaining for (c) NES and (d) SOX2. Bar graph showing the percentage of NES (e) and SOX2 (f) positive cells in each treatment group. Control cells (cells cultured without API) displayed few cells staining for both markers: (a) NES (p=0.00103) and (b) SOX2 (p=0.00421). Scale bar=50 µm. The values are expressed as the mean±SEM; n=3.
To investigate whether API interferes with cell proliferation or cell death, NPCs (after 18 days of culture) were stained for phosphorylated histone H3 (PH3), a G2/mitosis-specific marker, and cleaved caspase-3 (CASP3). API treatment did not affect the percentage of cells stained for PH3 (Supplementary Fig. 3a–c), but it decreased cell death as evidenced by the reduced percentage of cells stained for CASP3 (Supplementary Fig. 3d–f). In order to confirm that API effects were not cell-specific, we also applied API to two different human induced pluripotent stem (iPS) cell lines, obtaining similar results (Supplementary Fig. 2).
ERs affect neural differentiation induced by API
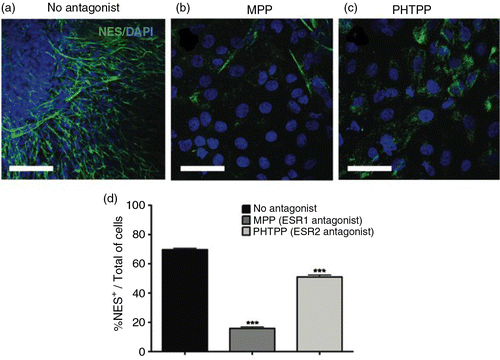
Considerable evidence confirms a role for ER signaling in the differentiation and maturation of neurons (Citation22, Citation23). Based on these observations, we postulated that the pro-estrogenic effects of API could account for the induced neural differentiation observed here. To investigate the involvement of ER signaling on API-induced neural commitment in human embryonic stem (hES) cells, antagonists of either the ESR1 or ESR2 forms of the ER were added to cultures before and during API treatment. Neural commitment was then measured at Day 18. Pharmacological antagonism of ESR1 achieved by using methyl-piperidino-pyrazole (MPP), a selective and widely used antagonist of this receptor, strongly inhibited NES+ expression. While in control cultures (no MPP) API treatment resulted in an average of 69.7% NES+ cells (a), the addition of MPP reduced the number of NES+ cells to 15.8% (b and d). This difference represents a statistically significant decrease in NES+ cells, attributable to ESR1 signaling inhibition (d and Supplementary Fig. 4a). Furthermore, blocking signaling of ESR2 with pyrazolo[1,5-a]pyrimidine (PHTPP) also reduced the number of NES+ cells from 69.7 to 51.0%, again representing a reduction in API effectiveness (c and d). Supplementary Fig. 4a.
Fig. 2 Estrogen receptor (ER) activation is required for API-induced differentiation into neural progenitors. Representative immunofluorescence images staining for (a) NES (green) counterstained with DAPI (blue). hES cells were treated with 10 µM API for the six initial days of culture in the presence of ER antagonists applied starting 2 h prior to API treatment. The antagonists used were (b) 10 nM methyl-piperidino-pyrazole (MPP; ESR1) and (c) 1 µM pyrazolo[1,5-a]pyrimidine (PHTPP; ESR2). API-induced neural differentiation was inhibited by ER antagonists. A reduction in the number of NES+ cells was observed after treatment with MPP (one-way ANOVA, p=0.0000833769) and with PHTPP (one-way ANOVA, p=0.00478). (d) Bar graph showing the percentage of NES+ cells in each treatment group; the values are expressed as the mean±SEM; n=3. Scale bar=50 µm.
RAR/RXRs are involved in API-induced neural differentiation
Previously, it was shown that FAB, which is formed by two API molecules, was able to increase neurogenesis in murine embryonic stem cells by stimulating the expression of RARs (Citation2). It is also known that activation of ERs promotes upregulation of RARs (RAR/RXRs), which have also been shown to be essential for neuronal differentiation (Citation24). Therefore, we hypothesized that API would also induce the expression of RAR in human cells. Indeed, we observed that API treatment increased the expression of RARA (b and c) compared to the control conditions (a and c). We also observed an increase in RARB (d and e) and RXRG (d and h) in the API-treated group. In contrast, the expression levels of RARG (d and f) and RXRA (d and g) were not affected by API, and RXRB was not detected (Fig. 3d). To confirm the involvement of RARs on human neural commitment induced by API, we applied antagonists to cultured cells 2 h before and during API differentiation (Day 0 to Day 6). Antagonists tested were ER50891 (RARA), LE135 (RARB), MM11253 (RARG), and UVI3003 (RXRs) (). In general, RAR antagonism strongly inhibited the effects of API on NES expression (Supplementary Fig. 4 and Fig. 4b). In this series of experiments, API induced NES expression in an average of 84.2% of cells (a and f). However, ER50891 efficiently reduced the number of NES+ cells, reaching only 16.0% of NES+ cells (b and f).
Fig. 3 API modulates retinoic acid receptor (RAR) expression. Images of dual labeling for NES (green) and RARA (red) show more intense staining for both proteins in hES cells (b) treated with 10 µM API compared to (a) untreated control cells. (c) The fluorescence intensity as a percentage of the total number of cells shows significantly increased RARA staining in API-treated cells compared to untreated controls (p=0.00047). (d) Western blotting showed increased expression of RARB (p=0.00276) and RXRG (p=0.00633), but not for RARG1 (p=0.10777), RXRA (p=0.70516), or RXRB in treated cells. Quantification of Western blotting for (e) RARB, (f) RARG1, (g) RXRA, and (h) RXRG are shown. The results were normalized relative to a control group considered as 100% and at least three independent experiments. The values are expressed as the mean±SEM; n=3, t-test. Scale bar=50 µm.
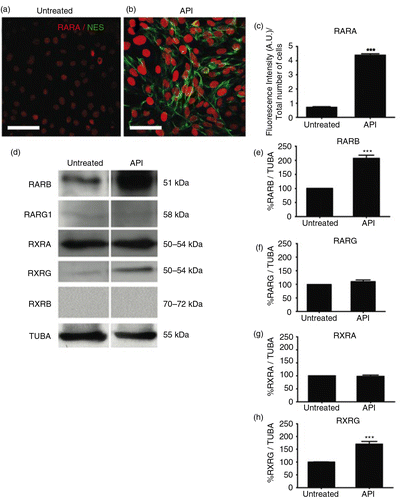
Fig. 4 RARs participate in neural differentiation. hES cells were treated with 10 µM API for the first 6 days of culture (18 days total) (a). RAR antagonists were applied to cell cultures starting 2 h before and during the entirety of API treatment. Images show immunofluorescence staining for NES (green) counterstained with DAPI (blue). API-induced neural differentiation was inhibited by RAR antagonists. A reduction in the number of NES+ cells was observed after treatment with (b) 10 µM ER 50891 (p=0.00701), (c) 10 µM LE 135 (p=0.000281), (d) 10 µM MM 11253 (p=0.001171), and (e) 10 µM UVI 3003 (p=0.001787) compared to (a) cells treated with API alone. Quantification is shown in (f). The values are expressed as the mean±SEM; n=3 (one-way ANOVA). Scale bar=50 µm.
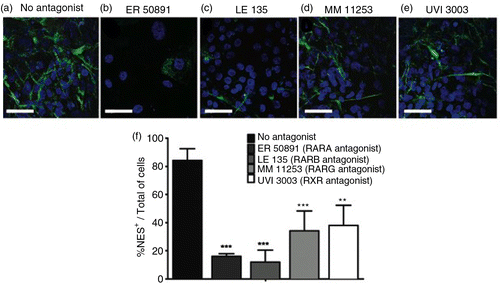
Blocking RARB signaling also attenuated the effects of API. LE135 reduced the number of NES+ cells, resulting in only 11.9% NES+ cells (c and f). Blocking the signaling of RARG (with MM 11253) and RXRs (with UVI 3003) reduced the number of NES+ cells after API treatment to 34.1 and 38.1%, respectively (d–f). The reduction in the number of NES+ cells observed after treatment with antagonists (f) indicates that RARs participate, at least partially, in the pro-differentiation effects of API.
NPCs induced by API become neurons
To investigate whether API-induced NPCs would be able to further differentiate into neurons, we isolated neural rosettes and allowed cells to differentiate for an additional 25 days. During these 25 days, we observed numerous processes growing from the cells, condensed soma and long neurites (b), similar to what is observed in neuronal primary cultures. These cells became positive for neuronal markers, such as β-tubulin-III (TUBB3, j), microtubule-associated protein 2 (MAP2, d), polysialylated-neural cell adhesion molecule (PSA-NCAM, l), neurofilament 200 (NEF, n), myelin basic protein (MBP, n), and calretinin (CALB2, h). API untreated controls could not be investigated further to check for mature neuronal markers, since these cultures do not form rosettes to be manually picked until Day 35. For this reason and also because the cells started to detach from the plate, we were not able to compare the amounts of markers between the two groups after this time point (Supplementary Fig. 5a and c).
Fig. 5 API induces human neuronal differentiation. Neural progenitors obtained from hES cells treated with API were allowed to differentiate for an additional 25 days in medium without API (see Methods in the Supplementary file). Undifferentiated hES cells were used as a negative control. Phase-contrast microscopy (a, b) or immunofluorescence staining for the following neuronal markers: (c, d) microtubule-associated protein 2 (MAP2), (e, f) synapsin 1 (SYN1), (g, h) calretinin (CALB2), (i, j) β-tubulin class III (TUBB3), (k, l) polysialic acid form of neural cell adhesion molecule (PSA-NCAM), (m, n) neurofilament (NEF), and (m, n) myelin basic protein (MBP), (o, p) synaptophysin (SYP), and (o, p) DLG4. Scale bar=50 µm.
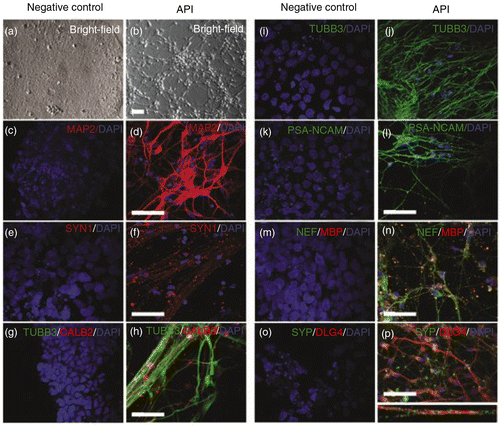
Next, these neurons were investigated to see if they displayed any evidence of functionality. Indeed, neurons expressed synaptic markers such as synapsin I (SYN1, f), discs large homolog 4 (DLG4), also known as postsynaptic density protein 95 (PSD95), and synaptophysin (SYP, p). Co-localization of presynaptic SYP and postsynaptic DLG4 (p) was also observed, suggesting that API-induced neurons were able to form synapses. Undifferentiated hES cells were used as negative controls (a) for all neuronal markers (c, e, g, i, k, m, and o).
API induces distinct subtypes of neural cells
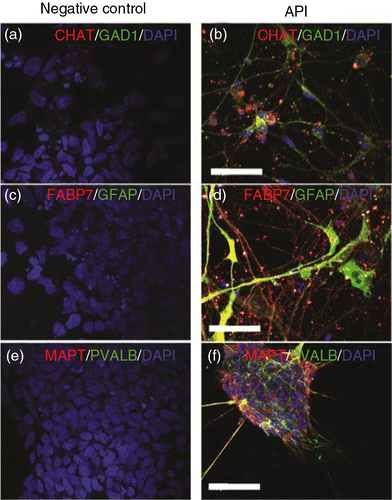
Among the different subtypes of neurons generated by treatment with API, cholinergic neurons (CHAT-positive; b and Supplementary Fig. 6) and GABAergic neurons (GAD1-positive, b) could be observed, including some cells that stained positive for parvalbumin (PVALB+, f), besides glial fibrillary acidic protein (GFAP, Supplementary Fig. 6) and brain lipid-binding protein (FABP7), which are glial markers (d). Hence, cell types generated after API exposure were heterogeneous, indicating that API can be used as the starting point for obtaining cultures of diverse cell subtypes. Control cells (pluripotent hES cells) were negative for all tested neuronal and glial markers (a, c, and e, and Supplementary Fig. 6).
Fig. 6 Distinct neuronal and glial markers after API neuronal differentiation. Neuronal diversity induced by API treatment is characterized by the presence of specific neuronal subtype markers such as (a, b) choline acetyltransferase (CHAT), (a, b) glutamate decarboxylase (GAD1), (c, d) glial fibrillary acidic protein (GFAP), (c, d) brain lipid-binding protein (FABP7), (e, f) parvalbumin (PVALB), and (e, f) tau protein (MAPT). Pluripotent hES H9 cells were used as a control and were negative for all neuronal and glial markers (a, c, e). Scale bar=50 µm.
Neurons treated with API show increased synaptogenesis
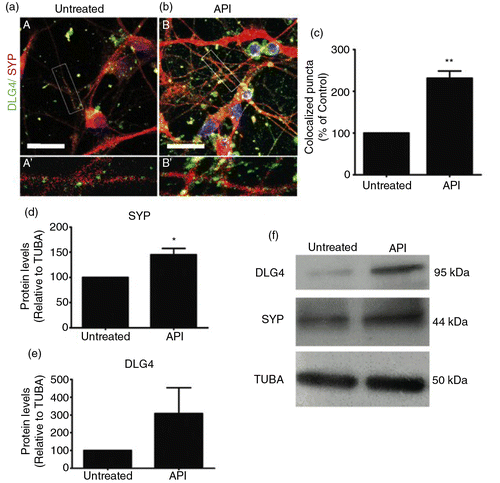
The results reported so far have consistently shown that API efficiently induces neural differentiation when applied to pluripotent stem cells. However, we were unable to check whether API further differentiates more neuronal precursors into mature neurons, because control cultures simply did not reach any neuronal differentiation spontaneously during the time points investigated. To circumvent this technical problem, we generated neurons by another method (RA treatment for 6 days, followed by a further 45 days in culture) and then treated them with 1 µM API for 72 h (Supplementary Fig. 1e). Control and API-treated cultures were then immunostained for DLG4 and synaptophysin to analyze synapse formation, markers of advanced neuronal maturation. As shown by confocal fluorescence microscopy, hES-derived neurons exposed to API exhibited a pronounced increase in co-localized synaptophysin and DLG4 compared to untreated neurons (a–c), suggesting that treatment of neurons with API improves synaptogenesis. API treatment increased SYP (d and f) by 74.3% compared to RA-induced neurons without API post-treatment (d and f). The treatment also increased DLG4 expression (f), but this increase was not statistically significant (e).
Fig. 7 API augments formation of synapses. Confocal microscopy of dual staining for the synaptic markers SYP (red) and DLG4 (green). Retinoic acid-induced neurons were treated with 1 µM API for 72 h. Untreated (A and A′) and API-treated (B and B′) cells presented neuronal morphology and were positively stained for SYP and DLG4. (b) API-treated cells showed more intense staining for both markers compared to (a) the untreated group. Insets show high-magnification images of selected areas in A and B with co-localized puncta in yellow (A′ and B′). (c) Higher co-localization indexes were measured after API treatment (t-test, p=0.0016; n=3). (d–f) Levels of synaptic protein expression were evaluated by Western blotting. Western blotting showed increased expression of SYP (p=0.0063287) but not DLG4 (p=0.287319) in treated cells. The results were normalized relative to a control group considered as 100% and at least three independent experiments. The values are expressed as the mean±SEM. Scale bar=50 µm.
Discussion
In this study, we investigated whether treatment with API was able to induce neural conversion of human pluripotent stem cells through activation of ERs and RAR/RXRs.
Neuronal differentiation of human pluripotent stem cells after API exposure gave rise to neural progenitors expressing NES and SOX2, as well as to neurons. This neural conversion was dependent, at least partly, on ER activation, since ER antagonists were capable of reducing differentiation of these cells. It is well known that estrogen may affect cell growth, proliferation, and differentiation (Citation25). In the nervous system, estrogen can also prevent cell death (Citation26–Citation28) or accelerate peripheral nerve regeneration (Citation29), as well as stimulate neurite outgrowth (Citation23, Citation30). It is worthy of mention, though, that flavonoids such as Icaritin, which can also activate ERs, direct mouse pluripotent stem cells toward neuronal differentiation in an ER-independent manner (Citation10). These results suggest that not necessarily all flavonoids that bind to ERs will have an effect mediated by these receptors, at least in murine cells.
Activation of ERs is also known to upregulate RARs and RXRs (Citation24). The fact that API promotes differentiation through activation of ERs raises the possibility that the increase in the expression of RARs (RARA, RARB, and RXRG) is a consequence of the activation of these receptors by the flavonoids (Citation31). Previously, it was shown that FAB, a product of the oxidative coupling of two API molecules, also increased the expression of RARs, RARA, and RARB, potentiating neuronal differentiation promoted by RA in mouse pluripotent stem cells (Citation2). Future works should clarify this correlation between RARs and ERs.
It is widely known that RARs are relevant to neurogenesis, since they are present in the subventricular zone of adult mice (Citation32) and, when sequentially activated, RARB and RARA lead to neuronal differentiation in cultured NPCs (Citation33). Additionally, the introduction of the human RARB deletion mutant (βΔC) into mouse embryonic stem cells resulted in desensitization to RA-induced differentiation (Citation34). Furthermore, RXRG is differentially expressed during remyelination of oligodendrocytes (Citation35). Our data showed that API treatment increased not only the expression of RXRG, but also that of MBP. Indeed, knockdown of RXRG or the receptor blockade inhibited oligodendrocyte differentiation in vitro, while administering RXR agonists to demyelinated cerebellar slice cultures or aged rats, caused an increase in remyelinated axons (Citation35). We demonstrated that blocking RARs reduced the effects of API, which suggests that human pluripotent stem cells require the recapitulation of important steps of in vivo differentiation. It is noteworthy that some ER and RAR/RXR antagonists prevented differentiation more than others, suggesting that these receptors do not contribute to differentiation to the same extent. Although we found that several types of ERs and RAR/RXRs are involved in API-induced differentiation of hES cells, more studies are needed to determine whether additional receptors participate in this process and which are the downstream biochemical cascades activated. API gave rise to NPCs that generated heterogeneous and mature neuronal cells (e.g. cholinergic and GABAergic neurons), indicating that it can be used as a starting point for obtaining enriched and diverse cultures of neurons that are affected in various brain disorders, such as amyotrophic lateral sclerosis, multiple sclerosis, and schizophrenia.
Finally, we also observed that API stimulates the formation of synapses in human neurons. After all, human neurons differentiated from pluripotent stem cells usually mature and form synapses at a slow pace in vitro(Citation36). This observation suggests that API could be used as a tool for neural differentiation, to accelerate neuronal maturation and synaptogenesis, and to make more robust in vitro models. These results endorse that API is not only a potent neurogenic agent but also a strong synaptogenic driver.
Strong evidence indicates estrogen's ability to regulate synapse structure and function (Citation23, Citation37). Shum et al. 2015 (Citation38) showed that human iPS-derived neurons were responsive to 17β-estradiol and that treatment with 17β-estradiol for 24 h resulted in an increase in dendritic branching. Estrogens may delay the onset or ameliorate the severity of psychiatric and neurodegenerative disorders such as schizophrenia (Citation39, Citation40), depression (Citation41, Citation42), Alzheimer's disease (Citation43, Citation44), and Parkinson's disease (Citation45, Citation46); however; the use of estrogen-based therapies is limited by the increased risk of estrogen-dependent tumors and cardiovascular problems. An alternative approach would be to mimic estrogenic-mediated positive effects by modulating specific ERs with other estrogenic compounds (Citation47, Citation48), such as some flavonoids classified as selective ER modulators (SERMs). Therefore, API is a strong candidate for replacing estrogen in neuronal differentiation strategies.
Conflict of interest and funding
The authors report no conflicts of interest, including personal or financial.
Authors' contributions
CSS, BSP, and SD performed the experiments. CSS, BSP, SLC, HLB, and SKR designed the experiments. BSP, SD, and SKR wrote the manuscript.
Supplementary Material
Download MS Word (3 MB)Acknowledgements
This work was supported by the Brazilian Development Bank (BNDES), Funding Authority for Studies and Projects (FINEP), Rio de Janeiro State Research Foundation (FAPERJ), and the National Council for Scientific and Technological Development (CNPq). The authors thank Ismael Gomes, Severino Galdino, and Grasiella M Ventura for technical assistance.
Notes
To access the supplementary material for this article, please see Supplementary files under ‘Article Tools’
References
- Koo HM, Mohamed S. The flavonoids (myricetin, quercetin, kaempferol, luteolin, apigenin) content of edible tropical plants. J Agr Food Chem. 2001; 6: 3106-12.
- Paulsen BS, Souza CS, Chicaybam L, Bonamino MB, Bahia M, Costa SL. Agathisflavone enhances retinoic acid-induced neurogenesis and its receptors alpha and beta in pluripotent stem cells. Stem Cells Dev. 2011; 20: 1711-21.
- Han JY, Ahn SY, Kim CS, Yoo SK, Kim SK, Kim HC. Protection of apigenin against kainate-induced excitotoxicity by anti-oxidative effects. Biol Pharm Bull. 2012; 35: 1440-6.
- Zhao L, Wang JL, Wang YR, Fa XZ. Apigenin attenuates copper-mediated β-amyloid neurotoxicity through antioxidation, mitochondrion protection and MAPK signal inactivation in an AD cell model. Brain Res. 2013; 1492: 33-45.
- Zhao L, Wang JL, Liu R, Li XX, Li JF, Zhang L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer's disease mouse model. Molecules. 2013; 18: 9949-65.
- Zand RS, Jenkins DJ, Diamandis EP. Steroid hormone activity of flavonoids and related compounds. Breast Canc Res Treat. 2000; 62: 35-49.
- Seol HS, Sato K, Murakami H, Toyomizu M, Akiba Y. Changes in gene expression involved in energy utilization during chicken follicle development. Anim Reprod Sci. 2006; 95: 283-94.
- Long X, Fan M, Nephew KP. Estrogen receptor-alpha-interacting cytokeratins potentiate the antiestrogenic activity of fulvestrant. Cancer Biol Ther. 2011; 5: 389-96.
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci USA. 2001; 98: 7093-100.
- Wang Z, Wang H, Wu J, Zhu D, Zhang X, Ou L. Enhanced coexpression of beta-tubulin III and choline acetyltransferase in neurons from mouse embryonic stem cells promoted by icaritin in an estrogen receptor-independent manner. Chem Biol Interact. 2009; 179: 375-85.
- Okada M, Makino A, Nakajima M, Okuyama S, Furukawa S, Furukawa Y. Estrogen stimulates proliferation and differentiation of neural stem/progenitor cells through different signal transduction pathways. Int J Mol Sci. 2010; 10: 4114-23.
- Okada M, Murase K, Makino A, Nakajima M, Kaku T, Furukawa S. Effects of estrogens on proliferation and differentiation of neural stem/progenitor cells. Biomed Res. 2008; 3: 163-70.
- Rishi AK, Shao ZM, Baumann RG, Li XS, Sheikh MS, Kimura S. Estradiol regulation of the human retinoic acid receptor alpha gene in human breast carcinoma cells is mediated via an imperfect half-palindromic estrogen response element and Sp1 motifs. Cancer Res. 1995; 21: 4999-5006.
- Elgort MG, Zou A, Marschke KB, Allegretto EA. Estrogen and estrogen receptor antagonists stimulate transcription from the human retinoic acid receptor-alpha 1 promoter via a novel sequence. Mol Endocrinol. 1996; 5: 477-87.
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999; 19: 14: 5792-801.
- Okada H, Tsutsumi A, Imai M, Nakajima T, Yasuda K, Kanzaki H. Estrogen and selective estrogen receptor modulators regulate vascular endothelial growth factor and soluble vascular endothelial growth factor receptor 1 in human endometrial stromal cells. Fertil Steril. 2010; 8: 2680-6.
- Galluzzo P, Marino M. Nutritional flavonoids impact on nuclear and extranuclear estrogen receptor activities. Genes Nutr. 2006; 1: 161-76.
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990; 60: 585-95.
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003; 39: 749-65.
- Navarro-Nunez L, Rivera J, Guerrero JA, Martinez C, Vicente V, Lozano ML. Differential effects of quercetin, apigenin and genistein on signalling pathways of proteaseactivated receptors PAR1 and PAR4 in platelets. Br J Pharmacol. 2009; 6: 1548-56.
- Long X, Fan M, Bigsby RM, Nephew KP. Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-α-dependent and –independent mechanisms. Mol Cancer Ther. 2008; 7: 2096-108.
- Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003; 4: 46-56.
- Srivastava DP, Woolfrey KM, Penzes P. Insights into rapid modulation of neuroplasticity by brain estrogens. Pharmacol Rev. 2013; 65: 1318-50.
- Prins GS, Chang WY, Wang Y, van Breemen RB. Retinoic acid receptors and retinoids are up-regulated in the developing and adult rat prostate by neonatal estrogen exposure. Endocrinology. 2002; 143: 3628-40.
- Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009; 30: 212-22.
- Sehara Y, Sawicka K, Hwang J-Y, Barrantes AL, Etgen AM, Zukin SR. Survivin is a transcriptional target of STAT3 critical to estradiol neuroprotection in global ischemia. J Neurosci. 2013; 33: 12364-74.
- Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol. 2013; 9: 241-50.
- Cardona-Rossinyol A, Mir M, Caraballo-Miralles V, Llado J, Olmos G. Neuroprotective effects of estradiol on motoneurons in a model of rat spinal cord embryonic explants. Cell Mol Neurobiol. 2013; 33: 421-32.
- Islamov RR, Hendricks WA, Jones RJ, Lyall GJ, Spanier NS, Murashov AK. 17Betaestradiol stimulates regeneration of sciatic nerve in female mice. Brain Res. 2002; 943: 283-6.
- Rozovsky I, Wei M, Stone DJ, Zanjani H, Anderson CP, Morgan TE. Estradiol (E2) enhances neurite outgrowth by repressing glial fibrillary acidic protein expression and reorganizing laminin. Endocrinology. 2002; 143: 636-46.
- Mak P, Leung YK, Tang WY, Harwood C, Ho SM. Apigenin suppresses cancer cell growth through ERb. Neoplasia. 2006; 8: 896-904.
- Haskell GT, LaMantia AS. Retinoic acid signaling identifies a distinct precursor population in the developing and adult forebrain. J Neurosci. 2005; 25: 7636-47.
- Goncalves MB, Agudo M, Connor S, McMahon S, Minger SL, Maden M. Sequential RARbeta and alpha signalling in vivo can induce adult forebrain neural progenitor cells to differentiate into neurons through Shh and FGF signalling pathways. Dev Biol. 2009; 326: 305-13.
- Chatzi C, van den Brink CE, van der Saag PT, McCaig CD, Shen S. Expression of a mutant retinoic acid receptor beta alters lineage differentiation in mouse embryonic stem cells. Stem Cells Dev. 2010; 19: 951-60.
- Huang JK, Jarjour AA, NaitOumesmar B, Kerninon C, Williams A, Krezel W. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 2011; 14: 45-53.
- Shi Y, Kirwan P, Livesey FJ. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc. 2012; 7: 1836-46.
- Sellers K, Raval P, Srivastava DP. Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition. Front Neuroendocrinol. 2014; 36: 72-89.
- Shum C, Macedo SC, Warre-Cornish K, Cocks G, Price J, Srivastava DP. Utilizing induced pluripotent stem cells (iPSCs) to understand the actions of estrogens in human neurons. Horm Behav. 2015; 74: 228-42.
- Gogos A, Van den Buuse M. Comparing the effects of 17beta-oestradiol and the selective oestrogen receptor modulators, raloxifene and tamoxifen, on prepulse inhibition in female rats. Schizophr Res. 2015; 168: 634-9.
- Labouesse MA, Langhans W, Meyer U. Effects of selective estrogen receptor alpha and beta modulators on prepulse inhibition in male mice. Psychopharmacology. 2015; 232: 2981-94.
- Hajszan T, Szigeti-Buck K, Sallam NL, Bober J, Parducz A, Maclusky NJ. Effects of estradiol on learned helplessness and associated remodeling of hippocampal spine synapses in female rats. Biol Psychiatry 2010; 67(2): 168-74 [PubMed CentralFull Text]
- Bredemann TM, McMahon LL. 17beta estradiol increases resilience and improves hippocampal synaptic function in helpless ovariectomized rats. Psychoneuroendocrinology. 2014; 42: 77-88.
- Zhang X, Wang J, Xing Y, Gong L, Li H, Wu Z. Effects of ginsenoside Rg1 or 17beta-estradiol on a cognitively impaired, ovariectomized rat model of Alzheimer's disease. Neuroscience. 2012; 220: 191-200.
- Zhao L, Mao Z, Chen S, Schneider LS, Brinton RD. Early intervention with an estrogen receptor beta-selective phytoestrogenic formulation prolongs survival, improves spatial recognition memory, and slows progression of amyloid pathology in a female mouse model of Alzheimer's disease. J Alzheimers Dis. 2013; 37: 403-19 [PubMed Abstract] [PubMed CentralFull Text]
- Bourque M, Dluzen DE, Di Paolo T. Signaling pathways mediating the neuroprotective effects of sex steroids and SERMs in Parkinson's disease. Front Neuroendocrinol. 2012; 33: 169-78.
- Rodriguez-Perez AI, Dominguez-Meijide A, Lanciego JL, Guerra MJ, Labandeira-Garcia JL. Inhibition of Rho kinase mediates the neuroprotective effects of estrogen in the MPTP model of Parkinson's disease. Neurobiol Dis. 2013; 58: 209-19.
- Hughes ZA, Liu F, Marquis K, Muniz L, Pangalos MN, Ring RH. Estrogen receptor neurobiology and its potential for translation into broad spectrum therapeutics for CNS disorders. Curr Mol Pharmacol. 2009; 2: 215-36.
- Zhao L, O'Neill K, Diaz Brinton R. Selective estrogen receptor modulators (SERMs) for the brain: current status and remaining challenges for developing NeuroSERMs. Brain Res Brain Res Rev. 2005; 49: 472-93.

