Abstract
Cell transplantation therapy using human pluripotent stem cell (PSC)–derived midbrain dopaminergic (mDA) neurons is soon expected to be available for patients with Parkinson's disease (PD). Highly efficient and reproducible protocols for the induction of mDA neurons for clinical application have already been reported, and the therapeutic potential and safety of these cells have been studied in parkinsonian animal models as preclinical trials. However, a new strategy that improves the survival and functional quality of the grafted mDA neurons is needed to achieve maximal efficacy of the cell transplantation therapy. One strategy would definitively be to adapt the brain's microenvironment with the use of small compounds, such as soluble factors and clinical drugs, in addition to current pharmacotherapies for PD. In this mini review, we focus on recent findings regarding the induction of mDA neurons from human PSCs toward clinical application and on a complementary strategy of drug treatment toward more efficient cell transplantation therapy for PD patients.
In context
Parkinson’s disease (PD) is a common neurodegenerative disorder that is characterized by the selective degeneration of midbrain dopaminergic (mDA) neurons. The loss of mDA neurons causes resting tremor, rigidity, bradykinesia, gait disturbances, and postural instability. Earlier, the main treatment for PD was pharmacotherapy using levodopa and dopamine receptor agonists. The efficacy of pharmacotherapy is gradually lost during long-term treatment, however, and side effects, such as the on–off phenomenon, wearing-off phenomenon, and drug-induced dyskinesia, begin to appear in later stages. In addition, pharmacotherapy cannot recover the lost mDA neurons. Therefore, cell transplantation therapy, which originally used aborted human embryonic tissue but has now expanded to other pluripotent stem cells (PSCs) sources, was developed to restore the lost mDA neurons in PD patients as a therapeutic option. In this study, we review recent progress in cell transplantation therapies and examine how drug treatment can improve PD patient outcome.
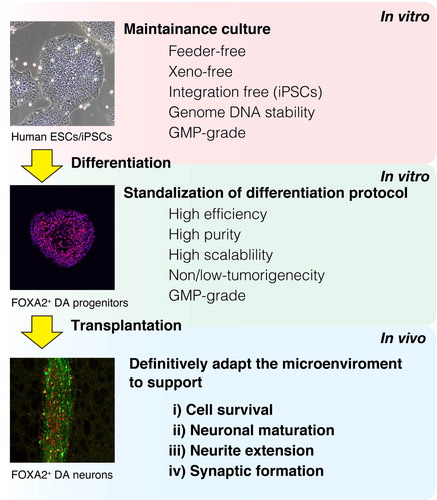
In the 1980s in Sweden and the United States, cell transplantation therapy for patients with Parkinson's disease (PD) was first performed with the grafting of human midbrain tissue from aborted fetuses (Citation1–Citation3). Fetal midbrain tissue allografts were shown to improve the motor function in some PD patients, an effect that lasted many years (Citation4–Citation9). However, strict ethical and logistic concerns limit the use of aborted fetal tissue for cell transplantation therapy. In addition, collecting donor cells from aborted embryonic tissue is technically difficult. Therefore, aborted fetal tissue is hard to standardize as donor cells. Human pluripotent stem cells (PSCs) such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are an alternative for cell transplantation therapy. These cells proliferate limitlessly and offer more advantages as donor cells than do human aborted embryonic tissue (Citation10–Citation12). Recent reports have indicated that human PSC–derived midbrain dopaminergic (mDA) neurons improved the rotational behavior of 6-hydroxydopamine (6-OHDA)–lesioned hemi-parkinsonian rat model long-term (Citation13–Citation16). However, most grafted cells die following transplantation as a result of mechanical and oxidative stress and a depletion of trophic factors (Citation17). To provide efficient cell transplantation therapy, it is necessary to maintain the survival of the grafted mDA neurons (Citation18, Citation19). In addition, conditions that promote the maturation, axonal growth, and innervation of the mDA neurons would help restore the lost neuronal function (Citation20–Citation23) (). However, since the microenvironment of the adult brain can only suboptimally facilitate the above phenomena, extensive research has considered small molecules, such as soluble factors or chemical compounds, to definitively adapt the microenvironment appropriately. Clinically, candidate factors must diffuse into the brain parenchyma by crossing the blood–brain barrier in order to exert a pharmacological effect. Therefore, already-approved drugs are preferred candidates. Here we review recent progress regarding the induction method of mDA neurons from hPSCs and drug treatment that supports the quality of cell transplantation therapy for PD patients.
Fig. 1 Cell transplantation strategy using human PSCs for PD patients. PSCs are cultured and then differentiated into DA progenitors. These progenitors are transplanted. In order to promote the efficacy of the grafted DA neurons, the brain microenvironment should be definitively adapted to promote the survival, maturation, neurite extension, and synaptic formation of the grafted cells.
Induction of transplantable mDA neurons from human PSCs
Human PSC is the preferred cell source for cell transplantation therapy instead of human fetal tissue, in part because of its ability for self-renewal and to differentiate into every type of somatic cell in the body. Human ESCs were first established from inner cell mass at the blastocyst stage (Citation24), and good manufacturing practice (GMP)–grade human ESC lines were recently established on human recombinant protein toward clinical application (Citation25, Citation26). Human iPSCs came several years later and were first generated by reprogramming somatic cells using four transcription factors (Citation27, Citation28). Although that original iPSC method is not compatible with clinical application, human iPSCs have since been established using safe methods that avoid gene transduction into the genome DNA (Citation29, Citation30) and are now available for clinical application. An additional benefit of iPSCs is that since they are derived from somatic cells, they can be used for autologous cell transplantation therapy, which could eliminate the need for immunosuppression (Citation31, Citation32).
Knowledge on how mDA neurons develop in vivo has allowed for the generation of mDA neurons from human PSCs in vitro. Indeed, recent protocols for the induction of mDA neurons control regional identity by mimicking midbrain organogenic processes (Citation33). For example, the inhibition of a dual smad signal with a transforming growth factor-β (TGF-β)/activin/nodal inhibitor and a bone morphogenic protein inhibitor drastically improved the commitment to neuronal lineage from undifferentiated human PSCs (Citation34, Citation35). Additionally, correct midbrain specification was induced by an optimal concentration of a glycogen synthase kinase 3β (GSK3β) inhibitor that dose-dependently activates Wnt signaling to induce LMX1A expression in FOXA2+ floor plate precursors (Citation13, Citation14). The protocol based on dual smad inhibition and GSK3β inhibition was reproducibly reported from other groups to induce mDA neurons from human PSCs (Citation36, Citation37). However, in general, differentiation protocols have inefficiencies that limit their clinical application without additional actions. Indeed, the differentiated cells are not homogeneous and may still contain unwanted cells such as residual undifferentiated cells or immature neural progenitor cells, which may cause neural overgrowth or tumor formation after grafting. Cell sorting technology can eliminate the unwanted cells and purify mDA neurons by using transgenic cell lines or antibodies against cell surface antigens (Citation38–Citation42). Recently, cell sorting using CORIN, a floor plate marker, provided an efficient collection of mDA progenitors and the elimination of contaminating undifferentiated cells and immature neural cells. In addition, scalable monolayer differentiation was achieved by using recombinant human laminin511-E8 fragment (Citation16). The mDA progenitors that were induced by this protocol survived well, differentiated into mature mDA neurons, and contributed to improve motor function in a parkinsonian rat model after striatal transplantation without any tumor formation or neural overgrowth. Before these protocols can be used for clinical application, however, they will need to use defined human components (xeno-free) and chemically defined materials. Additionally, safe cell maintenance and differentiation should be performed under GMP, and guidelines and safety regulations for clinical transplantation are still awaited (Citation37, Citation43–Citation46).
Successful cell transplantation therapy supported by clinical drugs
Factors that promote the survival, maturation, axonal growth, and neuronal innervation of the grafted DA neurons will enhance cell transplantation therapy for PD. For this purpose, several small molecules have been reported (Citation18–Citation21, Citation47) (Citation48).
Neuronal innervation and functional synaptic formation between grafted DA neurons and host striatal neurons are crucial for restoring the lost neuronal function of PD patients (Citation22, Citation49). Indeed, several technologies, such as rabies virus–mediated monosynaptic tracing, optogenetics, and DREADD (designer receptor exclusively activated by designer drugs) have been developed to evaluate the synaptic connection between the grafted DA neurons and host striatal neurons (Citation50–Citation52). Recently, it has been reported that the systemic administration of an estradiol derivative, estradiol-2-benzoate (E2B), promotes the activation of integrin α5, which is highly expressed in striatal neurons innervated by mDA neurons in adult rodent, and facilitates the behavioral recovery of 6-OHDA-lesioned rats via synaptic formation between the grafted iPSC-derived DA neurons and host striatal neurons (Citation23). Moreover, the expression level of integrin α5 was preserved in postmortem putamen of PD patients. These findings suggested that systemic administration of E2B modifies a microenviromental cue for synaptic formation via activation of integrin α5 in host striatal neurons, thus potentially providing pharmacotheraputic benefit in humans.
Conclusion and future directions
Although cell transplantation therapy for PD is approaching clinical application, there remain several issues that need to be resolved first. For example, human PSC–derived DA neurons need to be generated under GMP standards. Also, regulatory issues are still required for safe clinical trials. In addition, it is important to optimize the donor cells and also the host brain environment. For the latter, a research platform for drug discovery to definitively adapt the microenvironment is needed. All these innovations should lead to successful cell transplantation therapy for PD patients.
Conflict of interest and funding
The authors declare no conflict of interest. This study was supported by the following grants: a grant from the Research Center Network for Realization of Regenerative Medicine from the Japan Agency for Medical Research and Development (JT) and The Kyoto University Foundation (KN).
Acknowledgements
We thank Dr. Peter Karagiannis for critical reading of the manuscript.
References
- Brundin P, Strecker RE, Lindvall O, Isacson O, Nilsson OG, Barbin G et al. Intracerebral grafting of dopamine neurons. Experimental basis for clinical trials in patients with Parkinson's disease. Ann N Y Acad Sci. 1987; 495: 473-96. [ PubMed Abstract]
- Lindvall O, Rehncrona S, Brundin P, Gustavii B, Astedt B, Widner H et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson's disease. A detailed account of methodology and a 6-month follow-up. Arch Neurol. 1989; 46: 615-31. [ PubMed Abstract]
- Hauser RA, Freeman TB, Snow BJ, Nauert M, Gauger L, Kordower JH et al. Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol. 1999; 56: 179-87. [ PubMed Abstract]
- Widner H, Tetrud J, Rehncrona S, Snow B, Brundin P, Gustavii B et al. Bilateral fetal mesencephalic grafting in two patients with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). N Engl J Med. 1992; 327: 1556-63. [ PubMed Abstract]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001; 344: 710-19. [ PubMed Abstract]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003; 54: 403-14. [ PubMed Abstract]
- Mendez I, Viñuela A, Astradsson A, Mukhida K, Hallett P, Robertson H et al. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008; 14: 507-9. [ PubMed Abstract] [PubMed CentralFull Text]
- Hallett PJ, Cooper O, Sadi D, Robertson H, Mendez I, Isacson O. Long-term health of dopaminergic neuron transplants in Parkinson's disease patients. Cell Rep. 2014; 7: 1755-61. [ PubMed Abstract] [PubMed CentralFull Text]
- Kefalopoulou Z, Politis M, Piccini P, Mencacci N, Bhatia K, Jahanshahi M et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014; 71: 83-7. [ PubMed Abstract] [PubMed CentralFull Text]
- Lindvall O, Kokaia Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson's disease. Trends Pharmacol Sci. 2009; 30: 260-7. [ PubMed Abstract]
- Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, Bramoulle Y et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson's disease. Cell Stem Cell. 2014; 15: 653-65. [ PubMed Abstract] [PubMed CentralFull Text]
- Lindvall O. Clinical translation of stem cell transplantation in Parkinson's disease. J Intern Med. 2016; 279: 30-40. [ PubMed Abstract]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011; 480: 547-51. [ PubMed Abstract] [PubMed CentralFull Text]
- Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012; 1: 703-14. [ PubMed Abstract]
- Sundberg M, Bogetofte H, Lawson T, Jansson J, Smith G, Astradsson A et al. Improved cell therapy protocols for Parkinson's disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells. 2013; 31: 1548-62. [ PubMed Abstract]
- Doi D, Samata B, Katsukawa M, Kikuchi T, Morizane A, Ono Y et al. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2014; 2: 337-50.
- Sortwell CE, Pitzer MR, Collier TJ. Time course of apoptotic cell death within mesencephalic cell suspension grafts: implications for improving grafted dopamine neuron survival. Exp Neurol. 2000; 165: 268-77. [ PubMed Abstract]
- Rosenblad C, Martinez-Serrano A, Björklund A. Glial cell line-derived neurotrophic factor increases survival, growth and function of intrastriatal fetal nigral dopaminergic grafts. Neuroscience. 1996; 75: 979-85. [ PubMed Abstract]
- Koyanagi M, Takahashi J, Arakawa Y, Doi D, Fukuda H, Hayashi H et al. Inhibition of the rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J Neurosci Res. 2008; 86: 270-80. [ PubMed Abstract]
- Parish CL, Castelo-Branco G, Rawal N, Tonnesen J, Sorensen AT, Salto C et al. Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice. J Clin Invest. 2008; 118: 149-60. [ PubMed Abstract]
- Kauhausen JA, Thompson LH, Parish CL. Chondroitinase improves midbrain pathway reconstruction by transplanted dopamine progenitors in parkinsonian mice. Mol Cell Neurosci. 2015; 69: 22-9. [ PubMed Abstract]
- Peng SP, Schachner M, Boddeke E, Copray S. Effect of cell adhesion molecules on the neurite outgrowth of induced pluripotent stem cell-derived dopaminergic neurons. Cell Reprogram. 2016; 18: 55-66. [ PubMed Abstract]
- Nishimura K, Doi D, Samata B, Murayama S, Tahara T, Onoe H et al. Estradiol facilitates functional integration of iPSC-derived dopaminergic neurons into striatal neuronal circuits via activation of integrin α5β1. Stem Cell Rep. 2016; 6: 932-44.
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282: 1145-7. [ PubMed Abstract]
- Rodin S, Antonsson L, Niaudet C, Simonson OE, Salmela E, Hansson EM et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun. 2014; 5: 3195 [ PubMed Abstract]
- Canham MA, Van Deusen A, Brison DR, De Sousa PA, Downie J, Devito L et al. The molecular karyotype of 25 clinical-grade human embryonic stem cell lines. Sci Rep. 2015; 5: 17258 [ PubMed Abstract] [PubMed CentralFull Text]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131: 861—72
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318: 1917-20. [ PubMed Abstract]
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011; 8: 409-12. [ PubMed Abstract]
- Nakagawa M, Taniguchi Y, Senda S, Takizawa N, Ichisaka T, Asano K et al. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep. 2014; 4: 3594 [ PubMed Abstract] [PubMed CentralFull Text]
- Morizane A, Doi D, Kikuchi T, Okita K, Hotta A, Kawasaki T et al. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a nonhuman primate. Stem Cell Rep. 2013; 1: 283-92.
- Hallett PJ, Deleidi M, Astradsson A, Smith GA, Cooper O, Osborn TM et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson's disease. Cell Stem Cell. 2015; 16: 269-74. [ PubMed Abstract] [PubMed CentralFull Text]
- Arenas E, Denham M, Villaescusa JC. How to make a midbrain dopaminergic neuron. Development. 2015; 142: 1918-36. [ PubMed Abstract]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009; 27: 275-80. [ PubMed Abstract] [PubMed CentralFull Text]
- Morizane A, Doi D, Kikuchi T, Nishimura K, Takahashi J. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J Neurosci Res. 2011; 89: 117-26. [ PubMed Abstract]
- Denham M, Bye C, Leung J, Conley BJ, Thompson LH, Dottori M. Glycogen synthase kinase 3β and activin/nodal inhibition in human embryonic stem cells induces a pre-neuroepithelial state that is required for specification to a floor plate cell lineage. Stem Cells. 2012; 30: 2400-11. [ PubMed Abstract] [PubMed CentralFull Text]
- Sundberg M, Isacson O. Advances in stem-cell-generated transplantation therapy for Parkinson's disease. Expert Opin Biol Ther. 2014; 14: 437-53. [ PubMed Abstract]
- Fukuda H, Takahashi J, Watanabe K, Hayashi H, Morizane A, Koyanagi M et al. Fluorescence-activated cell sorting-based purification of embryonic stem cell-derived neural precursors averts tumor formation after transplantation. Stem Cells. 2006; 24: 763-71. [ PubMed Abstract]
- Pruszak J, Ludwig W, Blak A, Alavian K, Isacson O. CD15, CD24, and CD29 define a surface biomarker code for neural lineage differentiation of stem cells. Stem Cells. 2009; 27: 2928-40. [ PubMed Abstract] [PubMed CentralFull Text]
- Chung S, Moon JI, Leung A, Aldrich D, Lukianov S, Kitayama Y et al. ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proc Natl Acad Sci USA. 2011; 108: 9703-8. [ PubMed Abstract] [PubMed CentralFull Text]
- Ganat YM, Calder EL, Kriks S, Nelander J, Tu EY, Jia F et al. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J Clin Invest. 2012; 122: 2928-39. [ PubMed Abstract] [PubMed CentralFull Text]
- Bye CR, Jönsson ME, Björklund A, Parish CL, Thompson LH. Transcriptome analysis reveals transmembrane targets on transplantable midbrain dopamine progenitors. Proc Natl Acad Sci USA. 2015; 112: E1946-55. [ PubMed Abstract] [PubMed CentralFull Text]
- Peng J, Liu Q, Rao MS, Zeng X. Survival and engraftment of dopaminergic neurons manufactured by a good manufacturing practice-compatible process. Cytotherapy. 2014; 16: 1305-12. [ PubMed Abstract]
- Carpenter MK, Rao MS. Concise review: making and using clinically compliant pluripotent stem cell lines. Stem Cells Transl Med. 2015; 4: 381-8. [ PubMed Abstract] [PubMed CentralFull Text]
- Morizane A, Takahashi J. Cell therapy for Parkinson's disease. Neurol Med Chir (Tokyo). 2016; 15: 102-9.
- Daley G, Hyun I, Apperley J, Barker R, Benvenisty N, Bredenoord A et al. Setting global standards for stem cell research and clinical translation: the ISSCR guidelines. Stem Cell Rep. 2016; 6: 787-97.
- Yoshikawa T, Samata B, Ogura A, Miyamoto S, Takahashi J. Systemic administration of valproic acid and zonisamide promotes differentiation of induced pluripotent stem cell-derived dopaminergic neurons. Front Cell Neurosci. 2013; 7: 11 [ PubMed Abstract] [PubMed CentralFull Text]
- Nishimura K, Murayama S, Takahashi J. Identification of neurexophilin 3 as a novel supportive factor for survival of induced pluripotent stem cell-derived dopaminergic progenitors. Stem Cells Transl Med. 2015; 4: 932-44. [ PubMed Abstract] [PubMed CentralFull Text]
- Sørensen AT, Thompson L, Kirik D, Björklund A, Lindvall O, Kokaia M. Functional properties and synaptic integration of genetically labelled dopaminergic neurons in intrastriatal grafts. Eur J Neurosci. 2005; 21: 2793-9.
- Grealish S, Heuer A, Cardoso T, Kirkeby A, Jönsson M, Johansson J et al. Monosynaptic tracing using modified rabies virus reveals early and extensive circuit integration of human embryonic stem cell-derived neurons. Stem Cell Rep. 2015; 4: 975-83.
- Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson's disease model. Nat Biotechnol. 2015; 33: 204-9. [ PubMed Abstract]
- Chen Y, Xiong M, Dong Y, Haberman A, Cao J, Liu H et al. Chemical control of grafted human PSC-derived neurons in a mouse model of Parkinson's disease. Cell Stem Cell. 2016; 18: 817-26. [ PubMed Abstract] [PubMed CentralFull Text]

