Abstract
Aim: The aim of this study was the synthesis of steroid compounds with heterocyclic rings and good anticancer properties. Materials & methods: The synthesis, in silico and in vitro anticancer testing of novel pyridin-2-yl estra-1,3,5(10)-triene derivatives was performed. Results: All synthesized compounds have shown promising results for, antiproliferative activity, relative binding affinities for the ligand binding domains of estrogen receptors α, β and androgen receptor, aromatase binding potential, and inhibition of AKR1C3 enzyme. Conclusion: 3-Benzyloxy (17E)-pycolinilidene derivative 9 showed the best antitumor potential against MDA-MB-231 cell line, an activity that can be explained by its moderate inhibition of AKR1C3. Molecular docking simulation indicates that it binds to AKR1C3 in a very similar orientation and geometry as steroidal inhibitor EM1404.
Tweetable abstract
The series of pyridine-containing estra-1,3,5(10)-triene derivatives was synthesized. One novel derivative stood out by its excellent activity against the MDA-MB-231 cell line. This activity can be explained by its moderate inhibition of the AKR1C3 enzyme.
Graphical abstract
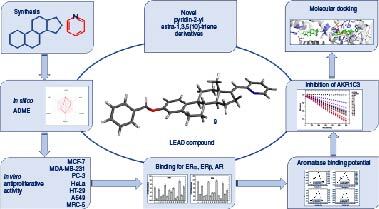
The synthesis of a series of novel pyridin-2-yl analogs was described.
In silico absorption, distribution, metabolism and excretion testing has indicated optimal properties for all synthesized compounds.
Antiproliferative activity was tested against six tumor cell lines, most of the compounds had strong antiproliferative activity against at least one tumor cell line.
Binding to ERα, ERβ, androgen receptor (AR) and aromatase, and inhibition of AKR1C3 were tested in vitro.
Molecular docking simulations were performed against ERα, AR and AKR1C3.
3-Benzyloxy (17E)-pycolinilidene derivative 9 showed the most promising antitumor potential against the MDA-MB-231 cell line.
This activity can be explained by its moderate inhibition of AKR1C3 enzyme.
Molecular docking simulation indicates that this novel compound binds to AKR1C3 in a very similar orientation and geometry as steroidal inhibitor EM1404.
Author contributions
Conception, drafting or design of the work and revising it critically for important intellectual content: I Kuzminac; acquisition and analysis M Stevanović, S Bekić, D Jakimov and E Petri; interpretation of data for the work: I Kuzminac, M Stevanović, A Ćelić and E Petri; drafting the work and revising it critically for important intellectual content: E Petri and M Sakač.
Financial disclosure
The authors acknowledge the financial support of Provincial Secretariat for Higher Education and Scientific Research of the Autonomous Province of Vojvodina [Project: New steroid derivatives – potential chemotherapeutics, No. 142-451-3463/2023-01] and the Ministry of Science, Technological Development and Innovation of the Republic of Serbia [Grant No. 451-03-66/2024-03/200125 and 451-03-65/2024-03/200125]. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.

