Figures & data
Table 2. Fundamental IR spectral data of calix[Citation4]pyrrole compounds (1, 2, 3 and 4).
Figure 1. TGA-DSC of meso-tetramethyltetrakis-[4-hydroxyphenyl]calix[Citation4]-pyrrole (1).
![Figure 1. TGA-DSC of meso-tetramethyltetrakis-[4-hydroxyphenyl]calix[Citation4]-pyrrole (1).](/cms/asset/94474d46-3fde-4383-b7c0-cfc0c56ac572/tcme_a_884940_f0001_c.jpg)
Figure 2. TGA/differential thermogravimetry-DTA of meso-tetramenthyltetrakis-[4-(2-ethoxy)ethoxyphenyl] calix[Citation4]pyrrole polymer (4).
![Figure 2. TGA/differential thermogravimetry-DTA of meso-tetramenthyltetrakis-[4-(2-ethoxy)ethoxyphenyl] calix[Citation4]pyrrole polymer (4).](/cms/asset/8125044d-54d0-460a-ac66-b81bf2215aa8/tcme_a_884940_f0002_c.jpg)
Table 3. Thermal analysis data, TG, DSC/DTA for the compounds (1, 3 and 4).
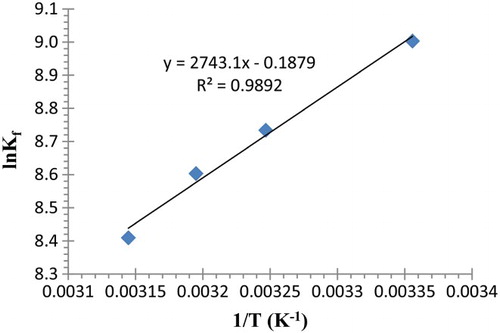
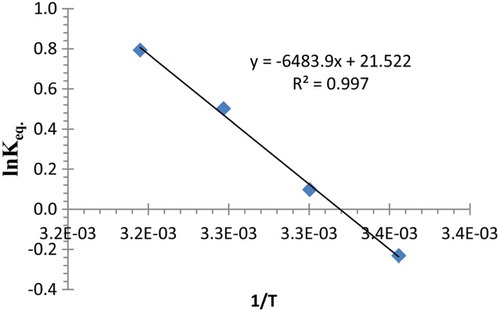
Table 4. Values of Kf for complexation of Pb2+ with (2) obtained at different temperatures in acetonitrile.
Table 5. Thermodynamic parameters for complexation of Pb2+ with (2) in acetonitrile.
Figure 6. Plot of the variation in Kf for complexation of Pb2+ with (2) as a function of (1/T) in acetonitrile.