Figures & data
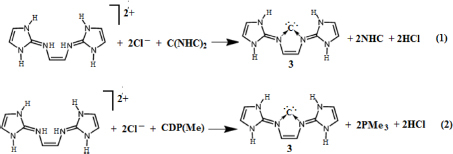
Figure 1 Schematic representation of carbodicarbene, (1) C(NHC)2 and (2) CDP.
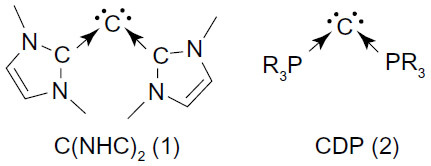
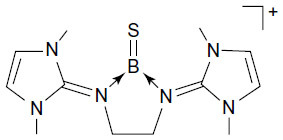
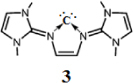
Figure 2 Schematic representation of bis(imidazolin-2-iminium) cation (A) and the boron cation compound (B).
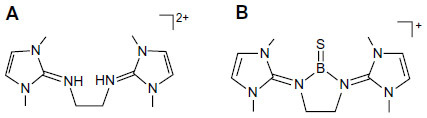
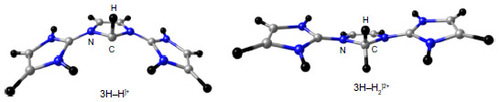
Figure 4 Optimized geometries at BP86/TZVP level of theory and relative energies (kcal/mol at MP2/aug-cc-pVDZ//BP86/TZVP) of different conformers of 3H.
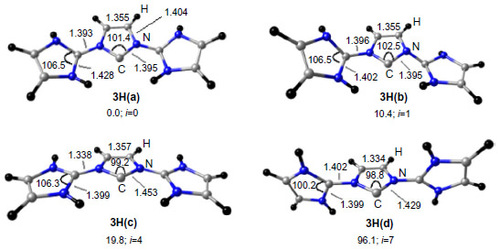
Figure 5 Shape of frontier Kohn–Sham orbitals of 3H and orbital energies ε in eV at the BP86/TZVP level of theory.
Table 1 MP2/aug-cc-pVDZ//BP86/TZVP calculated proton affinities (in gas phase); PAs (kcal/mol) of 1–3
Figure 7 BP86/BSI (BSI = TZVP for H, C, N, Cl and SDD for Au) optimized protonated derivatives of 3H.
Abbreviation: SDD, Stuttgart/Dresden.
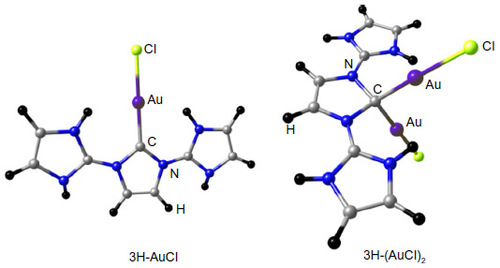
Table 2 Calculated reaction Gibbs free energies (kcal/mol) of reactions 1 and 2 at T=298.15 K and P=1 atm