Abstract
Background
In the single-arm CHRYSALIS trial, advanced non-small cell lung cancer patients harboring epidermal growth factor receptor (EGFR) exon 20 insertion (Exon 20ins) showed durable responses to amivantamab, an EGFR-MET bispecific antibody targeting tumors with EGFR Exon 20ins. This study compared the effectiveness of amivantamab to real-world systemic anti-cancer therapies in Japan.
Patients and methods
External control patients were selected by applying CHRYSALIS eligibility to Japanese patients from LC-SCRUM-Asia. External control patients were included for every qualifying line of therapy after platinum-based chemotherapy. Propensity score weighting was applied to external control patients to adjust for differences in baseline characteristics. Outcomes were compared between external control patients, and all and Asian-only CHRYSALIS patients using weighted Cox proportional hazards regression models for progression-free survival (PFS), time to next therapy (TTNT), and overall survival (OS), and generalized estimating equations with repeated measurements for overall response rate (ORR).
Results
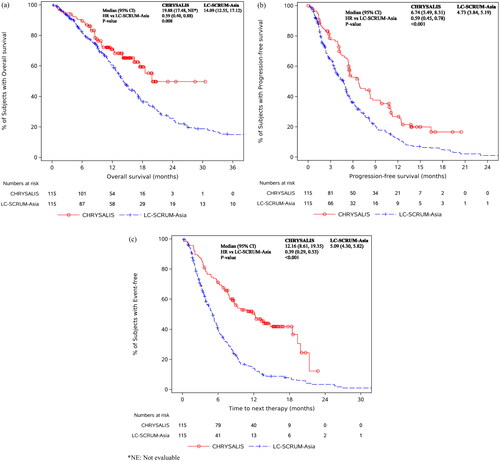
One hundred fifteen CHRYSALIS and 94 external control patients were identified. Compared to external control patients, amivantamab-treated patients had significantly longer OS (median OS 19.88 vs 14.09 months, HR [95% CI] 0.59 [0.40–0.88]), PFS (median PFS 6.74 vs 4.73 months, HR 0.59 [0.45–0.78]), TTNT (median TTNT 12.16 vs 5.09 months, HR 0.39 [0.29–0.53]), and significantly higher ORR (41.7% vs 14.1%). Analyses of amivantamab-treated Asian patients (n = 61) showed similar clinical benefits.
Conclusion
In the absence of clinical evidence from randomized clinical trials, this study reflects the benefit of amivantamab after platinum-based chemotherapy for advanced non-small cell lung cancer patients harboring EGFR Exon 20ins, compared to current real-world therapies.
Introduction
Lung cancer is the leading cause of cancer deaths worldwide, with an estimated 2.2 million new cases and 1.8 million deaths in 2020 [Citation1]. In Asia, there were 1.3 million new cases and 1.1 million deaths recorded in 2020 [Citation2]. Non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancer cases, with 5-year survival estimates ranging from 78% in stage IA disease to 6% in stage IV disease based on the eighth edition of the Tumor Node Metastasis (TNM) staging system developed by the International Association for the Study of Lung Cancer [Citation3–5].
Epidermal growth factor receptor (EGFR)-activating mutations are detected in approximately 30–50% of patients with NSCLC in Asia [Citation6]. Exon 19 deletions and exon 21 L858R point mutations account for 85–90% of all EGFR mutations in NSCLC [Citation7–10]. These common mutations have shown good clinical response to EGFR tyrosine kinase inhibitors (TKIs), but uncommon mutations of the EGFR gene in NSCLC are associated with a poorer response to EGFR TKIs [Citation8,Citation10–13]. Approximately 4–12% of primary EGFR gene mutations are exon 20 insertions (Exon 20ins), and these constitute the most frequent uncommon mutations [Citation14–17].
Limited real-world data so far indicate that advanced NSCLC (aNSCLC) patients with EGFR Exon 20ins are largely resistant to EGFR TKIs. Multiple studies in the Asian population (China, Taiwan, and India) showed that among metastatic patients with different EGFR exon 20 mutation subtypes, EGFR Exon 20ins showed the worst progression-free survival and overall survival with first-generation EGFR TKIs in the first line of treatment as well as subsequent lines [Citation18–20]. Structural analysis of EGFR Exon 20ins suggests that changes in the molecule’s drug-binding pocket shape reduced the ability of inhibitor molecules to bind to EGFR [Citation21]. Third-generation EGFR TKI osimertinib has been shown to overcome the reduced drug sensitivity conferred by some EGFR Exon 20ins in vitro and in vivo [Citation22–24]. However, the resistance of EGFR Exon 20ins to EGFR TKI therapy remains a therapeutic challenge [Citation25]. As such, platinum-based chemotherapy is the standard first-line of therapy for most aNSCLC patients with EGFR Exon 20ins [Citation26,Citation27]. However, prognosis for these patients remains poor; progression after platinum-based chemotherapy eventually occurs [Citation28,Citation29]. Thus, there is a high unmet need for effective treatments for aNSCLC patients with EGFR Exon 20ins.
Amivantamab is a fully human EGFR and MET bispecific antibody with immune cell-directing activity [Citation30]. In the Phase 1 CHRYSALIS trial, amivantamab demonstrated robust and durable anti-tumor activity with manageable safety profile in aNSCLC patients with EGFR Exon 20ins who had progressed on or after platinum-based chemotherapy [Citation30,Citation31]. However, as CHRYSALIS was a non-randomized, single-arm trial, a study was conducted to identify a real-world control arm from three databases in the United States (US) to provide comparative evidence of the clinical benefit of amivantamb [Citation32]. In the study, the number of Asian patients was more prevalent in the amivantamb-treated cohort than in the US-based dataset. This study aimed to derive a clinically similar external control (EC) cohort with Asian patients. Thus, the LC-SCRUM-Asia database in Japan was utilized to assess the clinical benefit of amivantamab in aNSCLC patients with EGFR Exon 20ins who progressed on or after platinum-based chemotherapy in comparison to contemporary systemic anticancer therapy approaches in real-world practice in Japan.
Materials and methods
Data sources
This was a comparative study utilizing data from the CHRYSALIS trial and Japanese cohort from the LC-SCRUM-Asia database. CHRYSALIS (ClinicalTrials.gov identifier: NCT02609776) sought to evaluate the safety, pharmacokinetics, and preliminary efficacy of amivantamab in NSCLC patients with EGFR Exon 20ins and progressed on or after platinum-based chemotherapy [Citation33]. The methods and initial findings have been previously reported [Citation30].
LC-SCRUM-Asia is a genetic screening project conducted by the National Cancer Center of Japan to perform screening of treatment target genes in lung cancer patients. There are more than 200 medical institutions across Japan participating in LC-SCRUM-Asia. Patients enrolled in LC-SCRUM-Asia have clinical stage II-IV or recurrent lung cancer. The database collects information on cancer biomarkers, patient clinical characteristics, anti-cancer treatment history, and longitudinal clinical outcomes [Citation34]. Tumor response and disease progression are evaluated by investigators according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Between March 2015 and September 2020, EGFR mutations were detected in 1,491 NSCLC patients. Among these patients, 148 (9.9%) patients had EGFR Exon 20ins, while 598 (40.1%) and 524 (35.1%) patients had exon 19 deletions and exon 21 L858R point mutations, respectively.
Study populations
The CHRYSALIS trial cohort for this study consisted of aNSCLC patients (≥18 years) with EGFR Exon 20ins who received the recommended Phase 2 dose of amivantamab. They had previously received platinum-based chemotherapy after metastatic NSCLC diagnosis or in the 12 months before metastatic NSCLC diagnosis, and had progressed on or after platinum-based chemotherapy. Additional inclusion and exclusion criteria can be found in Supplementary Materials S1. Eligible patients enrolled in the trial with a clinical cut-off date of 30 March 2021 were included in the trial cohort for this study.
The LC-SCRUM-Asia EC cohort for this study comprised adult aNSCLC patients with EGFR Exon 20ins who met clinically relevant eligibility criteria for the CHRYSALIS trial at enrollment into LC-SCRUM-Asia database. Patients were eligible if they received platinum-based chemotherapy following the diagnosis of locally advanced or metastatic NSCLC or in the 12 months before locally advanced or metastatic NSCLC diagnosis, and were treated with at least one line of systemic anticancer therapy (SACT) after platinum-based chemotherapy. Additional inclusion and exclusion criteria can be found in Supplementary Materials S1. Eligible patients enrolled in the LC-SCRUM-Asia database between March 2015 and September 2020 were included in the EC cohort.
Study variables and outcomes
Demographic characteristics (age, sex, race, and smoking history) and treatment characteristics (number of lines of therapy [LOTs] received, and SACT regimens prescribed) were recorded for patients in the CHRYSALIS trial cohort and in the EC cohort. The outcomes evaluated in this study were overall survival (OS), progression-free survival (PFS), time to next therapy (TTNT), and overall response rate (ORR). OS was measured from the index date to the date of death from any cause. PFS was defined as the interval between the index date and the date of disease progression or death from any cause, whichever occurred first, while TTNT was defined as the interval between the index date and the initiation of subsequent SACT or death, whichever occurred first.
For the CHRYSALIS trial cohort, the index date was defined to be the date of receiving the first dose of amivantamab. For the EC cohort, the index date was defined according to the approach proposed by Hernan et al. where dates of each qualifying LOT after platinum-based chemotherapy were considered [Citation35]. Thus, each EC patient identified from LC-SCRUM-Asia database could be included multiple times, once for each qualifying LOT. Patients were censored at the last known date of visit if they were lost to follow-up, alive at the end of the follow-up period (for OS), alive and progression-free at the end of the follow-up period (for PFS), or alive and without a record of subsequent SACT at the end of the follow-up period (for TTNT). ORR was defined as the percentage of patients who achieved a confirmed best overall response of complete response or partial response.
Statistical analyses
The propensity score weighting approach, average treatment effect on the treated (ATT), was utilized to weight EC patients to the distribution of baseline covariates in CHRYSALIS (age, sex, smoking history, and number of prior LOTs) to adjust for differences in baseline characteristics between CHRYSALIS and EC patients. Standardized differences between CHRYSALIS and EC patients were calculated before and after baseline covariate adjustment. An absolute standardized difference of <10% after adjustment for each baseline covariate in the propensity score model was considered to indicate good balance [Citation36].
Descriptive statistics were used to describe the baseline characteristics of the two cohorts. Categorical data (including treatments received by EC patients, stratified by LOT) were presented using frequencies and proportions, while continuous data were presented using means and standard deviations. To assess for differences between cohorts, Student’s t-test was used to compare means of continuous variables while Chi-Square test was used to compare proportions of categorical variables.
Clinical outcomes including OS, PFS, and TTNT were described using weighted Kaplan-Meier (KM) curves and compared between the CHRYSALIS and EC cohorts using weighted Cox proportional hazards regression models. Median time-to-event and hazard ratios (HRs) with associated 95% confidence intervals (95% CIs) were estimated. ORR was compared between the two cohorts using weighted generalizing estimating equations with repeated measurements, and odds ratio (OR) with associated 95% CI was estimated.
To investigate the clinical benefit of amivantamab in Asian patients, analyses described previously were repeated with the CHYSALIS trial cohort restricted to patients enrolled from Asia. Sensitivity analyses were conducted to investigate the impact of using different weighting approaches to adjust for differences in the distribution of baseline covariates between CHRYSALIS patients and EC patients to compare clinical outcomes. Stabilized probability of treatment weights (sIPTW), average treatment effect for the overlap population (ATO), average treatment effect for the control (ATC), doubly robust estimator and unweighted Cox/logistic regression models were evaluated. Sensitivity analyses were also conducted to evaluate the impact of using the date of initiation of the first LOT after platinum-based chemotherapy as the index date for the EC cohort on the comparison of clinical outcomes between CHRYSALIS and EC patients. A risk set adjustment method or delayed entry model was implemented for OS to adjust for bias arising from left truncation. A p-value <0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc., North Carolina, USA).
Ethics approval
The CHRYSALIS trial was approved by an Independent Ethics Committee and carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All patients provided written informed consent. The use of LC-SCRUM-Asia data was approved by the institutional review board of National Cancer Center Hospital East. Patients had provided consent on the use of data for future research during enrollment into LC-SCRUM-Asia.
Results
A total of 115 CHRYSALIS patients and 94 LC-SCRUM-Asia patients were identified and included in the study after propensity score matching. summarizes the baseline characteristics of the two cohorts of patients. While age, sex, and smoking history were comparable between CHRYSALIS and unweighted EC patients, unweighted EC patients had significantly more prior number of LOTs compared to CHRYSALIS patients (p < 0.001). Good balance of covariates between the CHRYSALIS and LC-SCRUM-Asia patients was achieved after propensity score matching, with an absolute standardized difference of < 10% achieved after adjustment for each baseline covariate included in the propensity score model (Figure S1 in Supplementary Materials S2).
Table 1. Baseline characteristics of CHRYSALIS and LC-SCRUM-Asia EC patients with (weighted) and without propensity score matching (unweighted).
Treatments received by EC patients after platinum-based chemotherapy are summarized in . Among 309 qualifying LOTs contributed by 94 EC patients, docetaxel, IO, and EGFR TKIs comprised 62.8% of all LOTs.
Table 2. Treatment patterns of LC-SCRUM EC patients in post-platinum lines by each LOT.
shows the KM curves comparing OS, PFS, and TTNT of the CHRYSALIS and EC cohorts. Compared to EC patients, amivantamab-treated CHRYSALIS patients had significantly longer OS (HR [95% CI]: 0.59 [0.40, 0.88]; median OS: 19.88 vs 14.09 months), PFS (HR [95% CI]: 0.59 [0.45, 0.78]; median PFS: 6.74 vs 4.73 months), and TTNT (HR [95% CI]: 0.39 [0.29, 0.53]; median TTNT: 12.16 vs 5.09 months). ORR was also significantly higher in amivantamab-treated patients than in EC patients (41.7% vs. 14.1%; OR [95% CI]: 4.40 [2.62, 7.40]).
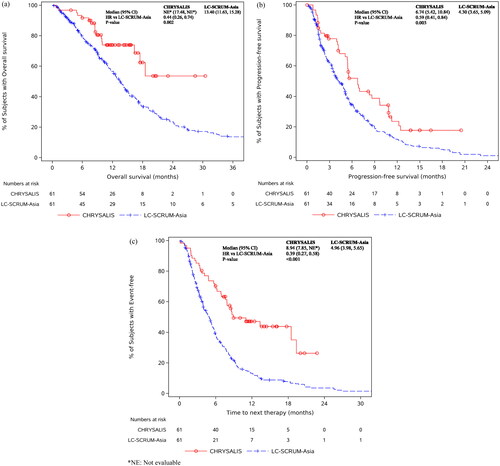
Figure 1. KM curves for amivantamab-treated CHRYSALIS and LC-SCRUM-Asia EC patients for (a) overall survival (OS), (b) progression-free survival (PFS), and (c) time to next therapy (TTNT). *NE: Not evaluable.
Asian patients
There were 61 Asian patients in the CHRYSALIS cohort and 94 LC-SCRUM-Asia patients included in the subgroup analyses. summarizes the baseline characteristics of the Asian CHRYSALIS and EC cohorts. Unweighted EC patients had significantly more prior number of LOTs compared to Asian CHRYSALIS patients (p = 0.048). There were no significant differences between cohorts for the other variables. A good balance of covariates between the Asian CHRYSALIS and LC-SCRUM-Asia patients was achieved after propensity score matching, with an absolute standardized difference of < 10% achieved after adjustment for each baseline covariate included in the propensity score model (Figure S2 in Supplementary Materials S2).
Table 3. Baseline characteristics of Asian CHRYSALIS and LC-SCRUM-Asia EC patients with (weighted) and without propensity score matching (unweighted).
shows the KM curves for OS, PFS and TTNT for the Asian CHRYSALIS and EC cohorts. Similar to the results comparing the overall CHRYSALIS and EC cohorts compared to EC patients, amivantamab-treated Asian patients had significantly longer OS (HR [95% CI]: 0.44 [0.26, 0.74]; median OS: not evaluable vs 13.40 months), PFS (HR [95% CI]: 0.59 [0.41, 0.84]; median PFS: 6.74 vs 4.30 months), and TTNT (HR [95% CI]: 0.39 [0.27, 0.58]; median TTNT: 8.94 vs 4.96 months). Similarly, ORR was also significantly higher in amivantamab-treated Asian patients than in EC patients (44.3% vs. 13.3%; OR [95% CI]: 5.18 [2.80, 9.60]).
Sensitivity analyses
Sensitivity analyses were conducted to investigate the impact of using different weighting approaches to adjust for differences in the distribution of baseline covariates between CHRYSALIS patients and EC patients and to evaluate the impact of using the first LOT after platinum-based chemotherapy (instead of all qualifying LOTs) for the EC cohort on the comparison of clinical outcomes between CHRYSALIS and EC patients. Statistically significant differences in clinical outcomes comparing all CHRYSALIS patients and Asian CHRYSALIS patients to EC patients respectively remained despite the use of different weighting approaches. Detailed results are presented in Tables S1 and S2 of Supplementary Materials S2. With the use of the first LOT after platinum-based chemotherapy, PFS and TTNT remained significantly longer, and ORR remained significantly higher in CHRYSALIS patients than in EC patients. There was no significant difference in OS between CHRYSALIS and EC patients. Detailed results are presented in Table S3 of Supplementary Materials S2.
Discussion
This study was a comparative analysis of amivantamab in a clinical trial and contemporary SACT in real-world practice in Japanese aNSCLC patients with EGFR Exon 20ins. The study found that amivantamab-treated patients had significantly better clinical outcomes than EC patients who received current therapies. Findings were consistent using the subgroup analyses of Asian patients from the CHRYSALIS trial, and in sensitivity analyses where different propensity weighting approaches and the use of first LOT after platinum-based chemotherapy were evaluated.
First-line platinum-based chemotherapy is recommended for most aNSCLC patients with EGFR Exon 20ins, with no clear recommendations or guidelines after the failure of platinum-based chemotherapy [Citation26]. Based on the real-world data in our current study, the analyses of treatment patterns indeed showed the majority of aNSCLC patients (77.4%) received platinum-based therapy as the first line treatment, while for the second line treatment, platinum-based therapy (26.4%), docetaxel (26.4%), and IO (19.1%) were the top three most common therapies. When identifying aNSCLC patients who progressed on or after platinum-based therapies only, docetaxel, IO, and EGFR TKIs were the top three most common therapies received in Japan (). The results showed that IO and EGFR TKIs are still commonly prescribed in the real-world setting despite established evidence of poor response to immune checkpoint inhibitors and EGFR TKIs among NSCLC patients with EGFR Exon 20ins [Citation37]. There is a lack of effective treatment options after the failure of platinum-based chemotherapy, which accentuates the need to seek out new treatment strategies after the failure of platinum-based chemotherapy for aNSCLC patients with EGFR Exon 20ins.
Amivantamab received approval and conditional approval by the US Food and Drug Administration and the European Medicines Agency, respectively, in 2021 for use in adult patients with locally advanced or metastatic NSCLC with EGFR Exon 20ins whose disease progressed on or after platinum-based chemotherapy. As a new treatment option, amivantamab showed promising results in the CHRYSALIS trial, where aNSCLC patients with EGFR Exon 20ins post-platinum chemotherapy received amivantamab and demonstrated durable responses [Citation30]. In this study, amivantamab-treated CHRYSALIS patients had significantly longer OS, PFS, and TTNT, and significantly higher ORR than EC patients from LC-SCRUM-Asia in Japan. The results corroborated a previously conducted study using the same cohort of CHRYSALIS trial patients, and a pooled external cohort of patients from three US-based real-world databases [Citation32].
The previous study conducted using real-world data from the US noted that Asian patients were more prevalent in the amivantamab-treated cohort than in the US real-world databases [Citation32]. This was due to the CHRYSALIS trial being initiated in South Korea before expanding to the US and other countries [Citation30]. It is noteworthy that the current study utilized an Asian database to derive an EC cohort, and additional analyses were conducted to compare Asian CHRYSALIS patients with EC patients who were all of Asian descent. However, it should be noted that while the EC patients were ethnically similar to CHRYSALIS patients, LC-SCRUM-Asia patients were all Japanese. Findings of better clinical outcomes in amivantamab-treated patients persisted in the subgroup analyses of Asian patients. Our study provides additional evidence of the clinical benefit of amivantamab compared to current therapies.
The primary propensity score weighting approach used in this study to adjust for differences in baseline characteristics between CHRYSALIS patients and LC-SCRUM-Asia EC patients was ATT. The use of other weighting approaches was explored with sensitivity analyses. Regardless of the weighting approach used, there were statistically significant differences in clinical outcomes comparing CHRYSALIS patients to EC patients, with CHRYSALIS patients showing better clinical benefit in OS, PFS, TTNT, and ORR. The consistency of results between main and sensitivity analyses, and across all clinical outcomes raises confidence in the robustness of the study’s findings, further supporting the effectiveness of amivantamab as compared to current therapies.
This study used all qualifying LOTs from LC-SCRUM-Asia database patients to compare clinical outcomes between CHRYSALIS patients and EC patients, as proposed by Hernan et al. [Citation35]. In sensitivity analyses, the use of first LOT after platinum-based chemotherapy also showed that amivantamab-treated CHRYSALIS patients had significantly better clinical outcomes (PFS, TTNT, and ORR) than EC patients treated with real-world therapies. Even though there was no significant difference in OS between amivantamab-treated patients and EC patients, the consistency of results between main and sensitivity analyses for the other clinical outcomes indicates a clinical benefit of amivantamab compared to current therapies. While it would be interesting to compare amivantamab with specific therapies at different LOTs, we were limited by the small number of patients for each specific therapy and LOT given the rarity of the mutation.
A strength of this protocol-driven study was the comparison of individual, patient-level data from a real-world data source with clinical trial data. This enabled assessment of the clinical benefit of amivantamab against current therapies in the absence of randomized clinical trials. The purposeful collection of detailed lung cancer-related data from the LC-SCRUM-Asia database makes it well-suited as an external comparator for single-arm lung cancer clinical trials. This study should also be evaluated within its limitations. Not all information collected in the CHRYSALIS trial was available in the LC-SCRUM-Asia database, a common challenge with real-world databases. Thus, some eligibility criteria used to identify EGFR Exon 20ins patients for the CHRYSALIS trial could not be applied to the LC-SCRUM-Asia database. As this was a non-randomized study, possible bias could be attributed to limited baseline characteristics to assess comparability between the two groups, and unidentified confounders which were not adjusted for. For example, patients enrolled in CHRYSALIS had Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0 or 1 and had no untreated brain metastases. In contrast, patients from LC-SCRUM-Asia included in this study were allowed to have unknown ECOG PS, and had no requirements specified for brain metastases. Information on ECOG PS and brain metastases was only captured at enrolment into LC-SCRUM-Asia, thus limiting the identification of patients having these characteristics. To note, an analysis was conducted to include brain metastases and ECOG PS as covariates in the propensity score weighting approach, and similar results were obtained. Another limitation, again inherent to real-world data sources, was that some data and outcomes may not be captured in as standardized of a manner or assessed as frequently compared to protocol-driven clinical trials. For example, physician assessments of treatment response may not be consistent and could reduce comparability between cohorts.
An area for consideration in future research would be to investigate the effects of EGFR Exon 20ins variants. It has been reported in the literature that some clinical characteristics have been associated with specific EGFR Exon 20ins variants, and that the variants had varying degrees of sensitivity to therapies [Citation37–42]. However, these were not explored in the current study.
Conclusion
Amivantamab-treated patients had significantly longer OS, PFS, and TTNT, and significantly higher ORR than patients treated with real-world therapies in the post-platinum-based chemotherapy setting in Japan. This reflects the benefit of amivantamab after platinum-based chemotherapy for aNSCLC EGFR Exon 20ins patients, compared to current therapies, and highlights the need for more targeted treatments for this patient population. At the time of publication, the phase III trial PAPILLON (NCT04538664) evaluating amivantamab in combination with chemotherapy in the frontline setting had recently been reported to have met its primary endpoint of PFS, providing further evidence of the efficacy of amivantamab in aNSCLC EGFR Exon 20ins patients [Citation43].
Supplemental Material
Download MS Word (1.3 MB)Acknowledgements
The authors would like to thank the patients, their families, collaborators in 183 institutions as well as collaborating pharmaceutical companies for their participation in LC-SCRUM-Asia. LC-SCRUM-Asia's collaborating pharmaceutical companies are Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Janssen, Kyowa Kirin, Medical & Biological Laboratories, Merck Serono, Merus, MSD, Novartis, Ono, Pfizer, Sumitomo Dainippon, Taiho, and Takeda.
Disclosure statement
T. M. Kim received honoraria from or held an advisory role at AstraZeneca, Boryung, F. Hoffman-La Roche Ltd/Genentech, Inc, IMDBx, Inc., Janssen, Novartis, Regeneron, Samsung Bioepis, Sanofi, Takeda, Yuhan, and received research funding from AstraZeneca-Korea Health Industry Development Institute, outside of the submitted work.
N. Girard received honoraria and/or consulting fees from AbbVie, Amgen, AstraZeneca, BMS, Boehringer-Ingelheim, Daachi, Janssen, Lilly, Mirati, MSD, Novartis, Novartis, Pfizer, Roche, Sanofi, Takeda, and received grants from MSD and AstraZeneca paid to institution outside of the submitted work.
Zhuo, D. Y. Yu, Y. Yang, and M. Murota are full-time employees of Janssen. J. Zhuo received long-term incentives from Janssen. G. K. M. Low was a full-time employee of Janssen when this study was performed and received long-term incentives from Janssen. She is not an employee of Janssen currently.
C.T.K. Lim and N. J. Kleinman are full-time employees of IQVIA.
C. Cho is the founder of DAAN Biotherapeutics, has stock ownership in Bridgebio Therapeutics, Cyrus Therapeutics, Gencurix Inc, Interpark Bio Convergence, J INTS Bio, Kanaph Therapeutic Inc, TheraCanVac Inc, received consulting fees and/or research funding from Abion, AbbVie, AstraZeneca, Bayer, BeiGene, Blueprint Medicines, Boehringer-Ingelheim, BMS, Bridgebio Therapeutics, CHA Bundang Medical Center, Champions Oncology, CJ, CJ Bioscience, CJ Blossom Park, CureLogen, Cyrus Therapeutics, Dizal Pharma, Dong-A ST, Eli Lilly, Genexine, GI-Cell, GIInovation, Guardant, Hanmi, HK Inno. N, Imnewrun Biosciences Inc., Interpark Bio Convergence Corp, ImmuneOncia, Janssen, JINTSbio, Kanaph Therapeutics, LG Chem, Medpacto, MOGAM Institute, MSD, Novartis, Nuvalent, Oncternal, Onegene Biotechnology, Ono, Oscotec, Pfizer, RandBio, Regeneron, Roche, Takeda, Therapex, Yuhan, and received royalty from Champions Oncology, Crown Bioscience, Imagen, outside of the submitted work.
Data availability statement
Due to the nature of the research and due to ethical/legal/commercial restrictions, supporting data is not available.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660.
- Ferlay J, Ervik M, Lam F, et al. Global cancer Observatory: cancer today. Lyon, France: International Agency for Research on Cancer 2020. [Internet]. 2020 [cited 2022 Jun 3]. Available from: https://gco.iarc.fr/today/home.
- Collins LG, Haines C, Perkel R, et al. Lung cancer: diagnosis and management. afp. 2007;75:56–63.
- Woodard GA, Jones KD, Jablons DM. Lung cancer staging and prognosis. In: Reckamp KL, editor. Lung Cancer: Treatment and Research [internet]. Cham: Springer International Publishing; 2016 p. 47–75.
- Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2017;12(7):1109–1121. doi: 10.1016/j.jtho.2017.04.011.
- Zhang Y-L, Yuan J-Q, Wang K-F, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48):78985–78993. doi: 10.18632/oncotarget.12587.
- Hsu W-H, Yang JC-H, Mok TS, et al. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol. 2018;29(suppl_1):i3–i9. doi: 10.1093/annonc/mdx702.
- Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13(1):e23-31–e31. doi: 10.1016/S1470-2045(11)70129-2.
- Russo A, Franchina T, Ricciardi G, et al. Heterogeneous responses to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with uncommon EGFR mutations: new insights and future perspectives in this complex clinical scenario. Int J Mol Sci. 2019;20(6):1431. doi: 10.3390/ijms20061431.
- Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(Suppl 1):S24–S31. doi: 10.1038/onc.2009.198.
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314.
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938.
- Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol. 2020;61:167–179. doi: 10.1016/j.semcancer.2019.09.015.
- Takeda M, Sakai K, Hayashi H, et al. Clinical characteristics of non-small cell lung cancer harboring mutations in exon 20 of EGFR or HER2. Oncotarget. 2018;9(30):21132–21140. doi: 10.18632/oncotarget.24958.
- Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013;8(2):179–184. doi: 10.1097/JTO.0b013e3182779d18.
- Riess JW, Gandara DR, Frampton GM, et al. Diverse EGFR exon 20 insertions and Co-Occurring molecular alterations identified by comprehensive genomic profiling of NSCLC. J Thorac Oncol. 2018;13(10):1560–1568. doi: 10.1016/j.jtho.2018.06.019.
- Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220–229. doi: 10.1158/1535-7163.MCT-12-0620.
- Chen D, Song Z, Cheng G. Clinical efficacy of first-generation EGFR-TKIs in patients with advanced non-small-cell lung cancer harboring EGFR exon 20 mutations. Onco Targets Ther. 2016;9:4181–4186. doi: 10.2147/OTT.S108242.
- Wu J-Y, Yu C-J, Chang Y-C, et al. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17(11):3812–3821. doi: 10.1158/1078-0432.CCR-10-3408.
- Kate S, Chougule A, Joshi A, et al. Outcome of uncommon EGFR mutation positive newly diagnosed advanced non-small cell lung cancer patients: a single center retrospective analysis. Lung Cancer (Auckl). 2019;10:1–10. doi: 10.2147/LCTT.S181406.
- Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20–selective kinase inhibitor in non–small cell lung cancer. Nat Med. 2018;24(5):638–646. doi: 10.1038/s41591-018-0007-9.
- Piotrowska Z, Wang Y, Sequist LV, et al. ECOG-ACRIN 5162: a phase II study of osimertinib 160 mg in NSCLC with EGFR exon 20 insertions. J Clin Oncol. 2020;38(15_suppl):9513–9513. doi: 10.1200/JCO.2020.38.15_suppl.9513.
- Floc’h N, Martin MJ, Riess JW, et al. Antitumor activity of osimertinib, an irreversible Mutant-Selective EGFR tyrosine kinase inhibitor, in NSCLC harboring EGFR exon 20 insertions. Mol Cancer Ther. 2018;17(5):885–896. doi: 10.1158/1535-7163.MCT-17-0758.
- Lee Y, Kim TM, Kim D-W, et al. Preclinical modeling of osimertinib for NSCLC with EGFR exon 20 insertion mutations. J Thorac Oncol. 2019;14(9):1556–1566. doi: 10.1016/j.jtho.2019.05.006.
- Yang Z, Yang N, Ou Q, et al. Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhibitor osimertinib in non-small cell lung cancer patients. Clin Cancer Res. 2018;24(13):3097–3107. doi: 10.1158/1078-0432.CCR-17-2310.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Small Cell Lung Cancer 2022 [Internet]. NCCN. 2022 [cited 2022 Jun 3]. Available from: https://www.nccn.org/guidelines.
- Huang C-Y, Ju D-T, Chang C-F, et al. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine. 2017;7(4):23. doi: 10.1051/bmdcn/2017070423.
- Choudhury NJ, Schoenfeld AJ, Flynn J, et al. Response to standard therapies and comprehensive genomic analysis for patients with lung adenocarcinoma with EGFR exon 20 insertions. Clin Cancer Res. 2021;27(10):2920–2927. doi: 10.1158/1078-0432.CCR-20-4650.
- Yasuda H, Park E, Yun C-H, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5(216):216ra177. doi: 10.1126/scitranslmed.3007205.
- Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR exon 20 Insertion-Mutated Non-Small-Cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391–3402. doi: 10.1200/JCO.21.00662.
- Janssen Research & Development, LLC. A Phase 1, first-in-human, open-label, dose escalation study of JNJ-61186372, a human bispecific EGFR and cMet antibody, in subjects with advanced non-small cell lung cancer [Internet]. clinicaltrials.gov; 2022 [cited 2022 Jun 7]. Report No.: NCT02609776. Available from: https://clinicaltrials.gov/ct2/show/NCT02609776.
- Minchom A, Viteri S, Bazhenova L, et al. Amivantamab compared with real-world therapies in patients with advanced non-small cell lung cancer harboring EGFR exon 20 insertion mutations who progressed after platinum-based chemotherapy. Lung Cancer. 2022;168:74–82. doi: 10.1016/j.lungcan.2022.03.005.
- Haura EB, Cho BC, Lee JS, et al. JNJ-61186372 (JNJ-372), an EGFR-cMet bispecific antibody, in EGFR-driven advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2019;37(15_suppl):9009–9009. doi: 10.1200/JCO.2019.37.15_suppl.9009.
- Goto K. Development of nationwide genomic screening project (LC-SCRUM-Japan) for the establishment of cancer precision medicine. Annals Oncol. 2017;28:ix5. doi: 10.1093/annonc/mdx570.001.
- Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758–764. doi: 10.1093/aje/kwv254.
- Morgan CJ. Reducing bias using propensity score matching. J Nucl Cardiol. 2018;25(2):404–406. doi: 10.1007/s12350-017-1012-y.
- Remon J, Hendriks LEL, Cardona AF, et al. EGFR exon 20 insertions in advanced non-small cell lung cancer: a new history begins. Cancer Treat Rev. 2020;90:102105. doi: 10.1016/j.ctrv.2020.102105.
- Cardona AF, Rojas L, Zatarain-Barrón ZL, et al. EGFR exon 20 insertion in lung adenocarcinomas among hispanics (geno1.2-CLICaP). Lung Cancer. 2018;125:265–272. doi: 10.1016/j.lungcan.2018.10.007.
- Yang G, Li J, Xu H, et al. EGFR exon 20 insertion mutations in Chinese advanced non-small cell lung cancer patients: molecular heterogeneity and treatment outcome from nationwide real-world study. Lung Cancer. 2020;145:186–194. doi: 10.1016/j.lungcan.2020.03.014.
- Hirose T, Ikegami M, Endo M, et al. Extensive functional evaluation of exon 20 insertion mutations of EGFR. Lung Cancer. 2021;152:135–142. doi: 10.1016/j.lungcan.2020.12.023.
- Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Sig Transduct Target Ther. 2019;4(1):10. doi: 10.1038/s41392-019-0038-9.
- Passaro A, Mok T, Peters S, et al. Recent advances on the role of EGFR tyrosine kinase inhibitors in the management of NSCLC with uncommon, non exon 20 insertions, EGFR mutations. J Thorac Oncol. 2021;16(5):764–773. doi: 10.1016/j.jtho.2020.12.002.
- Johnson&Johnson. Treatment with RYBREVANT® (amivantamab-vmjw) plus chemotherapy resulted in statistically significant and clinically meaningful improvement in progression-free survival in patients with newly diagnosed EGFR exon 20 insertion mutation-positive non-small cell lung cancer. [cited Aug 2023]. Available from: https://www.jnj.com/treatment-with-rybrevant-amivantamab-vmjw-plus-chemotherapy-resulted-in-statistically-significant-and-clinically-meaningful-improvement-in-progression-free-survival-in-patients-with-newly-diagnosed-egfr-exon-20-insertion-mutation-positive-non-small-cell-lung-cancer