ABSTRACT
This paper investigates the differences and similarities between European regulatory research integrity systems. The data collection process involved gathering information from public sources. A total of 27 European countries were included in the comprehensive dataset. Three determinants were examined: the legal structure of national research integrity regulation, the presence of national research integrity guidelines, and the provision of research integrity training by national research integrity offices. Qualitative content analysis was employed to identify relevant differences in national research integrity systems and the work of national research integrity offices. The findings suggest that the functions and powers of research integrity offices in Europe vary significantly, and there is extensive variation in the legal status and functions of national research integrity systems. We identify the major implications arising from these differences and explore what the challenges for harmonization of the European research integrity systems are. Our findings highlight the need for promoting dialogue between actors on an international level.
1. Introduction
International research collaboration has skyrocketed since the middle of this century’s first decade (Chen, Zhang, and Fu Citation2019). As research has become predominantly an international endeavor, it is now also commonly expected that the ethical norms guiding the work of researchers will be universal (B. D. Resnik and Shamoo Citation2011; TRUST Citation2018). However, even if a set of norms were universally recognized, local implementation and enforcement could still introduce substantial differences between regulatory environments. This has raised worries regarding fairness and applicable practices in general in international research collaborations (Desmond and Dierickx Citation2021; Godecharle, Nemery, and Dierickx Citation2013).
The aim of this paper is to analyze the extent to which the data on different regulatory environments regarding research integrity is complete and comparable so that classifications and categorizations based on this data can be made uniform – or if the data can reasonably be interpreted in more than a single fashion making classifications and categorizations of different regulatory environments merely indicative. In this paper, we have analyzed publicly available information on 27 research integrity systems around Europe and we looked for the similarities and differences between them.
The paper focuses on three distinctive determinants between research integrity systems and the role of national research integrity offices within these systems. The first dimension is the research integrity regulatory structure. The regulatory structures can vary from integration into national legislation, through self-regulatory structures where research integrity regulation is coordinated nationally with soft-law arrangements, and to voluntary systems where research misconduct is investigated, and research integrity promoted at the local level in research organizations and higher education institutions (ENRIO Citation2019b). The second dimension is the existence of national research integrity guidelines. The national guidelines for research integrity can take the form of a binding agreement on the research misconduct investigation process and/or a guiding document on recommendations for good research practices and the responsibilities of different research stakeholders. The guidelines may have national scope, or they might be codes of conduct produced by a research funding organization or an academy of sciences that has an aspirational role for all research organizations in a country (Desmond and Dierickx Citation2021). The third dimension is the research integrity training provided by a national research integrity office. Training can be perceived as a vehicle for bringing the guidelines into practice in research organizations. Some of the research integrity offices have been assigned the responsibility to provide training at national or subnational levels. The roles of research integrity offices vary from providing all higher education institutions with research integrity training materials to train-the-trainer programs, and to individual workshops and lectures on research integrity.
In this paper, a research integrity system is a set of bodies such as research integrity offices and institutions assigning research misconduct investigation and research integrity promotion to local research organizations and/or to a national oversight body. Accordingly, research integrity offices are defined as public administrative bodies, research funding organizations or academies of sciences with a national or subnational role in research misconduct investigation, drafting of codes of conduct for research integrity, research integrity promotion and/or research integrity training.
Guiding principles of research integrity and the definitions of falsification, fabrication, plagiarism (FFP) are widely shared in Europe (Desmond and Dierickx Citation2021) while in some Asian countries this concept is more spacious (e.g., in Korea, FFP also includes unethical authorship; see Chou, Lee, and Fudano Citation2023). However, at the institutional level, the divergencies become apparent (Desmond and Dierickx Citation2021; Godecharle, Nemery, and Dierickx Citation2013). All European national administrative structures have their own origins, and they are embedded in their own social, political, and economic environments.
We begin by examining the existing research literature on the varieties of research integrity systems in Europe. We describe the data collection on the publicly available information on the European research integrity systems and present the results of our analysis. We discuss our findings in connection with our effort of trying to pinpoint what constitutes differences and similarities between these systems, and what do these differences imply
2. Literature overview
2.1. Research integrity regulation in Europe
In the literature, there were a few attempts to delve into research integrity regulation in Europe. In their review of the national-level documents on research integrity regulation of all the 32 European Free Trade Association countries, Desmond and Dierickx (Citation2021) discuss the divergencies in research integrity codes of conduct in Europe. Their viewpoint is that there is a risk of injustice regarding how these codes of conduct are interpreted because research integrity norms are understood differently across Europe and there might not be common understanding of these principles. Worse yet, the even more skeptical argument that the ethical principles in codes of conduct are merely window dressing cannot be ruled out if no obligatory procedures for enforcing these principles are implemented in the codes of conduct.
Desmond and Dierickx (Citation2021) also point out that their text-analysis methodology is not sufficient to come into conclusions about the actual practices and contextual reasoning behind codes of conduct for research integrity. However, they introduce questions that would shed light on the topic, for example, what the purpose of legislation and sanctioning of research misconduct (retribution, deterrence, or rehabilitation) is and what system of incentives would be seen as desirable (Desmond and Dierickx Citation2021).
Perković Paloš et al. (Citation2023) have compared and categorized research ethics and integrity landscapes in 16 European countries. However, this study has limitations, i.e., it does not address the existing organizational differences within a country.
Previous studies use different categories to portray research integrity regulation in Europe. Also, the samples used differ. It is worth noticing that the European research integrity system has a range of origins and various legal standings. In some European countries, there is a self-regulatory system, in others a legislative one, and in some a voluntary-based system (ENERI consortium Citation2020). Not all national research integrity offices, for example, investigate misconduct cases or act as appeal bodies. This makes their raison d’être focused on promoting responsible conduct of research through training and advisory capacity (Nolte et al. Citation2023). Also, it is not uncommon that the establishing of national research integrity structures has been boosted by a public scandal (Sheldon Citation2011). This emphasizes the political and ad hoc nature of some regulatory systems. The public perception has also changed quite rapidly. In the 1990s when the first European national research integrity offices were established, research misconduct was explained in individual terms (“bad apples”) but during the last two decades, this explanation has more and more given way to systemic explanations (“bad systems”) (Huistra and Paul Citation2022). Likewise, roles of national research integrity offices (more specifically corresponding to the role of ombudspersons) varied, e.g., educator, facilitator, counselor (Fischbach and Gilbert Citation1995), conciliator, agent of change (Stieber Citation2000), organizational, advocate, corporate (Carl Citation2012). These differences and rapid changes in the research integrity environment also highlight that legal arrangements are born of political bargains and compromises, and each arrangement reflects the prevailing allocation of power and different accounts of desired outcomes (Reisman Citation2008).
The European research integrity landscape is a multifaceted one. As Kahneman et al. (Citation2021) have noted, complicated phenomena make our evaluations vulnerable to noise, i.e., undesirable variability in judgments of the same problem. The European research integrity landscape cannot be categorized in a singular fashion because researchers focus on different aspects, showing how complex the phenomenon is, and suggesting that we need many approaches to fully understand it. Considering these specifics, we aim to fill this gap by contributing to the field with the larger sample and considering certain organizational differences.
2.2. Research integrity in the broader landscape of research
Research integrity is an umbrella concept that gathers many related principles and attributes under its shelter. Because national research integrity offices use research integrity as a practice-oriented concept, it makes it hard to grasp what the normative content exactly is, and how it should guide researchers’ actions. Research integrity has been described as the “attitude and habit of researchers to conduct research according to appropriate ethical, legal and professional frameworks, obligations and standards” (ENERI Citation2019). The European Code of Conduct for Research Integrity, one of the internationally most influential guidelines to research integrity, states that research integrity aims to ensure TRUST in and validity of the methods and findings of research through regulations, tools, and good practices, and that the principles of reliability, honesty, respect, and accountability underpin research integrity efforts (ALLEA Citation2023). Even in these brief descriptions, it becomes apparent that research integrity is connected not just to prevention and investigation of research misconduct but to foundational values, such as truthfulness and fairness.
It is not self-evidently clear that a description that encompasses value-driven principles is one that is of highest practical value. However, it is evident that research integrity is connected to a broader set of values instead of a thin and technical set of rules on good research practices. There are also solid grounds for a broader conceptualization of research integrity.
In the past few decades, research integrity was mainly perceived as prevention of research misconduct (generally defined as FFP) in planning, conducting, or reviewing research work, or in reporting and disseminating its results (Anderson et al. Citation2013; D. B. Resnik et al. Citation2015). Besides actual misconduct we can find less severe, yet unacceptable violations of responsible conduct of research. The terminology describing these violations is not entirely fixed. Literature and national and discipline-specific codes of conduct usually use terms “unacceptable research practices” or “questionable research practices” to refer to activities, such as failing to provide authorship credit to all who contributed to the work, deliberately not discussing contrary evidence, not sharing data when this would be appropriate, and so forth.
If we consider the harm done in failing to meet the standards of research integrity, we notice that even when defined in the context of preventing research misconduct or unacceptable research practices, research integrity has its rationale in a broader ethical framework which maintains scientific excellence and fairness and retains public TRUST. Research integrity is not exclusively about preventing research misconduct. Rather, it is also about culture and environment, and it is about behaviors and expectations, and values of inclusion and collaboration (Downey and Veitch Citation2021). This can be clearly observed in internationally influential codes of conduct for research integrity.
Besides the European Code of Conduct for Research Integrity (ALLEA Citation2023), the view that research integrity is closely tied to the accuracy, predictability, and reproducibility of scientific findings is also reflected in other major international codes of conduct. The National Academies of Sciences, Engineering, and Medicine in the United States also positions research integrity in the wider ethical context of objectivity, honesty, openness, accountability, fairness, and stewardship, stating that “practicing integrity in research means planning, proposing, performing, reporting, and reviewing research in accordance with the values described above” (National Academies of Sciences, Engineering, and Medicine Citation2017, 38).
To account for the complexity of research integrity, we formulated our first research question: What are the implications of differences and similarities emerging in European research integrity system?
As we pointed out earlier in the paper, the literature suggests that there are obstacles in seeking to construct a unified presentation of the wholeness of the European research integrity system (Desmond and Dierickx Citation2021; Godecharle, Nemery, and Dierickx Citation2013). Therefore, our second research question is: What are the challenges for harmonization of European research integrity system?
3. Methodology
3.1. Data collection
We collected data from public sources:
Country Cards in Embassy of Good Science (Embassy of Good Science, Citation2023) that reflect data collected between July 2018 and June 2019 as well as later user additions via the wiki functions (n = 16);
Country reports containing descriptions of the research integrity landscape in European countries on the website of the European Network of Research Integrity Offices (ENRIO Citation2019b) that are updated upon demand (n = 24) by the ENRIO secretariat;
ENRIO member organization information sheets on ENRIO’s website (ENRIO Citation2023) that are provided by all new ENRIO members and updated whenever there are changes in the line or scope of organizations’ work (n = 23); and
Latest annual reports that are publicly available in English during the period of data collection in January–April 2023 (n = 10) and which are of the following organizations:
The Austrian Agency for Research Integrity (OEAWI) 2021
The Flemish Commission for Research Integrity VCWI Citation2021
The Danish Committee on Research Misconduct 2021
The Finnish National Board on Research Integrity TENK 2022
The German Research Ombudsman Citation2021
The Lithuanian Office of the Ombudsperson for Academic Ethics and Procedures 2022
The Norwegian National Research Ethics Committees FEK Citation2021
The Swedish National Board for Assessment of Research Misconduct (Npof) 2021
The Netherlands Research Integrity Network (NRIN) 2020
The Netherlands Board on Research Integrity (LOWI) 2021
All countries with a country report card and all countries which have an institution affiliated with the ENRIO have been included in this study. Overall, comprehensive data on 27 European countries were collected (supplemental material).
3.2. Data analysis
To analyze the data, we used qualitative content analysis to examine manifest or latent meanings in a particular text (Hsieh and Shannon Citation2005; Zhang and Wildemuth Citation2009). We used qualitative coding to identify excerpts relevant to the aim of this study. In this paper, the categories “legal status of research misconduct investigation,” “national guidelines” and “nationally coordinated research integrity training” refer to key characteristics of national research integrity systems. All three categories are represented by a set of codes.
We used deductive coding on two categories: legal status of research misconduct investigation and guidelines. Here, we developed first the codes based on the existing literature. The three codes for legal status were derived from the ENRIO Handbook: Recommendations for the Investigation of Research Misconduct (ENERI consortium Citation2020) and the codes indicating differences in national guidelines were identified in previous related studies (Desmond and Dierickx Citation2021; Perković Paloš et al. Citation2023). For the category “nationally coordinated research integrity training,” we developed codes from the source material (Vaismoradi, Turunen, and Bondas Citation2013) as well as inductively due to lack of relevant research. After identifying codes, we went through the data and assigned excerpts to codes. Datasets derived from the Embassy of Good Science and ENRIO Country Cards also supported the selected coding framework.
We used three different codes for each of the three categories (). We first extracted data from (1) the Country Cards and (2) the Country Reports and then complemented our dataset with (3) data from ENRIO members’ information sheets. Data from annual reports served to revise and amend our dataset, and to identify possible ambiguities. We focused on data related to the national regulatory body’s mandate to handle alleged research misconduct, the presence of national-level research integrity or other relating guidelines, and the provision of national-level training.
Table 1. The three categories and the respective codes.
We started our analysis from the Embassy of Good Science’s Country Cards. This database is most recently updated, and it served as the starting point in collecting national guidelines and descriptions of administrative structures of research misconduct investigation and research integrity training. Second, we analyzed the data from the ENRIO Country Reports and ENRIO membership information sheets to validate, revise or amend the observations made based on the Country Cards. ENRIO members’ information sheets especially further clarified the roles and competences of national research integrity offices. Annual reports provided information on the frequency and scope of consultations research integrity offices provide and form of education they provide.
Using qualitative content analysis (Hsieh and Shannon Citation2005), we pinpointed relevant differences in national research integrity systems and the line of work of the national research integrity offices. The following example is used to describe how codes are used in finding evidence on the differences between research integrity regulatory systems. In the Country Cards, we looked for descriptions of oversight-body on research integrity misconduct investigation and possible national guidelines, in this sample case Austria, and the observation came up with:
Austrian Agency for Research Integrity is also responsible for investigating cases of misconduct. Inquiry can be initiated by the members of the Agency and individuals, whereupon the Agency will decide its competence to bring statements in each case. However, those statements do not have any legal influence and it is up to each institution to bring decision about further actions in the cases of allegations of research misconduct. Besides the Agency, cases of misconduct at universities are handled by research integrity committees or similar bodies. Some cases of proven misconduct were published in media. (Self-regulation) (Highlight added.)
This extract provided us with information on the existence of a national self-regulatory system in Austria, but not if the misconduct is investigated in the national body on the first instance or if Austrian Agency for Research Integrity acts as an appellate body. In the Country Cards we observed about Austria that “Total of 5 guidelines were found” but this did not suffice for our coding scheme as the scope of the guidelines were not clear.
The ENRIO Country Report on Austria validated the first observation and gave further information on the remit of the national research integrity office:
The scope of the Commission is nationwide. It can investigate research misconduct within member and non-member (public and private) research institutions in Austria. The Commission, too, can start an investigation on research misconduct on its own initiative in member and non-member organisations in Austria. The Commission’s remit (…) is not restricted to cases in first instance as it can also give opinions in second instance. (…) The Commission is not obligated to handle requests of (…) non-member organisation, but in general it will do so in cases of severe and founded allegations of research misconduct with a connection to Austria. The Commission may handle anonymous requests. The Commission will not handle alleged research misconduct cases of students on bachelor – or master level. (2) In second instance, the reporter or accused in first instance, disagreeing with the decision of the local research institution can ask the Commission for an opinion. (Self-regulation) (Highlight added.)
Observation in the OEAWI Annual Report 2021 provided us with information that the Austrian Guidelines for Good Scientific Practice have not only aspirational but an obligatory nature:
The Commission operates on the basis of its Rules of Procedure and the Guidelines for Good Scientific Practice. (National-level obligatory)
In this paper, we were able to use all categories and codes (see ) to map European research integrity systems accordingly.
3.3. Limitations
First, the data collection is limited to the aforementioned public sources; we recognize that some practices and ways of e.g., training may remain invisible in the public sources. Second, accuracy of the data depends on timely updates (which might be irregular, not up to date) as well as to the period of data collection in the spring of 2023. Therefore, the legislation revisions or establishment of new national research integrity systems taking place after the data collection period might affect our findings over time. These limitations remain beyond the scope of our study.
Second, when annual reports were not found in English (n = 6), Google Translate was used to translate the original texts (Npof Citation2021; The Danish Board on Research Misconduct Citation2021; FEK Citation2021; NRIN Citation2021; the German Research Ombudsman Citation2021; VCWI Citation2021). Google Translate might be an insufficient tool to translate nuances adequately but for the purposes of this study, the translation was sufficient.
4. Results
4.1. Divergencies in national-level research integrity systems in Europe
What is striking is that with only three categories, namely competence in handling allegations of research misconduct, national-level research integrity guidelines, and national coordination of research integrity training, we can draw a map of European research integrity systems in which hardly any national research integrity systems are similar. On the contrary, triple-categorizing European countries might be overly simplified and may not present adequately the pluralist nature of the national research integrity systems, whose categorization is genuinely open to multiple interpretations.
Some characteristics were represented across datasets: applicable laws, scope of research integrity guidelines, and forms of training were indicated unambiguously. Data from both repositories, however, lacked explicated information on contextual differences between the countries. Annual reports and ENRIO membership information provided some information on contextual differences, but their scope was limited to the role of the national research integrity office.
4.1.1. Competence in handling allegations of research misconduct
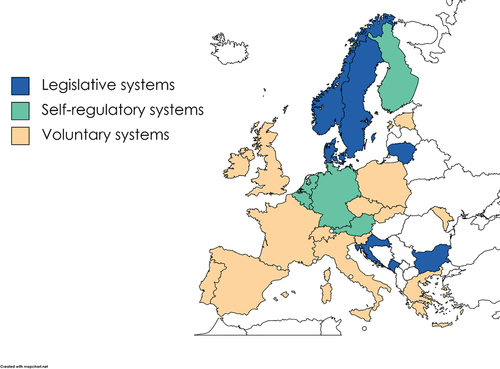
A distinction has been made between self-regulation, legislative, and voluntary-based national research integrity systems (). Within the last 20 years, a number of European countries have established statutory regulation of research, although they are still largely based on self-regulation or mutual agreements (ENERI consortium Citation2020). Legislative systems have a law where research misconduct or the process of research misconduct investigation is regulated. Self-regulatory systems are inherent in countries where there is a mutual agreement between research performing and funding organizations on research misconduct investigation and a national body that gives first or second instance opinions/decisions on misconduct cases, or that acts as an oversight body. In voluntary-based systems, there might be a national code of conduct or a widespread consensus on research integrity norms, but research organizations do not disclose misconduct cases to a national research integrity office or to other oversight body. Local and national handling of cases have their respective advantages even though the benefits of a national oversight body are well established (ENERI consortium Citation2020) and include more comprehensive due process and national harmonization of handling research misconduct cases.
In nationally coordinated self-regulatory systems, there is usually a national oversight body while the first instance of research misconduct investigation is handled locally at the level of research performing organizations or research funding organizations.
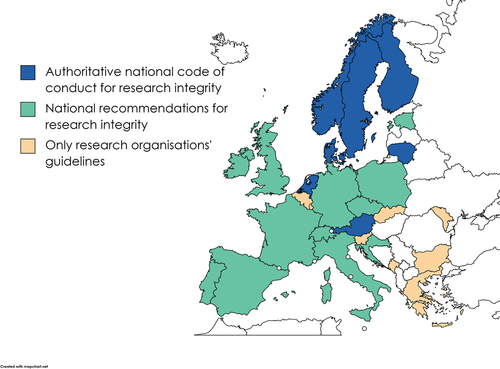
4.1.2. National-level research integrity guidelines
Countries can have either a national code of conduct on research integrity or research integrity guidelines drafted by a national academy of sciences or a national research funding organization (). Both have a national scope but there is the difference that guidelines for national academies of sciences and national research funding organizations can be directly applied only to research funded by the funding organization or research conducted by the employees of the academy. Their role in a national context is merely aspirational whereas a national code of conduct is applicable to all or many research organizations and higher education institutions in a country. The distinction between a code of conduct and guidelines remains ambiguous, with these terms frequently utilized interchangeably; however, a code of conduct often connotes implications beyond mere recommendation, signifying enforceable mandates in some contexts, whereas guidelines may oscillate between enforceability and serving merely as catalysts for individual ethical contemplation for researchers. Further development of this demarcation is beyond the scope of this paper.
The existence of national guidelines does not necessarily indicate that there would be a nationally coordinated self-regulatory system for research misconduct investigation. Guidelines merely state what should be considered for research to be responsible while being in accordance with the principle of academic self-regulation (ENERI consortium Citation2020). National oversight bodies, therefore, imply that at least some authority is transferred from local to national level.
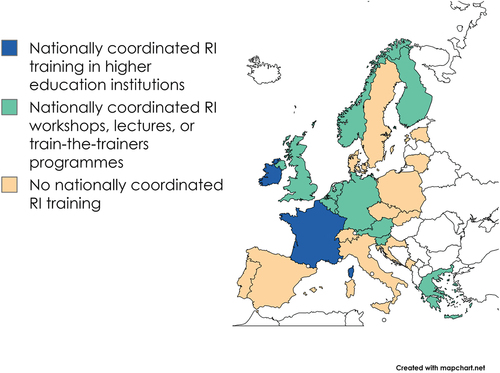
4.1.3. National coordination of research integrity training
In Europe, there is a wide variety of how research integrity training is nationally coordinated. We distilled three ways of coordination used by national research integrity systems: research integrity training is nationally coordinated; national research integrity office provides researchers and other stakeholders with workshops, lectures and/or training programs; and no nationally coordinated training in research integrity is provided ().
It should be acknowledged that a wide array of practices and levels of involvement remain invisible in the categorization. While some organizations provide train-the-trainers programs and participate in disseminating Horizon Europe program-funded projects’ deliverables on research integrity training (e.g., Austria), other organizations have more modest pedagogical aims and they focus on events and seminars (e.g., Belgium, Lithuania). Also, there are kinds of training that are rather relevant to awareness-raising in a general sense (e.g., Norway’s combination of topic-specific guidelines and guidance to their implementation).
Most national research integrity offices provide research organizations and policymakers with consultation even when they do not directly offer training or seminars. This is another approach of training their mind-set on research integrity through awareness-raising.
4.2. The implications of differences and similarities emerging in European research integrity system
Above, we identified more differences than similarities in European research integrity systems. Our categorization tends to be more sensitive to differences rather than similarities. This is due to the fact that our study focuses on existing research integrity structures and more informal exchanges of information, good practices, and leadership initiatives between national research integrity offices, remain largely unnoted. This characteristic may lead to a greater focus on the variances that exist between national research integrity structures rather than the commonalities.
Despite the noted differences, it is important to recognize that there are certain national research integrity policies that have been adopted across all the countries included in our study, and the European Code of Conduct for Research Integrity (ALLEA Citation2023) is acknowledged as a normative source in research integrity. Even if not directly implemented by a national research integrity office, these policies and norms are usually upheld by other national bodies, such as national research funding organizations or academies of science. Legal systems play a crucial role in enforcing these policies, offering a framework within which research integrity can be maintained. However, the observed presence of research integrity policies in all studied countries, even amidst diverse enforcement mechanisms and levels of legal leverage, implies that there is a consensus on the pivotal role of research integrity for the excellence of science.
We have observed national differences in the approach to creation and enforcement of research integrity norms, sharing research misconduct cases to a national oversight body, and national coordination of research integrity training. These differences underscore the complexity of balancing national legal frameworks with the shared principles of research integrity. Despite these challenges, the widespread adoption of core integrity policies reflects a global acknowledgment of their importance to the scientific community.
Most importantly, we have observed that organizations seek international cooperation. In our study, we have focused on ENRIO, but there are also other networks relevant to many research integrity offices, such as the European Network for Academic Integrity (ENAI) and the Network for Education in Research Quality (NERQ). Observation in NRIN’s annual report (Citation2021) stresses the importance of international cooperation:
The NRIN’s affiliation with ENRIO is valuable, because it is essential for developments in the field of scientific integrity to keep abreast of international developments: contact with European sister organizations are therefore important.
Also, observation in OeAWI’s annual report 2021 suggests that there is straightforward benefit in cooperation at the European level:
The OeAWI has benefited tremendously from this network [ENRIO] and will become a founding member in the proposed organization. (a non-profit association based in Brussels)
When employing annual reports as data sources, it is imperative to acknowledge that they are not neutral or purely informational; they are crafted with the explicit intention of communicating to specific audiences, such as shareholders, potential funders, and regulatory bodies. This purposeful crafting introduces biases, as information is selectively presented to highlight successes and secure funding. Therefore, researchers must critically evaluate these documents, understanding that the presentation of data is influenced by organizational goals. The rhetorical orientation of annual reports, while strategic in nature, does not undermine the observation that organizations place significant importance on international cooperation, nor does it diminish the recognized value inherent in learning from their European colleagues.
In conclusion, the implications of differences and similarities emerging in European research integrity system becomes apparent in the management of the pluralism of European research integrity regulation. Research projects are usually international, and disputes in them have, also, the potential to cross the borders of national regulatory systems. National research integrity offices across different countries exhibit a diverse range of powers and capacities to influence national research policy. These differences underscore the complexity and heterogeneity of how research integrity is governed and enforced at the national level, highlighting the importance of understanding the unique contexts within which these structures operate. Differences emerging in the European research integrity system necessitate close interaction among national research integrity offices in order to understand different systems and regulatory environments. Our findings suggests that national research integrity offices perceive significant value in cooperation and mutual learning, highlighting a collective commitment to advancing research integrity across borders.
4.3. The challenges for harmonization of European research integrity system
Pressure for both increased regulation and harmonization of research integrity norms has greatly increased during the last decade (Desmond and Dierickx Citation2021). As can be seen from the European Commission’s calls for projects that advance research integrity training and the success of, e.g., the VIRT2UE project Training guide (https://www.embassy.science/wiki/Guide:Bbe860a3-56a9-45f7-b787-031689729e52), the need for research integrity training that crosses national boundaries has increased accordingly.
We might encounter two types of challenges to implementing the guidance in the pluralist structure of the European research integrity regulatory environment. The first challenge is emphasized in cross-border cases, when the jurisdiction of a case is contested or divided between separate organizations and national regulatory systems. When researchers’ affiliations are moving from one country to another and multiple organizations around the world are involved in the same research projects, it may be difficult to grasp which jurisdiction solving a particular case should belong to. In other words, which country should investigate a case of alleged research misconduct, what code of conduct should be applied, and so forth.
The second challenge concerns the definition and scope of research misconduct and what implications for justice there are if a divergence in norms and their interpretation in different national codes of conduct can be found. If similar misconduct cases are assessed differently in different European countries and if the severity of sanctions is dependent on the country in which the case is investigated, the fairness of research collaborations can be questioned. Also, if European academia is provided with research integrity training using diverse interpretations of content of, e.g., ethical principles, values, good research practices, it might pose a problem for raising shared understanding of appropriate ways to promote research integrity.
Neither of these challenges must be detrimental to aspirations for a harmonized guidance on good scientific practice and research integrity. Pluralist legal structures of research integrity regulation do not necessarily produce conflict and friction; they can also produce harmony and convergence, as can be seen from, e.g., the case of European human rights law (Krisch Citation2010). It can be asked whether this has happened despite, or perhaps because of, the pluralist structure. In European Union law, harmonization can be explained with shared legal history and compatible legal culture and deep structure of law. Yet, in a more international, and even cosmopolitan, context the analysis of conflict and convergence cannot be solely explained by legal histories.
5. Discussion
Even though European divergencies in research integrity regulation run deep, schemes for fruitful co-operation and co-learning between national research integrity offices have been established, especially regarding cross-border cases with the challenge of legal pluralism (Nolte et al. Citation2023). National research integrity systems are interconnected. They are not immutable but are responsive to the challenges that the academic community at large faces.
To perceive how research integrity norms and regulatory environments have converged, we should investigate how the norms actually work in national and local contexts. Even though there are no two identical research integrity regulatory systems in Europe, there is a family resemblance (Wittgenstein Citation1953) of functions national research integrity offices strive to achieve. European research integrity offices’ mandates are connected by a series of overlapping similarities, where no one feature is common to all of them. Some of the overlapping similarities derived from our findings are the prevention of research misconduct by drafting guidelines and codes of conduct; promotion of responsible research practices through training; ensuring legal certainty in research misconduct investigations through transparent investigation procedures, training research integrity advisors and acting as national appeal bodies; and affecting national research policy through policy advice. It is possible to trace some characteristics, such as the occurrence of research misconduct, the training initiatives and monitoring the training outcomes, or issues emerging in the handling of cross-border cases. These characteristics can be used to observe the provenance of differences and similarities between the national research integrity systems.
Furthermore, reads in the ENRIO handbook: Recommendations for the Investigation of Research Misconduct, that “the balance between self-regulation and regulations differs very much between countries in Europe due to different traditions and values. This does not leave room for common recommendation although it is recommended to discuss and evaluate the balance between (external) regulations and (internal) self-regulations” (ENERI consortium Citation2020, 8). Also, in our study it became clear that the European research integrity system includes a mix of internal and external regulation. It is evident that national research integrity offices find value in working together and learning from each other.
The influence of a particular national research integrity office on national research integrity regulation depends on multiple factors ranging from history, legal structures and economics to the personal capabilities and preferences of the people in charge of the operations. The political climate or the individual commitment of research integrity practitioners can have a significant effect on how research integrity is perceived in a given country (e.g., Nolte et al. Citation2023).
In methodological terms, the categorization of different systems, while feasible, may yield diverse outcomes based on the aspects emphasized in each classification. If we align our categories to the ones suggested by Perković Paloš et al. (Citation2023), it becomes clear that abstractions on differences and similarities among European research integrity regulatory systems can vary substantially.
These differences emerge particularly with regard to the interpretation of what constitutes a national code of conduct and what is defined as obligatory research integrity training. We interpret that some of the differences were due to ambivalence in translating concepts in different languages. For example, Perković Paloš et al. (Citation2023) concluded that in Norway there is no mandatory research integrity training, only mandatory research ethics training, but it is unclear if this interpretation fully considers that the Norwegian term “forskningsetikk” (research ethics) encompasses research integrity. Some ambivalences were due to particular national contexts that remain invisible in much of the documentation used in the Country Cards. For example, it was concluded that Finland does not have a national code of conduct (Perković Paloš et al. Citation2023, 11) which ignores the Finnish context of nationally coordinated self-regulation through which all Finnish research organizations and higher education institutions have adopted TENK’s code of conduct for research integrity, making it de facto a national code.
The categorizations are not clear-cut even in the cases that would seem obvious. For example, Finland is explicitly a self-regulatory system (Spoof Citation2018) but the Finnish Universities Act 558/2009 dictates that Finnish universities must arrange their activities so as to ensure a high international standard in research in conformity with research integrity (Yliopistolaki 558/2009 Citation2009). In this case, the law supports the self-regulatory system but does not provide the definition of research integrity, the process of investigation, or other norms relating to research misconduct investigation or research integrity promotion.
In Norway, on the other hand, the law gives a short definition of research misconduct (Lov om organisering av forskningsetisk arbeid Citation2017) and the preparatory work of the Act identified a non-exhaustive list of unacceptable practices that can be considered to be serious enough to be investigated in research misconduct procedures (Langtvedt Citation2020). The law and its preparatory work leave much discretion to local committees and the national research integrity office on how to define other research integrity-related concepts further, consider the severity of actions, and put research misconduct investigation into practice.
Also, the categorization between self-regulatory and voluntary systems is not a strict one but is rather fluid. We can take the UK’s research misconduct investigation system as an example. In the UK, the national research integrity office provides research organizations with experts who are available to serve as external members on panels investigating or adjudicating claims of research misconduct (ENRIO Citation2019a). The national research integrity office promotes research integrity at national level and their expertise can also be used for research misconduct investigation at a local level. Yet, as the information about actual research misconduct cases is not shared with other research institutions or disclosed to an oversight body, the UK does represent a voluntary system. Still, it should be emphasized, that in the UK and other voluntary systems, the regulation is not straightforward but is rather complicated and multifaceted. Research organizations may be liable, for example, to deal with quality assurance agencies to which they must disclose sensitive information.
Differences in the regulatory structures of national research integrity landscapes, the existence of national codes of conduct, and national-level coordination of research integrity training all have an impact in the way the ethical and legal norms are perceived and put into action in practice (Nolte et al. Citation2023; Pizzolato and Dierickx Citation2021; Tauginienė and Gaižauskaitė Citation2022).
6. Conclusions
We analyzed data ambiguity to assess different regulatory environments regarding research integrity. This allowed us to explore the implications of differences and similarities emerging in the European research integrity system, as well as the challenges for its harmonization. We argued that the functions and powers of European research integrity offices differ greatly. Nevertheless, their categorization is yet possible at some extent, but there is great variation between legal status of national research integrity systems. If we tried to categorize these systems in detail, most countries would constitute their own category.
Hence, the pluralist nature is manifested in the European research integrity system. Some national organizations focus on research integrity promotion and training and provide no research misconduct investigation, whereas some organizations focus only on research misconduct investigation. Some European countries have national guidelines or laws and their preambles defining research misconduct, while other European countries have no national-scope guidelines but only organizations’ own guidelines for research misconduct investigation and research integrity promotion.
Ultimately, we conclude that research integrity policies of the existing (and newly appearing) national research integrity offices should be carefully reconsidered to boost the cross-border laying of uniform research integrity standards. Such practice is necessary for multiple purposes, e.g., to shape a homogenous understanding on research integrity through education and training and share it with all relevant stakeholders, to smoother apply the line of global (or at least European) thinking in (multinational) research conduct and dissemination. Even if a full-scale synchrony may not be realistic, it would be important that the actors across nations and research integrity systems understand other perspectives that may emerge in the policies of other countries and identify the reasons that may cause the divergencies. To further promote understanding about different views of research integrity that constitute national policies, it would be important that actors can engage in dialogue with each other. Stakeholders such as the World Conference on Research Integrity and ENRIO play a pivotal role in advancing this.
Given the aforesaid, national research integrity offices might benefit from this study’s findings while consistently seeking to achieve more similarities than differences in their practice. Likewise, the countries that have not yet established national research integrity offices might benefit from these findings while developing research integrity policy without more ado by addressing the challenges discussed. Speaking with one voice of research integrity, at least in Europe, would help.
Authors’ contribution
All authors contributed to the conceptualization design of the study. LT and KV contributed to literature review. KV, LT and EL contributed to methodological design of the study. KV contributed to data collection and data analysis. All authors contributed to writing and editing the manuscript. All authors read and approved the final manuscript.
Ethical approval
This study was conducted in compliance with the national guidelines of TENK (Finland) as well as the guidelines of University of Helsinki and Hanken School of Economics.
Acknowledgments
For providing comments on the manuscript draft, we thank Natalie Evans, Assistant Professor, Ethics, Law & Medical Humanities (Vanderbilt University Medical Center).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Additional information
Funding
References
- ALLEA. 2023. European Code of Conduct for Research Integrity. Revised Edition. https://allea.org/wp-content/uploads/2023/06/European-Code-of-Conduct-Revised-Edition-2023.pdf.
- Anderson, M. S., M. A. Shaw, N. H. Steneck, E. Konkle, and T. Kamata. 2013. “Research Integrity and Misconduct in the Academic Profession.” In Higher Education: Handbook of Theory and Research: Volume 28, edited by M. B. Paulsen, 217–261. Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-94-007-5836-0_5.
- Carl, S. 2012. “Toward a Definition and Taxonomy of Public Sector Ombudsmen.” Canadian Public Administration 55 (2): 203–220. https://doi.org/10.1111/j.1754-7121.2012.00208.x.
- Chen, K., Y. Zhang, and X. Fu. 2019. “International Research Collaboration: An Emerging Domain of Innovation Studies?” Research Policy 48 (1): 149–168. https://doi.org/10.1016/j.respol.2018.08.005.
- Chou, C., I. L. Lee, and J. Fudano. 2023. “The Present Situation of and Challenges in Research Ethics and Integrity Promotion: Experiences in East Asia.” Accountability in Research 1–24. https://doi.org/10.1080/08989621.2022.2155144.
- The Danish Board on Research Misconduct. 2021. Årsberetning 2021. The Danish Board on Research Misconduct.
- Desmond, H., and K. Dierickx. 2021. “Research Integrity Codes of Conduct in Europe: Understanding the Divergences.” Bioethics 35 (5): 414–428. https://doi.org/10.1111/bioe.12851.
- Downey, F., and R. Veitch. 2021. “Research Integrity—It’s About More Than Misconduct.” Forensic Sciences Research 6 (4): 283–284. https://doi.org/10.1080/20961790.2021.1971383.
- Embassy of Good Science. 2023. Facts & Figures. https://embassy.science/wiki/Special:BrowseData/Report.
- ENERI. 2019. EUREC – ENERI – European Network of Research Ethics and Research Integrity. http://www.eurecnet.org/eneri/.
- ENERI consortium. 2020. ENRIO Handbook: Recommendations for the Investigation of Research Misconduct. ENRIO. http://www.enrio.eu/wp-content/uploads/2019/03/INV-Handbook_ENRIO_web_final.pdf.
- ENRIO. 2019a. Country Report: United Kingdom. http://www.enrio.eu/country-reports/united-kingdom/.
- ENRIO. 2019b. Country Reports Archive. http://www.enrio.eu/country-reports.
- ENRIO. 2023. “Members.” http://www.enrio.eu/members/.
- FEK. 2021. Årsrapport 2021. De nasjonale forskningsetiske komiteene FEK.
- Fischbach, R. L., and D. C. Gilbert. 1995. “The Ombudsman for Research Practice: A Proposal for a New Position and an Invitation to Comment.” Science and Engineering Ethics 1 (4): 389–402. https://doi.org/10.1007/BF02583257.
- The German Research Ombudsman. 2021. Jahresbericht 2021. The German Research Ombudsman.
- Godecharle, S., B. Nemery, and K. Dierickx. 2013. “Guidance on Research Integrity: No Union in Europe.” Lancet 381 (9872): 1097–1098. https://doi.org/10.1016/S0140-6736(13)60759-X.
- Hsieh, H.-S., and S. E. Shannon. 2005. “Three Approaches to Qualitative Content Analysis.” Qualitative Health Research 15 (9): 1277–1288. https://doi.org/10.1177/1049732305276687.
- Huistra, P., and H. Paul. 2022. “Systemic Explanations of Scientific Misconduct: Provoked by Spectacular Cases of Norm Violation?” Journal of Academic Ethics 20 (1): 51–65. https://doi.org/10.1007/s10805-020-09389-8.
- Kahneman, D., O. Sibony, and C. R. Sunstein. 2021. Noise: A Flaw in Human Judgement. Little Brown Spark.
- Krisch, N. 2010. Beyond Constitutionalism: The Pluralist Structure of Postnational Law. Oxford University Press. https://doi.org/10.1093/acprof:oso/9780199228317.001.0001.
- Langtvedt, N. J. 2020. The Act on Ethics and Integrity in Research. Forskningsetikk. https://www.forskningsetikk.no/en/resources/the-research-ethics-library/legal-statutes-and-guidelines/the-act-on-ethics-and-integrity-in-research/.
- Lov om organisering av forskningsetisk arbeid, LOV-2017-04-28-23. 2017. https://lovdata.no/dokument/NL/lov/2017-04-28-23?q=Forskningsetikkloven.
- National Academies of Sciences, Engineering, and Medicine. 2017. Fostering Integrity in Research. National Academies Press. https://doi.org/10.17226/21896.
- Nolte, H., K. Videnoja, L. Tauginienė, H. Czesnick, and S. Rutiku. 2023. “ENRIO’s Leading Pathway to Research Integrity Promotion.” In Handbook of Academic Integrity, edited by S. E. Eaton, 1–18. Singapore: Springer Nature. https://doi.org/10.1007/978-981-287-079-7_168-1.
- Npof. 2021. Årlig rapport. Npof. https://npof.se/wp-content/uploads/2022/06/Arlig-rapport-Npof-2021.pdf.
- NRIN. 2021. Jaarverslag 2020 & Jaarplan 2021. Netherlands Research Integrity Network (NRIN). https://www.nrin.nl/images/NRINJaarverslag20_Jaarplan21.pdf.
- Perković Paloš, A., R. Roje, V. Tomić, and A. Marušić. 2023. “Creating Research Ethics and Integrity Country Report Cards: Case Study from Europe.” Accountability in Research 1–35. https://doi.org/10.1080/08989621.2022.2163632.
- Pizzolato, D., and K. Dierickx. 2021. “Stakeholders’ Perspectives on Research Integrity Training Practices: A Qualitative Study.” BMC Medical Ethics 22 (1): 67. https://doi.org/10.1186/s12910-021-00637-z.
- Reisman, W. M. 2008. “On the Causes of Uncertainty and Volatility in International Law.” In The Shifting Allocation of Authority in International Law: Considering Sovereignty, Supremacy and Subsidiarity, edited by T. Broude and Y. Shany, 1st ed., 33–50. Oxford: Hart Publishing; Bloomsbury Collections.
- Resnik, D. B., T. T. Neal, A. A. Raymond, and G. E. Kissling. 2015. “Research Misconduct Definitions Adopted by U.S. Research Institutions: Introduction.” Accountability in Research 22 (1): 14–21. https://doi.org/10.1080/08989621.2014.891943.
- Resnik, B. D., and A. E. Shamoo. 2011. “The Singapore Statement on Research Integrity.” Accountability in Research 18 (2): 71–75. https://doi.org/10.1080/08989621.2011.557296.
- Sheldon, T. 2011. “Dutch Research Community Is Shocked by Cases of Scientific Misconduct.” British Medical Journal 343: d7690. https://doi.org/10.1136/bmj.d7690.
- Spoof, S.-K. 2018. A Framework for Self-Regulation in Research Integrity: The Finnish Model, Step by Step. Responsible Research. https://vastuullinentiede.fi/en/planning/framework-self-regulation-research-integrity-finnish-model-step-step.
- Stieber, C. 2000. “57 Varieties: Has the Ombudsman Concept Become Diluted?” Negotiation Journal 16 (1): 49–57. https://doi.org/10.1023/A:1007546404573.
- Tauginienė, L., and I. Gaižauskaitė. 2022. “Jumping with a Parachute – Is Promoting Research Integrity Meaningful?” Accountability in Research, 1–26. https://doi.org/10.1080/08989621.2022.2044318.
- TRUST. 2018. The TRUST Code – a Global Code of Conduct for Equitable Research Partnerships. https://doi.org/10.48508/GCC/2018.05.
- Vaismoradi, M., H. Turunen, and T. Bondas. 2013. “Content Analysis and Thematic Analysis: Implications for Conducting a Qualitative Descriptive Study.” Nursing and Health Sciences 15 (1): 398–405. https://doi.org/10.1111/nhs.12048.
- VCWI. 2021. Jaarverslag 2021. VCWI. https://www.vcwi.be/sites/default/files/VCWI_jaarverslag2021.pdf.
- Wittgenstein, L. 1953. Philosophical investigations. Philosophische Untersuchungen. Macmillan.
- Yliopistolaki 558/2009. 2009. https://www.finlex.fi/fi/laki/ajantasa/2009/20090558.
- Zhang, Y., and B. M. Wildemuth. 2009. “Qualitative Analysis of Content.” In Applications of Social Research Methods to Questions in Information and Library Science, edited by B. M. Wildemuth, 308–319. Westport, CT: Libraries Unlimited.