ABSTRACT
Objectives: Many polyphenols such as EGCG from green tea, curcumin, apigenin, resveratrol or the alkaloid berberine show in-vitro activity that is much higher than FDA and EU approved drugs. And yet, while mice studies show excellent results, human clinical trials have so far been quite disappointing.
Methods: In this review paper we accordingly present data from scientific literature and publicly available databases that support further development of nanoformulations for enhanced natural antioxidants bioavailability and activity.
Results: Nanobubbles, and nanoparticles may enhance the polyphenols bioavailability and special coatings can be used to avoid liver fast inactivation. Zeolites have also been tested as carriers for bioactive compounds from the Mediterranean food. Other polyphenols can be used with nanoemulsions for synergistic antioxidants treatment. Finally, nanobubbles in the presence of ferric or copper ions at low pH as in human stomach or lysosomes can produce small amount of hydroxyl radicals, stimulating activation of Nrf2 transcription factor and detox enzymes.
Discussion: A number of nanoformulations, including nanobubbles loaded with natural antioxidants may be used with low carbs and protein diet as a support to chemotherapy or treatment of advanced tumors. Good results in animal studies of neurodegenerative diseases and type II diabetes were also observed.
Nanobubbles and nanoparticles may enhance the polyphenols’ bioavailability
Nanobubbles may stimulate the activation of Nrf2 and detox enzymes
Armoured oxygen nanobubbles may enhance radiotherapy or chemotherapy effects
KEY POLICY HIGHLIGHTS
1. Introduction
Chronic diseases, such as type II diabetes, Alzheimer's, cardiovascular diseases, and cancer, represent a substantial burden to society. Prevention is the only way to avoid these often-devastating conditions. A healthy lifestyle, including regular aerobic and resistance exercise, low fat and sugar food and beverages and low caloric intake, may help accordingly, in the prevention of many chronic conditions connected particularly to the development of obesity and ageing [Citation1,Citation2]. Early intervention with drugs, ‘functional foods’ or nutraceuticals may help to slow down the development of age-related conditions. Food polyphenols have been specifically studied within many in vitro and in vivo studies as a non-prescription intervention to slow down ageing. Yet, polyphenol-rich food does not provide therapeutic levels of polyphenols and acts rather in a long-term preventive manner. Many polyphenol supplements (nutraceuticals) have been thus developed for mass production and sales. The supplement industry is booming, with billions of dollars made every year. However, recent research points to a limitation of polyphenol concentrates which is poor bioavailability [Citation3]. Such molecules do not even get engulfed by gut cells and end up at a large per cent in faeces. This is because polyphenols, in general, are poorly bioavailable, show poor gastric solubility, and gastric residence time, and are metabolized fast in the liver. The bioavailability of such natural products that can be used in the prevention and treatment of many conditions as adjuvants should thus be improved. Nanotechnology is a perfect way to achieve such goals [Citation4,Citation5]. Various -nano and -micro encapsulation techniques have been tested for the improvement of polyphenol delivery and increased bioavailability. Nano and -micro carriers, may, for example, be inorganic solid nanoparticles, such as zeolites or metal oxides, nanometals (gold and silver), quantum dots, organic emulsions, liposomes, multilayer coatings (polymers and inorganic materials– composites), surfactant micelles, extracellular vesicles (lipid bi- and multi-layers), nanobubbles, coated (‘armoured’ nanobubbles), etc. [Citation6]. We will, however, focus on the mechanisms of action of some of the most potent polyphenols including epigallocatechin gallate (EGCG) from green tea, curcumin from turmeric, alkaloid berberine, chlorogenic acid, and some products from Mediterranean food such as olive oil polyphenols and accompanying nano-formulations, particularly nanobubbles for the enhancement of their bioavailability.
1.1. Nanobubbles
With significant development of nanotechnology, nanobubbles, used as ultrasound contrasting agents, are becoming more valuable in ultrasound imaging diagnosis and their application in drug delivery systems. Ultrasound medical technology is widely used due to as high safety, great speed and low cost. Additionally, nanobubbles are promising drug delivery systems due to their smaller size and prolonged circulation time in comparison with microbubbles [Citation1,Citation2]. Moreover, novel biocompatibility and non-toxicity show the great importance of their usage [Citation3].
A study at Tel Aviv University has proved that a combination of ultrasound and nanobubbles enables the removal of tumours without surgery, by destroying tumour cells in a targeted manner. The researchers, unlike the invasive treatment methods, which use the injection of microbubbles into the tumour directly, showed a way of destroying a tumour in a non-invasive manner, by applying nanobubbles directly into the bloodstream. However, this method has its advantages and disadvantages. On the one hand, it provides localized and spot-focused treatment, which shows great results in solid tumours deep within the body. This also offers the possibility of treatment of patients who are not good candidates for resection surgery. On the other hand, the disadvantage is that the high-intensity ultrasound waves used in this type of treatment may induce the damage of nearby healthy tissue4.
Furthermore, the study by S. Liu et al. [Citation5] has applied nanobubble technology to hydrogen water in vivo and in vitro, which can act as a new therapeutic antioxidant. The application of hydrogen nanobubbles in water with different reactive oxygen species has offered basic data for future applications of hydrogen nanobubbles in different fields of biomedicine. Additionally, there is poor wound healing that is often associated with aberrancies in tissue oxygenation, in which nanobubbles, as a new modality for tissue oxygen delivery, can be induced. Currently, several models of tissue oxygen delivery systems exist, such as Hyperbaric Oxygen Therapy (HBOT) and Topical Oxygen Therapy (TOT). Recent studies suggest that current wound healing treatment could be advanced with nanobubble delivery [Citation6].
The size of the nanobubble is relevant for delivery into the cell. For example, plasmid DNA (pDNA) was increasingly transferred into the cytoplasm of cells by the use of ultrasound in the presence of NB size higher than 200 nm [Citation7] In addition, nanobubbles below 200 nm delivering doxorubicin showed increased accumulation in the tumour interstitial space in a mouse model of LS-174 T colorectal cancer [Citation8]. Still, the size characterization of NB may be challenging and different methods have been developed for this specific purpose. One such method is the (1) resonant mass measurement (RMM) for bubbles with sizes higher than 200–300 nm, a (2) holographic nanoparticle tracking analysis (NTA) based on the creation of an interference pattern by combining the scattered light and that of an unobstructed reference beam and (3) high-frequency M-mode imaging to observe stochastic motion of NBs within an agarose gel with known pore sizes [Citation9].
To this date, nanobubbles are used mainly in drug delivery systems, tumour theranostics, brain drug delivery and vascular disease theranostics. Future offers challenges on how to introduce nanobubbles in many more fields that could have great results in biomedicine [Citation1].
1.2. The proposed mechanisms of action of polyphenols enhanced with nanobubbles and other nanocarriers
We propose a radical new theory of mechanisms of action of nutraceuticals and carriers used for their encapsulation. The mechanisms of action should be evaluated within a dual context of results in the literature showing both detrimental effects of antioxidants on health in humans and animals [Citation10,Citation11] and opposing in vitro, in vivo and human clinical trials results on polyphenols, nanobubbles and nanoparticle benefits to animal and human health [Citation12,Citation13]. The final effect of polyphenols on the molecular levels indeed, depends on many stoichiometric data underlying biological effects including, polyphenol concentration, expression levels of biomolecules in an organism, physiological status, and status of the immune and general antioxidative system. Accordingly, we suggest that polyphenols, nanobubbles and some nanoparticles such as micro- and mesoporous zeolites act as mild pro-oxidants and induce the activation of signal transduction cascades that activate organisms’ own cellular defences via final activation of transcription factor Nrf2, that modulates the activity of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation. Modification of the activity of other protein kinases, such as JNK, ERK1, etc. also play a role in the positive effects of polyphenols and their carriers [Citation12–14]. Some antioxidant effects do occur and result in the activation of other signal transduction cascades such as modification of the activity of PI3K kinase, protein kinase Akt and inhibition of the activation of mTOR complex. This promotes autophagy, detoxifies cells from old oxidized proteins and lipids, and induces apoptosis of old senescent cells [Citation15,Citation16]. Even with the current encapsulation techniques most polyphenols, nanobubbles and nanoparticles exert a large portion of their effects locally in the gut. Encapsulation indeed, enables adsorption of polyphenols and carriers by the gut microbiome and gut epithelial and/or immune and nerve cells [Citation17,Citation18]. These agents then particularly promote gut microbiome diversity [Citation19]. Along with healthy eating habits and fiber-rich food consumption production of beneficial metabolites such as short-chain fatty acids, SCFA is then enhanced [Citation20]. Polyphenols, nanobubbles and nanoparticles enhance anti-inflammatory effects of fiber-rich foods. This may help in almost any chronic condition if intervention happens early before tissue degeneration. This local activity is corroborated by the recent unveiling of the gut – pancreas, gut – liver, gut-immune system and gut-brain axis [Citation21–26]. The human gut includes small intestines starting with the duodenum and ileum, a large intestine that includes cecum and colon and finally rectum and anus. All those areas are inhabited by friendly commensal microbes and occasional pathogens. Cells inside the human gut are involved in so much more than just nutrient and water absorption. A complex interaction between gut cells, microbiota, food, and drinks dramatically affects the rest of the body including the brain (gut-brain axis). When something goes wrong in the gut, this may as well be the start of a mild chronic inflammatory response that can result in a variety of tissue degenerative diseases. Healthy eating, exercise, weight, and body fat percentage may start to be disrupted as a consequence of gut imbalance. Even mental illnesses can be exacerbated after gut malfunctions. New techniques such as spatially resolved single-cell multi-omics have recently been applied to gut research [Citation27]. The human cell atlas will inevitably identify some rare new cell types. Given that human intestines are very long this will present an enormous job. New biotech platforms such as, for example, the ALTIS Biosystems’ REPLIGUT model, provides human stem cells derived from various gut cells in monolayer culture for in vitro testing. Such an approach may help in the research of the gut since mice are not a good model system for human gut biology studies. 3D human organoids may also provide a good model for gut research [Citation28]. However, the gut-liver relationship involved in so many health issues such as glucose metabolism or detox processes is still difficult to address with current state-of-the-art techniques. Such model systems are essential for breakthroughs. At last, current microbiota research is usually based on analysis of the microbiome and cells that come out from the intestine through the faeces. But those are not necessarily representative of the situation inside the upper part of the gut, particularly in the small intestine. Polyphenols, nanobubbles and nanoparticles showed fantastic results on improvements of the health conditions in mice model systems, while in human clinical trials, they had very modest effects, if any.
1.3. Human gut structure and organization and polyphenols
The small intestine is dominated by rod-like villi with single-layer enterocytes that adsorb basic food components. Other cells, such as goblet cells and tuft cells, are less common. Epithelial cells are polarized, and their sides are connected via tight junctions. Proteins, such as occludins and claudins, are common and are involved in the formation of so-called zonula occludens (ZO). The tight junctions play a delicate role: they allow for the entrance of nutrients but generally inhibit the entrance of microbes or toxins. Enterocytes are rich in a variety of immune system attraction cell surface receptors such as TOLL receptors. Many innate immune system cells also reside nearby. Mucus-producing goblet cells contribute to the healthy gut-intestinal barrier as well. In addition, Paneth cells are involved in glycolysis and help stem cells in maturation and differentiation. A quite complex structure and biology of gut components is acknowledged. Polyphenols and nanocarriers were shown to interact with all those cells. In our opinion, apart from some large-scale omics approaches, the ALTIS REPLIGUT model may also be used to identify the modifications that happen upon polyphenol ingestion in such a complex system. So far, we simply do not know the whole complex picture and only partial knowledge is available. Another important gut structure is the so-called crypts of Lieberkuhn, simple tubular structures where stem cells proliferate and mature. In the distal ileum, lamina propria many innate response immune cells reside and from gut – associated lymphoid tissue (GALT). M cells, which are somewhat modified enterocytes, cover lymphatic nodules in the lamina propria. Some well-defined follicles inside the intestinal wall called Peyer's Patches (PP)s are formed oppositely to the mesenteric attachment. PPs do not have any protective layer and are in direct contact with commensal ‘good’ bacteria. PPs can respond very fast to any presence of microorganisms nearby. Probably they also can get in touch with nanocarriers and nutraceuticals and react to their presence. presents the human gut schematically [Citation29].
Figure 1. Schematic presentation of small intestine. Particles may enter the intestinal barrier from the small intestine lumen through microfold cells (M-cells) that present them to immunological cells (i.e. dendritic cells) in the lamina propria and the Peyer’s patches. Peyer’s patches are rich in T cells, macrophages, and activated antibody-secreting B and plasma cells. The single layer of the intestinal epithelium is protected by mucus produced by Goblett cells and containing mucin glycoproteins where immunoglobulin A (IgA) and antimicrobial peptides prevent interaction of microbiota with the cell surface. Disruption of the intestinal barrier leads to the secretion of pro-inflammatory cytokines by T-cells (adopted from [Citation29]).
![Figure 1. Schematic presentation of small intestine. Particles may enter the intestinal barrier from the small intestine lumen through microfold cells (M-cells) that present them to immunological cells (i.e. dendritic cells) in the lamina propria and the Peyer’s patches. Peyer’s patches are rich in T cells, macrophages, and activated antibody-secreting B and plasma cells. The single layer of the intestinal epithelium is protected by mucus produced by Goblett cells and containing mucin glycoproteins where immunoglobulin A (IgA) and antimicrobial peptides prevent interaction of microbiota with the cell surface. Disruption of the intestinal barrier leads to the secretion of pro-inflammatory cytokines by T-cells (adopted from [Citation29]).](/cms/asset/03183c34-a441-40ff-a6cf-0e55fd9c855e/yrer_a_2333619_f0001_oc.jpg)
Enteroendocrine cells include closed cells that do not reach the epithelium and open cells that can be found at the end of each villus. Such open cells have taste sensors and secrete various hormones that can regulate glucose metabolism, the pancreas, and the brain-gut axis. Given the effects of nanocarriers and polyphenols on animal and human physiology, those cells almost certainly are involved in the action of these substances. Gastric motility (irritable bowel syndrome, IBS) and effects on the brain and nerve vagus probably originate there. The M cells transport antigens inside, close to the CD4+ T lymphocytes. T cells, in turn, regulate the GALT response. It is almost certain that small nanobubbles and nanoparticles interact with M cells and are presented to the T cells. As we already mentioned, epithelial cells can secret pro or anti-inflammatory cytokines depending on the stimuli. It is almost certain that nanocarriers with polyphenols interact with the epithelial cells of the small intestines. The human ileum is home to more bacteria than any other part of the small intestines. ‘Good bacteria’ such as Lactobacillus, and Bacteroides are involved in bile acids and vitamins, such as K or B12, metabolism. Clostridium, Staphylococcus and Streptococcus can be good or bad depending on the circumstances. Firmicutes use fiber in food to produce butyrate which is very important for gut mucosa renewal. Further on, the large intestine comprises several areas such as the cecum, colon, rectum, and anus. Its mucosa is very different from the small intestine and has smooth surfaces with no villi. However, crypts are present and are numerous. The simple columnar epithelium has openings at the top. Those columnar absorptive cells mainly reabsorb water and electrolytes. To eliminate semisolid faeces, thick mucus protects the intestine walls. This is accomplished by a large number of goblet cells. Whether nanocarriers interact with the signal transduction cascades involved in water reabsorption is, however, not known. Semi – processed food includes many various endotoxins and microbes so GALT is also present in the large intestine. Given the beneficial effects of polyphenols and nanocarriers on endotoxin-caused damage, it is almost certain that certain interactions with the GALT occur. Recently some small lymphatic vessels were observed in the cecum, but their role is not known.
When chronic inflammation is caused in the large intestines and colon area ulcerative colitis may develop. The proliferation of lymphatic and blood vessels, the so-called lymphangiogenesis is correlated to the expression of vascular endothelial growth factors (VEGFs) [Citation27]. It seems that nanocarriers loaded with polyphenols interact with the VEGFs and nanobubbles and polyphenols have already shown their potential to inhibition of lymphatic vessel formation in the large intestine [Citation28]. Concerning the local effects in the rectum, nanocarriers with polyphenols are shown to inhibit adenomas and polyps of the rectum [Citation30]. Polyphenols- and nanobubbles-rich water can also relieve itchy and painful anus symptoms. Similarly to polyphenols, the microporous zeolite may also alleviate symptoms correlated with pro–inflammatory conditions inside the small intestine, irritable bowel syndrome or rectum polyps as well [Citation31].
1.4. Interactions between microbiota, intestinal cells, food, and ‘naked’ and encapsulated polyphenols
Gut microbiota outnumbers gut cells and their variety. While most commensal microorganisms are beneficial, some pathogenic microbes enter the human digestive system daily. Therefore, gut epithelia adsorb nutrients and prevent microorganisms’ penetration inside the tissues and organs. Epithelial cells may indeed, sense microbiota changes and respond by the secretion of pro-inflammatory or anti-inflammatory molecules including cytokines, chemokines and other small molecules that crosstalk with the GALT, gut nervous system, enterocytes, commensal microbiota and pathogens ().
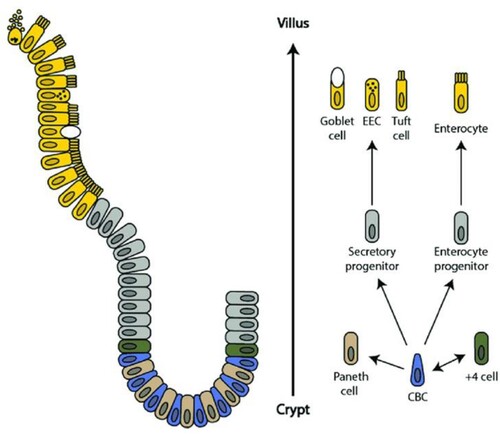
Figure 2. Schematic of the intestinal crypt-villus unit. The intestinal crypt-villus unit is maintained by multipotent crypt base columnar (CBC; Lgr5 +) and +4 cells (Hopx +, Bmi1 +, mTert +, Lrig +). These stem cells are found in the crypt and supply the villus with specialized intestinal cells, including enterocytes, goblet cells, enteroendocrine cells (EEC), and tuft cells, which are eventually shed at the villus tip. Conversely, Paneth cells are mature cells that remain in the crypt and modulate the stem cell environment. Figure adapted, with permission from Company of Biologists, from Beumer et al. 192. Available herein via license: Creative Commons Attribution 4.0 International from https://www.researchgate.net/publication/323367542_Comparative_regenerative_mechanisms_across_different_mammalian_tissues.
When something goes wrong exaggerated pro-inflammatory response and/or gut permeability occurs. This happens in patients with irritable bowel syndrome, Crohn‘s disease, inflammatory bowel disease (IBD), impaired glucose control, prediabetes, loss of weight control, autoimmune, neurodegenerative diseases and cancer [Citation32]. In a healthy individual thick hydrogel of mucus protects the cells from exposure to commensal and pathogenic microbes, food toxins, acids, caustics, antigens, etc. In the small intestine, where food absorption happens, the mucus should be loose and flexible to exert particular tribomechanical properties, such as, for example, a weak attractive surface force with the epithelial cells and the possibility to be removed when needed. Microbes on the other side, activate enzymes needed to loosen mucus. In the case of pathogen microbial enzymes, the mucus becomes largely porous, and patches of epithelial cells are increasingly temporarily exposed to food antigens, microbes, and abrasive food ingredients [Citation32].
In the large intestines, food leftovers are dehydrated, and faeces have higher viscosity and abrasiveness. Tribomechanical behaviour inside large intestines is completely different. To protect epithelial cells from abrasion mucus layer of the large intestine is much thicker and divided into at least two layers. The inner mucus layer is well organized, smooth, lamellar and non-porous. Attractive forces between epithelial cells and lower mucus layers are very strong with a large number of hydrogen bonds and well-hydrated hydrogel formation. The outer layer is much looser and its tribo-chemistry is much different. Microbes that live in the large intestines hydrolyze proteolytically mucins. This results in lower molecular weight of biopolymers. Recent polymer chemistry research with direct surface force measurements indeed showed that the higher the molecular weight, the stronger the attractive forces [Citation33]. Attractive forces between the outer and inner mucus layers are weak. We can reasonably conclude that such hydrogel is loose and faeces movement through the large intestine can move it. This is necessary because otherwise, free movement of materials would not be possible. How nanobubbles, nanoparticles and nanodroplets affect surface forces and tribomechanical behaviour inside the large intestine is a new area of research. However, nanoobjects, including polyphenols, modify the diversity of gut microbiota. Therefore, such products can indirectly modify gut mucus as well. Future research will show whether nanobubbles and nanoparticles can directly modify surface forces and gut viscosity. Nanobubble research, for example, showed that they can attach to porous hydrophilic materials by entrapment. The highest adsorption rates were observed for partially hydrophobic materials [Citation34,Citation35]. Mucus hydrogels are exactly partially hydrophobic porous hydrogels. Consequently, we expect that nanobubbles, nanoparticles and partially hydrophobic polyphenols such as curcumin will affect mucus behaviour. The immune system at those locations can then react with a low-grade inflammation. Accordingly, nanobubbles, and nanoparticles with polyphenols positively influence such conditions by stimulating the body own antioxidant and anti – inflammatory responses [Citation36]. Fibre and mucus-metabolizing microbes produce many valuable small molecules that can be transported even in the brain. Short-chain fatty acids (SCFA) such as acetate, propionate or butyrate are very beneficial for human health. Butyrate is an energy source for many cells inside the gut including colonocytes and seems to stimulate gene expression of many beneficial proteins including tight junctions occludins and claudins important for healthy gut barriers. This may prevent conditions such as ‘leaky gut’. Acetate seems to be protective against toxins. Acetate and propionate may also stimulate goblet cell proliferation and differentiation and mucin layer formation [Citation37]. The schematic presentation of gut-tight junctions is illustrated in .
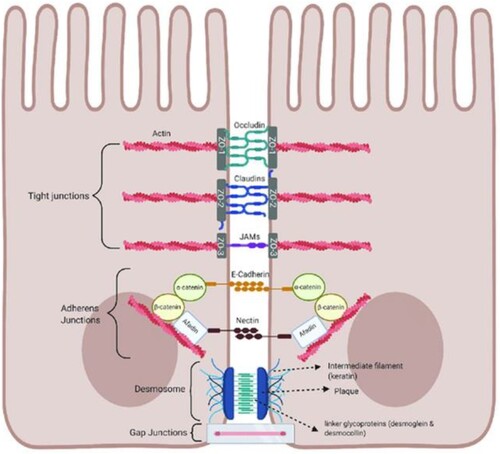
Figure 3. Schematic of tight junctions in the healthy gut. Tight junctions are at the apical end of junctional complexes composed of three transmembrane proteins: occludin claudins and junctional adhesion molecules (JAMs) that bind intracellular membrane proteins, zonula occludens (ZOs) which connect the transmembrane tight junction to the actin skeleton. Below the tight junctions are adherens junctions composed of transmembrane proteins, E-cadherin and nectin linked to the cytoskeleton by scaffolding proteins, catenin and afadin linked to actin filaments. Desmosomes are made up of the transmembrane liner glycoproteins, desmoglein and democollin which are cadherin proteins linked to intermediate keratin filaments. Gap junctions form a tunnel for small molecules to pass between adjacent epithelial cells. Image made using BioRender. Available via license: Creative Commons Attribution 4.0 International from https://www.researchgate.net/publication/348420396_Role_of_Metabolic_Endotoxemia_in_Systemic_Inflammation_and_Potential_Interventions.
Microbes can also metabolize bile salts and produce secondary bile salts that activate TGR5 receptors in the ileum and can help improve glucose metabolism, by various mechanisms including activation of the hypothalamus and stimulation of glucagon-like peptide 1 (GLP-1) [Citation37–40]. Microbes that metabolize lactate may stimulate enhanced oxidative phosphorylation inside mitochondria [Citation41]. This may in turn stimulate system cell maturation and crypt regeneration. It is also well-documented that microbiota may increase butyrate formation and metabolize polyphenols. The polyphenol metabolites have been then correlated with anticancer and anti-inflammatory effects, positive effects on the gut barrier integrity and local inflammation status.
In conclusion, a direct effect of nanobubbles and nanoparticles loaded with polyphenols may thus be exerted locally in the gut through interactions with the mucus and induction of protective low-grade pro-inflammatory response and through interactions with microbiota where microbiota polyphenol-metabolites underly a vast array of biological effects, including anti-cancer and anti-inflammatory effects. Caution is, however, needed as the individual gut microbiome status may play a role in nanocarriers uptake. Accordingly, an inflammatory status or gut pathologies that affect the gut endothelium integrity may affect the uptake and stability of the nanocarriers. Similarly, nanocarriers may also affect the microbiota status such as shown for carbon-based nanoparticles [Citation42,Citation43].
2. Nanotechnology enhancement of bioavailability and activity of polyphenols
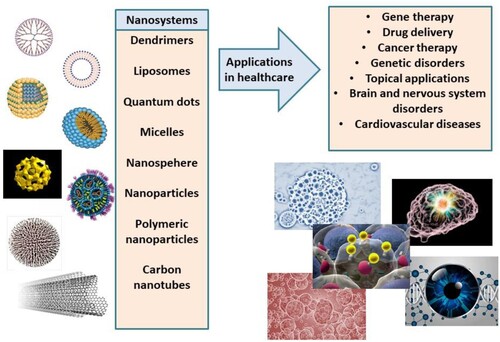
Polyphenols are often antioxidants or mild pro – oxidants. So, it is not surprising that contact with oxygen over time can reduce their bioactivity. Unpleasant taste, odour or smelly ‘burps’ are also quite common. Many polyphenols are difficult to process or are almost completely insoluble in water. Often, their gut transit rates are very fast, and they end up in faeces. When polyphenols are bioavailable, the liver metabolizes and inactivates them. Finally, the bioavailability of most polyphenols may be regarded as low in humans. Gaseous nanobubbles, nanoliquids as well as solid and semi-solid nanoparticles have been used to enhance polyphenols’ bioavailability. Polyphenols are, accordingly, entrapped inside these diverse nanoparticles, to increase their bioavailability profiles. Particularly interesting are studies with nano liquids or nanobubbles either those containing natural or synthetic ingredients. Gelatin, chitosan, alginate, albumin, casein, whey, and pea proteins are only a few examples of natural materials used for nanoencapsulation. Triglycerides (fats oils and grease), waxes and hard fats have all been used along with emulsifiers or surfactants such as lecithin. Synthetic polymers, surfactants and emulsions have also been applied for polyphenol entrapment. Polymers, such as poly (lactic-co-glycolides) [PLGA], have been approved by the FDA as well [Citation44]. Polymeric anhydrides, polyglycolides (PGA), poly cyanoacrylates, polycaprolactones, polylactides (PLA), polymalic acid, and polyorthoesters are among the most popular due to their high biocompatibility. Many products include mixtures of up to five different ingredients. Surfactants are often needed to stabilize or solubilize the matrix produced. Nonionic surfactants, zwitterionic surfactants as well as amphipathic alcohols and polyalcohols such as polyvinyl alcohol have also been used. Popular nonionic surfactants include polyoxyethylene alcohols, polyoxyethylene glycol alkyl ethers, alkyl ethoxylates, and alkyl phenol ethoxylates. The most popular zwitterionic surfactants are natural phospholipids. Some of the nanocarriers studied so far to enhance bioavailability and activity of polyphenols are presented in .
Figure 4. Nanosystems (nanocarriers) studied in nanomedicine and corresponding applications in healthcare.
Many reviews and recent books [Citation45,Citation46] have been published on the topic of nanocarriers for increased polyphenol bioavailability. Bioavailability depends on many factors, including gut absorption, systemic circulation, phase I detoxification in the liver, exclusion in kidneys, uptake in organs and particularly crossing the blood – brain barrier. The goal is to deliver polyphenols inside gut cells involved in the stimulation of immune cells inside the GALT which indirectly affects the rest of the body. Coating the nanocarriers with biocompatible molecules such as protein albumin can significantly enhance the uptake by enterocytes and other gut cells. It seems that albumin strongly adheres to stomach mucosa receptors. Several other biopolymers such as casein, chitosan, alginates etc. can disrupt tight junctions inside the epithelial tissue and enhance the absorption of encapsulated polyphenols temporarily. The mechanism is based on the fusion of encapsulated polyphenols with cell and organelle membranes or the active transport of encapsulated polyphenols. Nanobubble are partially hydrophobic polyelectrolytes (high surface charge) and can affect both these processes [Citation44].
Another aspect that should be thoroughly investigated during the development of nanocarriers is their influence on the final activity of polyphenols and other bioactive compounds. Liang et al tested the encapsulation of EGCG in zein/chitosan nanoparticles for controlled applications in food systems [Citation47]. Interestingly, they found that EGCG-encapsulated zein/CS NPs exhibited higher DPPH scavenging activity than the control EGCG without encapsulation. Another study aimed to construct and assess pea protein isolate nanocarriers for lipophilic polyphenols curcumin, quercetin, and resveratrol, showing that the ABTS and DPPH radical-scavenging activity of polyphenols was noticeably improved through complexation with pea protein isolate [Citation48]. Improved antioxidant properties, under accelerated degradation conditions and simulated digestion, were also observed for hydrophilic anthocyanins as part of novel nano-complexes utilizing ovalbumin and sulphated polysaccharides [Citation49]. The Historical Perspective written by Maqsoudlou concluded that antioxidant-loaded nanocarriers can be applied in many formulations with positive outcomes, i.e. higher and controlled release of antioxidant activity [Citation50].
3. Curcumin and its formulations for increased bioavailability
Curcumin is among the most studied polyphenols [Citation45]. Animal studies show a myriad of potential preventives and therapeutic activities even though human clinical trials were less successful. This may be the consequence of curcumin's high hydrophobicity and poor solubility in water. Accordingly, its gut absorption in humans is very limited. Importantly, it is metabolized by the liver as a xenobiotic which means it is cleared fast from the body. Curcumin is also prone to autooxidation of inactive products. Such bioactive molecule is an ideal candidate for nanotechnology encapsulation. In particular, nanobubbles and nanoparticles loaded with polyphenols, exhibit mild pro–oxidative activity which stimulates transcription factor Nrf2 signalling pathways and gene expression of many detox enzymes, mostly phase II antioxidant enzymes such as catalase, superoxide dismutase (SOD), or glutathione oxo-reductase. Other signalling pathways involve protein kinases such as PI3k, JAK-STAT, NFkB, ERK-1, JNK, protein kinase Akt, mTOR complex, AKAP kinase, etc. Therefore, curcumin promotes autophagy, detoxification, anti-inflammatory and anticancer signalling in many cell types [Citation12]. Moreover, curcumin’s pro-apoptotic activity of senescent or cancer cells is also stimulated by gene expression activation of the p53 tumour suppressor protein. Many p53-independent pro-apoptotic and anti-proliferative pathways are also stimulated by curcumin [Citation46]. Inhibition of pro-inflammatory cancer-promoting TNF-alfa, many interferons, and interleukins such as Il-6, are probably influenced by the inhibition of NFkB. Many other pathways are also influenced by curcumin. Curcumin modifies the activity of many growth factors such as epidermal growth factor (EGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor b1 (TGF-beta1), vascular endothelial growth factor (VEGF). The activity of numerous receptors and transcription factors is also modified [Citation51]. In all, the activity of over 100 targets can be increased or decreased by curcumin as shown in many studies in vitro. The issues with poor bioavailability of curcumin in mice and humans have been reviewed in great detail in [Citation45]. In short, oral administration of curcumin is low, and often most sophisticated detection techniques such as HPLC/MS/MS, where liquid chromatography is coupled to a new generation of mass spectrometers are needed to detect serum or tissue curcumin concentrations. In most cases, tissue doses of curcumin were sub 1 ng/ml [Citation52]. Even at very high doses over 10 grams a day, serum concentrations did not reach 4 micrograms/ml. Administering curcumin with other supplements such as piperine from black pepper (Piper nigrum) that modifies gut permeability temporarily resulted in somewhat higher serum concentrations. Piperine also modifies liver detox enzymes involved in say glucuronidation. At a dose of 2 grams of curcumin and 20 mg of piperine, up to 200 ng /ml of curcuminoids were detected in serum plasma at 0.75 h [Citation53]. Phospholipids such as lecithin are commonly used to enhance bioavailability of polyphenols.
3.1. Enhancing curcumin bioavailability with nanotechnology
Tribomechanical activation (grinding), spray drying with ultrasound and other high-energy treatments have been used to produce curcumin nanoparticles with sizes ranging from 30 nm to 380 nm [Citation54]. These nanopreparations increased the antimicrobial activity of curcumin by 10–15% but the final dosage needed for good antimicrobial activity was still large (up to 1 mg/ml). Nanoparticles of pure curcumin do not solve completely the bioavailability issue.
3.2. Hydrophilic nanoparticles and their mixtures with other reagents
Encapsulation of curcumin with different hydrophilic particle-based formulations has been tested. Acids, such as ascorbic acid, citric acid or glycyrrhizic acid, were tested to coat chitosan or other polyelectrolytes to prevent microbiota degradation of curcumin and stabilization of curcumin during stomach passage at very low pH [Citation54]. Nonionic and polyelectrolyte chitosan coatings significantly increased curcumin stability and bioavailability and decreased the fast serum clearance. Hydroxypropyl – methylcellulose used for artificial tears is a particularly good coating material [Citation55]. In Japan, the only drinkable curcumin formulation was prepared [Citation56,Citation57]. Potent emulsifier non-starch polysaccharide ‘gum ghatti’ from Indian plant Anogeisssus Latifolia was used at 4% with glycerin at 40–46% and water and curcumin. ‘Theracurmin’ drink delivered up to 300 ng/ml to serum at a dose of only 210 mg of curcumin. Negatively charged rape seed globulin protein cruciferin in acylated form has been used to coat cationic polyelectrolyte chitosan. This formulation was used for increasing the gut passage and longer residence time [Citation58].
3.2.1. Curcumin and micelles
Detailed studies have been performed with surfactant micellar systems loaded with curcumin. At low concentrations, surfactants are single molecules. However, at higher concentrations surfactant molecules self-assemble in solution and on the surfaces into aggregates where hydrophobic hydrocarbon chains form micelles in solutions or hemimicelles on the surfaces of nanoparticles or micronized particles. The bottom line, while such formulations increased the bioavailability of hydrophobic curcumin that gets incorporated inside the hydrophobic surfactant tails in serum and tumour tissues, the concentration of curcumin in tumour tissues is still in pg/ml [Citation58], and in vitro studies show that micromolar concentrations of curcumin are needed. Consequently, it can be concluded that many of the observed positive effects of curcumin occur in the gut.
3.2.2. Curcumin and amphipathic natural lipids, phospholipids, and liposomes
Natural amphipathic molecules are non-toxic and can be administered with minimum side effects [Citation57]. Lipid nanoparticles, bilayers, liposomes, and glycolipids have been used to increase the bioavailability of polyphenols. However, all those approaches showed a delivery capacity of only up to 60 ng/ml of curcumin in the human plasma. The amphipathic glycolipids with hydrophilic sophorolipid group and hydrophobic hydrocarbon chains increased curcumin serum concentration six times [Citation59] but this is still not satisfactory to reach concentrations required for a strong biological effect, as observed in the in vitro studies. Solid lipid particles were also used to increase curcumin serum concentrations but again, only up to 30 ng/ml [Citation60].
3.2.3. Curcumin and complex commercial compositions
Cureit, a complex composition with four different curcumin derivatives, is encapsulated in four different acidic (negatively charged) polysaccharides coated with turmerin turmeric antioxidant protein with some cationic groups. Some turmeric essential oils are also added to solubilize curcumin and enhance its bioavailability. While these compositions increased the bioavailability of curcumin to almost 100 ng/ml, it is still far away from the concentrations needed to reproduce in vitro results [Citation61]. Theracurmin drinks have a simple composition [Citation62]. A mixture of curcuminoids with essential turmeric sesquiterpenoid oils (less than 5%) provides the highest serum curcuminoid concentrations ever observed in human plasma. Up to 800 ng/l was accordingly, measured in human serum after the consumption of 4 times 500 mg of Biocurcumax [Citation62].
3.2.4. Curcumin and nanobubbles for enhanced bioavailability
Nanobubbles (NBs) are other colloidal systems that have been used to enhance curcuminoid bioavailability [Citation63]. Gas/liquid interfaces are ultrasound echogenic and have been FDA-approved for use in ultrasound diagnostics [Citation64]. NBs (1–1000 nm) are stable for months and have been used in over 20 applications from water treatment to diagnostic combined with theranostic approaches [Citation64]. In addition to pure ‘naked nanobubbles’ NBs can be coated with proteins, phospholipids, polymers or mixed layers of polymers and natural or synthetic surfactants. Such NBs are termed ‘armoured NBs’ and have significantly enhanced activity. Recently, it was reported that NBs with SF6 gas inside the hydrophobic core coated with dextran sulphate polysaccharide can enhance the bioavailability of curcumin. NBs loaded with curcuminoids were able to inhibit the growth of castration-resistant prostate cancer cells in vitro as well [Citation65]. A very complex PEGylated curcumin – PLGA – PVA NBs with natural micellar phospholipid surfactants were used to enhance the bioavailability of curcuminoids in the brain for Parkinson's disease treatment. For such applications, focused ultrasound was used to release encapsulated curcumin locally. The solubility of encapsulated curcumin in mice brains was accordingly, enhanced almost 3,000-fold [Citation66]. Further on, lipid-PLGA NBs are used as curcumin bioavailability enhancers as well mainly due to their softer nature in comparison with pure PEGylated nanoparticles [Citation67].
3.2.5. Curcumin nanoparticle and zinc oxide nanoparticle as therapeutic application
Many scientific studies prove the undeniable positive influence of natural antioxidants on the improvement of the health condition when suffering from Type 2 Diabetes Mellitus and Alzheimer's disease. The mentioned positive influence has been proven in many animal models that were carried out in different scientific centres. Scientists investigated the ability of a natural compound, a curcumin nanoparticle and a biomedical metal zinc oxide nanoparticle to alleviate hippocampal modifications in Type 2 Diabetes Mellitus-induced rats [Citation67]. Model rats that were treated with curcumin nanoparticle and zinc oxide nanoparticles, comparing them with other groups of diseased rats that were not treated, greatly improved their memory function and learning, improved redox and inflammation status, have lowered Bax, and upregulated Bcl2 expressions in the hippocampus. Both nanoparticles significantly improved histological lesions in the hippocampus. Scientific evidence points to the fact that curcumin nanoparticles and zinc oxide nanoparticles are potential neuroprotective agents to alleviate hippocampal toxicity associated with diabetic complications [Citation68,Citation69].
Diabetes mellitus is a growing worldwide problem today. It is a group of metabolic disorders characterized by loss of glucose homeostasis and insufficient production or action of insulin. According to many studies, the use of nanomaterials in the future is already giving certain positive results and they certainly represent the future in the treatment of such serious diseases. Today, nanomaterials are widely used in antidiabetic studies due to their unique properties. Studies using zinc nanoparticles have attracted special attention in modern scientific research. In particular, they pointed to the fact of the relationship between diabetes and the imbalance of zinc homeostasis. So far, very few studies have attempted to investigate the effect of zinc oxide nanoparticles on microRNA dysregulations in diseases such as diabetes. However, certain studies made a breakthrough and proved the therapeutic effect of zinc oxide nanoparticles on an animal model of diabetic rats induced by streptozotocin and also proved its role in microRNA dysregulation. Therapy based on zinc oxide nanoparticles for 15 days, at a dose of 5 mg/kg of body weight, resulted in significant improvements in blood insulin, glucose tolerance, and the structure and function of pancreatic β cells. Studies have also shown that zinc oxide nanoparticle therapy increases levels of enzymatic and non-enzymatic antioxidants in streptozotocin-induced diabetic rats. It was also found that zinc oxide nanoparticles specifically regulate the expression of pancreatic and liver microRNAs that participate in the development of diabetes. The results of such studies point that zinc oxide nanoparticles can certainly be used as a promising antidiabetic drug, and the antidiabetic effect of zinc oxide nanoparticles is certainly partially mediated by restoring the balance of oxidants/antioxidants and modulation at the microRNA level [Citation70–72].
4. EGCG polyphenol and its formulations for increased bioavailability
EGCG from green tea is a well-studied polyphenol that can exert both anti and pro–oxidant behaviour through Nrf2 and induction of phase II detox antioxidants enzymes. EGCG inhibits the JAK/STAT pathway with subsequent anti-proliferative, pro-apoptotic and anti-inflammatory effects in vitro and invivo [Citation73]. Also, the protein kinase Akt – mTOR-AKAP axis can be positively modified by EGCG towards enhanced apoptosis [Citation74–76].
4.1. Enhancing bioavailability of EGCG
Oral absorption of EGCG is very poor. Most of the ingested EGCG ends up in the large intestines where it is excreted with the feces. Absorption occurs through low-efficiency passive diffusion [Citation47] while excretion from cells occurs through efficient active mechanisms based on multidrug-resistance proteins MRP1 and MRP2. Small amounts of total EGCG can be adsorbed in the ileum part of the small intestines. Absorbed EGCG is then quickly metabolized in the gut, with subsequent liver methylation, glucuronidation and sulfation [Citation47]. Less than 1% of ingested EGCG enters the blood circulation. Colonic bacteria also metabolize EGCG. Efforts to increase the absorption of EGCG into gut cells have been unsuccessful so far. Molecular modifications increase the hydrophobicity of EGCG by partial methylation, acylation of 1-8 polyphenolic OH groups or glycosylation as reviewed in [Citation74]. Long-chain fatty acids acylation further on, can significantly increase membrane permeability and plasma concentration up to 3 times higher than EGCG itself.
4.1.1. EGCG and hydrophilic nanoparticles
As already explained for curcumin, cationic chitosan nanoparticles (NPs) and dual polyelectrolyte cationic–anionic NPs yield the best serum bioavailability enhancement [Citation47]. Among anionic partners used for this purpose, ferritin, zein, polyglutamic acid, polyphosphate, casein, and beta-lactoglobulin may be considered. Dual-layer nanoparticles are hydrophilic, small and can be either positively or negatively charged. In short, the best results observed were up to 3 times higher EGCG absorption rates than those of pure EGCG [Citation77–80]. Inorganic gold and silver nanoparticles have also been used as carriers to protect tea polyphenols and EGCG from degradation and to enhance their absorption [Citation81]. Ultrasound-enhanced incorporation of EGCG into gold nanoparticles yielded stable gold sols via electrostatic repulsions. Mesoporous silica and zeolite nanoparticles can also be used for EGCG protection and delivery [Citation82]. Nanohydroxyapatite/silica with EGCG has been used to prevent tooth decay, erosion and wear [Citation83]. Slow release of EGCG from mesoporous silica pores was tested to protect the exposed dentin in the patient’s mouth.
4.1.2. EGCG and amphipathic nano-lipids
Nano-lipids can have either hydrophobic or hydrophilic cores with hydrophilic lipid head shells. Surface groups can be anionic, cationic, zwitterionic or polar hydrophilic. Particle size can vary between 40 nm and 1,500 nm. Zeta potential can vary between −30 mV and +25 mV. Particles can be monodisperse or polydisperse. Such systems can improve EGCG solubility and encapsulation efficiency, stability, cell permeability, EGCG pharmacokinetics, synergistic delivery with other ingredients as well as bioavailability [Citation84]. Various techniques can be used for EGCG encapsulation inside nano-lipids such as mechanical cavitation, high energy mixing, ultrasound, radiofrequency and microwave thermal treatment, spray drying, etc. Liposomes have a hydrophilic shell and core, but hydrophobic tails are present in between the shell and core. Solid-lipid nanoparticles (SLN) use saturated fatty acid chains that are less flexible and semisolid. Nanostructure lipid carriers (NLC) are softer and more flexible. Many hybrid versions are also tested [Citation85].
4.1.3. EGCG and emulsions
Biological surfactants and emulsifiers such as polysorbate 80 are quite popular in the functional food arena. We strongly warrant against such an approach as food emulsifiers have been shown to negatively influence gut microbiome and gut leakage even with human gut studies [Citation86]. Another popular emulsifier Tween 80 is also identified in a negative context when it comes to the human gut microbiome [Citation87]. Some attempts have been made to emulsify polyphenols in soy oil/water emulsions using soy protein as an emulsifier [Citation88]. Ultrasound has been used to improve emulsion stability and EGCG encapsulation [Citation89]. Another similar approach is to use classical surfactant micelles. To avoid issues with the above-mentioned food surfactants, one can also apply proteins to lower the surface tension and create micelles. Micellar casein with some salts was accordingly, used to encapsulate EGCG very efficiently. Cationic chitosan can also be used to prepare cationic polymeric micelles. Finally, protamine cationic proteins can be used to simultaneously deliver siRNA and EGCG for tumour treatment [Citation90]. Cationic polymeric micelles combined with anionic polymers such as casein or lactoglobulin can be used as 100% natural nontoxic encapsulation reagents. Chitosan combination with small anionic molecules such as citric or ascorbic acid form nanogels that are also very efficient delivery systems [Citation91].
5. Chlorogenic acid and its formulations for increased bioavailability
Chlorogenic acid (5-O-caffeoylquinic acid) is both an anti and pro-oxidant. Chlorogenic acid, similarly to other phenolic compounds, remains in the gut after ingestion where it affects the immune system and microbiota. Still, it can be modified with nanotechnology to accumulate in the fat tissue. There it modifies signal transduction cascades as discussed below. This compound is present in many foods, most notably coffee beans, but only extract concentrates that are modified for enhanced bioavailability exert substantial in vivo effects. Chlorogenic acid has been used along with other nutraceuticals to fight obesity-related disorders such as oxidative stress, inflammation, glucose tolerance loss and additional weight gain even when exercising and eating healthy. Numerous in vivo studies including human clinical trials showed great potential of this molecule alone and particularly when combined with other polyphenols such as quercetin, resveratrol, etc. This was recently reviewed in great detail in ref [Citation92].
5.1. Enhancing the bioavailability of chlorogenic acid
Once again, cationic chitosan nanoparticles complexed with acidic chlorogenic acid or other negatively charged but larger molecules such as alginates deliver the best results. More complex compositions such as nanoparticles stabilized emulsions-Pickering emulsions have also been used. Essential oils such as cinnamaldehyde along with more negatively charged molecules such as tripolyphosphates have been used to enhance chitosan encapsulation of chlorogenic acid [Citation93–95]. Liposomes have also been successfully used to enhance the bioavailability of chlorogenic acid [Citation96]. We do not recommend solid – liquid nanoparticles, nano-emulsions and nano-micelles since recent research identified that most of those nonionic surfactants present in such formulations significantly interfere with the human gut microbiome [Citation97,Citation98]. In short, oppositely charged cationic–anionic natural polyelectrolytes such as cationic chitosan and various anionic polysaccharides or proteins seem to be the most efficient compositions with the least human side effects. Metal nanoparticles, nano-zeolites, solid–lipid nanoparticles, nano- and microemulsions and nano-micelles all show some toxicity and possible side effects.
6. Polyphenols from the Mediterranean diet and potential carriers for increased bioavailability
The Mediterranean diet is a dietary pattern that has, due to its health benefits, spread as a health-promoting trend worldwide. Mediterranean foods contain high amounts of antioxidants, significant amounts of fibres, vitamins and minerals, and monosaturated lipids which have a positive effect on human health. The primary benefits of the Mediterranean diet are health benefits related to the positive effects on the cardiovascular system, metabolic syndrome, cognition and cancer, which opens a broad range of possibilities in further biomedical research that connects medicine and the Mediterranean diet [Citation99]. Olive oil is considered one of the key healthy ingredients in the Mediterranean diet. Consumption of olive oil is associated with reduced risk of many non-communicable diseases. These health benefits are mainly correlated to health-promoting components of olive oil including polyphenols, squalene, tocopherols and pigments. The water insolubility and oxidation of olive oil hinder the wider application of olive oil in the food sector. Therefore, to improve storage stability and regulatory effects on gut microbiota, food-grade olive oil Pickering emulsions stabilized by starch/β-cyclodextrin complex nanoparticles were developed. The starch/β-cyclodextrin complex nanoparticle concentration and oil phase fraction had an important influence on the droplet size and storage stability of emulsions. Moreover, experiments done on animals suggested that food-grade olive oil Pickering emulsions can modulate the gut microbiota in mice by enhancing the richness and diversity, which ultimately results in high concentrations of health-promoting bacteria [Citation100]. This study has thus provided a new strategy for food-grade olive oil emulsions with amazing stability and positive health effects, which could potentially be applied as an animal fat substitute or as an example of a semi-finished product that needs to be further developed into other food products [Citation101]. Nanoparticles were also used to improve the bioavailability of compounds from olive oil with antioxidant properties. Squalene from olive oil has strong antioxidant activity, but due to its highly hydrophobic nature, and limited bioavailability. To enhance its delivery and improve its cellular uptake, squalene was encapsulated into PLGA nanoparticles. PLGA nanoparticles loaded with squalene protected mouse hepatocytes from oxidative and endoplasmic reticulum stresses by several molecular mechanisms [Citation102]. However, the authors noticed some limitations in the PLGA encapsulation efficiency of squalene. Another example is a study by Valentino et al. [Citation103], which provided new insights into the therapeutic effects of intra-articular injection of hydroxytyrosol-loaded chitosan nanoparticles embedded into thermosensitive hydrogels. Hydroxytyrosol, which has been released from hydrogel, could protect chondrocytes from ROS damage and inactivate inflammatory factors. Because of the positive results, this could potentially be an efficient delivery method for osteoarthritis treatment. Another naturally occurring polyphenol found in olive leaves that is known to be an antioxidant is oleuropein, which has unfortunately very high instability. Oleuropein has been incorporated in nanostructured lipid carriers to enhance its bioavailability and improve its antioxidant efficacy in lung epithelial cells. This research can encapsulate nanocarriers, which could ultimately lead to further design of formulations for pulmonary treatment [Citation104].
Another relevant research study is by Guo X. et al. [Citation105]. They have shown synergistic delivery of resveratrol and ultrasmall nanoparticles that are based on copper, by aptamer-functionalized ultrasound nanobubbles for the treatment of nonalcoholic fatty liver disease (NAFLD). NAFLD produces reactive oxygen species and oxidative stress in which antioxidative treatment could potentially help. These ultrasmall copper-based nanoparticles (CuNPs) are known for their many enzyme-like activities, such as scavenging oxygen species, thus providing a new strategy for the treatment of inflammatory diseases. One of the most known polyphenol compounds from red wine that has strong antioxidant properties is resveratrol and therefore, it is used in this specific research study. However, due to the poor water solubility and stability of resveratrol, a great need for stable and more effective delivery of this compound has emerged. Therefore, liver-targeted nanocarriers which are loaded with these bioactive compounds are considered to have a more effective approach. Guo X. et al. have ultimately found that the combination of these two components can effectively treat inflammation in NAFLD and opened a new door in its treatment options [Citation105].
An interesting inorganic material of natural provenience, namely the zeolite clinoptilolite, has also been increasingly studied for biomedical applications [Citation106–108] including applications as drug carriers [Citation109] (). While polyphenols from Mediterranean food show potent and exploitable pharmacological properties, their bioavailability remains questionable. Zeolite clinoptilolite may thus be regarded for such applications due to its incredible structural properties that bring additional benefits to health as well. Its structure consists of aluminium and silicon tetrahedra linked in a chemically stable crystal structure with pores and cavities filled with water and exchangeable cations. Accordingly, it acts as an adsorbent, ion exchanger and molecular sieve [Citation106]. Importantly, this is a non-toxic material that may be applied to animals and humans without harmful side effects. So far, several synthetic zeolites have been studied as drug carriers of polyphenols as they can be constructed in such a way that their shape corresponds to the molecule to be incorporated or adsorbed on the material. Some examples involving polyphenols include the curcumin-loaded zeolite Y nanoparticles/PCL-gelatin electrospun nanofibers [Citation110], the curcumin -oped zeolitic imidazolate framework nanoparticles by zinc ion-driven simultaneous coordination of curcumin and 2-methylimidazole [Citation111] and pH- and photothermal-responsive zeolitic imidazolate framework (ZIF-8) compound loaded with (-)-epigallocatechin-3-gallate and doxorubicin [Citation112]. Similarly, synthetic zeolites incorporating biophenols have been used as drug carriers in dentistry as well. For example, a synthetic preparation of silver-zeolite and a polyphenol-rich extract of the algae Ascophyllum nodosum has been applied and studied biofilm-related oral diseases with positive results [Citation113]. The data from all these studies show a clear loading capacity of zeolite materials for small molecules and their release from the material, including biophenols, but the issue of biocompatibility and potential toxic effects on the in vivo systems will remain a challenge. A zeolite clinoptilolite-based drug delivery system may thus, be regarded as a controlled release of polyphenols in the intestinal tract as a non-toxic alternative. So far, only a few studies have been conducted with clinoptilolite materials as carriers for polyphenols. For example, a polyphenol catechin-rich Acacia catechu extract by a clinoptilolite material Clinosorbent-5 (CLS-5) has been tested for drug delivery purposes. This zeolite clinoptilolite-based system showed catechin microencapsulation capacity in an acidic medium relevant for the human intestine as 32%. In addition, 88% maximum release efficiency was achieved after 24 h in the in vitro conditions mimicking the gastrointestinal tract [Citation114]. Good results were obtained as a proof of concept for oral delivery of small pharmaceutically active molecules in a study with a clinoptilolite-based carrier for pH-controlled oral delivery of aspirin. The results corroborate applications of clinoptilolite-based delivery systems as non-toxic naturally available microporous materials for oral delivery of small molecules [Citation115]. More studies in this field are accordingly needed where the properties of zeolite clinoptilolite’s unique inorganic structure will be deciphered for understanding and designing appropriate polyphenol molecules’ interactions with the material. Indeed, clinoptilolite comes in nature as a volcanic tuff that is subjected to diverse milling methods – micronization before biomedical applications. These give rise to materials with the increased active area and different physical–chemical properties, including changed affinity towards cations from the environment as shown for example in Kraljevic Pavelic et al. [Citation116]. Accordingly, each material should be first characterized for exact physical–chemical properties including pore size, surface structure and zeta potential to design a proper delivery/release system for biophenols. Recently, researchers started studies of nano-sized zeolite particles as these may be particularly appropriate for drug delivery and drug release. Accordingly, a nanocarrier has been recently produced by coating the natural clinoptilolite nanoparticles’ surface with a phosphatidylcholine layer [Citation108]. Phosphatidylcholine was tested previously, as an absorption enhancer with properties directed towards biological barrier permeation and improvement of drug diffusion [Citation117,Citation118].
7. Current issues and future considerations with polyphenols bioavailability
As already discussed in this review, polyphenols exert so many beneficial activities and may be regarded as systemic bioactive. Unfortunately, these extremely potent biological effects are only observed at high sub-micromolar to micromolar concentrations inside the affected tissues or tumours, which is not easily achievable from food or even classical medical formulations. Polyphenols are indeed, poorly absorbed into the gut cells and are often excreted from the body very fast. Modern nanotechnology approaches have been used to increase the bioavailability of polyphenols, such as curcumin, up to 30 times. While this is very impressive, we still have a long way to go to reach micromolar concentrations inside the tissues. Intravenous administration with ultrasound or magnetic-directed precise targeting may further help, particularly when it comes to cancer treatment. Some enhanced bioavailability nutraceutical compositions already exist on the market. Maybe berberine and chlorogenic acid applications along with healthy eating habits and exercise will soon be used in reducing the pro-inflammatory state of overweight and obese individuals. However, as animal studies and some clinical trials indicated, this only works if the patient is willing to change the lifestyle as well. Nanotechnology and polyphenols are not magic bullets that replace the consequences of someone's bad lifestyle. However, when combined with an appropriate low-inflammatory diet and exercise, such compositions may be a difference maker for hundreds of millions of those willing to change their lifestyle as a support to treat gut dysbiosis or metabolic syndrome. Complex compositions encompassing many ingredients may be the best choice. It seems that the nanotechnology approach that works best in such applications may be based on cationic polyelectrolytes such as chitosan combined with anionic polyelectrolytes such as alginate or bovine serum albumin or phosphatidylcholine: this does enhance bioavailability, stability and efficiency of many polyphenols. Still, careful toxicology evaluations are called in this area. If enhanced absorption of polyphenols inside the various gut cells is achieved, gut – pancreas, gut -liver, gut brain etc. axis can help enhance polyphenols' beneficial effects systemically. Our ability to work with 2D and 3D human gut cells systems and organoids will significantly enhance our ability to optimize polyphenol efficiency and study their biological effects when conjugated to nanocarriers. Finally, spatially resolved single-cell multi-omics approaches shall help identify mechanisms of polyphenol action in human biopsy tissues or organoids. In conclusion, a lot of research has still to be done before we can expect the first nanotechnology-enhanced polyphenols blockbuster nutraceutical or drug on the market.
Author contributions
Conceptualization, MČ and KP; writing – original draft preparation, MČ, SKP, ŽP, AA; AB; writing – review and editing, KP; visualization SKP. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data and references used for the preparation of the manuscript have been derived from public domain resources.
Additional information
Funding
References
- Jin J, Yang L, Chen F, et al. Drug delivery system based on nanobubbles. Interdiscip Mater. 2022;1:471–494. doi: 10.1002/idm2.12050
- Zullino S, Argenziano M, Stura I, et al. From micro- to nano-multifunctional theranostic platform: effective ultrasound imaging is not just a matter of scale. Mol Imaging. 2018;17:153601211877821. doi: 10.1177/1536012118778216
- Nitai AS, Mahmud R, Habib A, et al. A systematic review on nanobubbles in drug delivery: mechanism and applications. Biomed J Sci Tech Res. 2022;43(5):35041–35045.
- Combination of ultrasound and nanobubbles enables the removal of tumors without surgery. News-Medical.net. 2022. [cited 2023 June 6]. Available from: https://www.news-medical.net/news/20221121
- Liu S, Oshita S, Thuyet DQ, et al. Antioxidant activity of hydrogen nanobubbles in water with different reactive oxygen species both in vivo and in vitro. Langmuir. 2018;34:11878–11885. doi: 10.1021/acs.langmuir.8b02440
- Sayadi LR, Banyard DA, Ziegler ME, et al. Topical oxygen therapy & micro/nanobubbles: a new modality for tissue oxygen delivery. Int Wound J. 2018;15:363–374. doi: 10.1111/iwj.12873
- Kida H, Feril LB, Irie Y, et al. Influence of nanobubble size distribution on ultrasound-mediated plasmid DNA and messenger RNA gene delivery. Front Pharmacol. 2022;13:855495. doi: 10.3389/fphar.2022.855495
- Nittayacharn P, Yuan HX, Hernandez C, et al. Enhancing tumor drug distribution with ultrasound-triggered nanobubbles. J Pharm Sci. 2019;108(9):3091–3098. doi: 10.1016/j.xphs.2019.05.004
- Batchelor DVB, Armistead FJ, Ingram N, et al. The influence of nanobubble size and stability on ultrasound enhanced drug delivery. Langmuir. 2022;38(45):13943–13954. doi: 10.1021/acs.langmuir.2c02303
- Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Nat Acad Sci. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106
- Gomez-Cabrera MC, Domenech E, Romagnoli M, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142
- Rana A, Samtiya M, Dhewa T, et al. Health benefits of polyphenols: a concise review. J Food Biochem. 2022;46(10):e14264. doi: 10.1111/jfbc.14264
- Rajha HN, Paule A, Aragonès G, et al. Recent advances in research on polyphenols: effects on microbiota, metabolism, and health. Mol Nutr Food Res. 2022;66(1):e2100670. doi: 10.1002/mnfr.202100670
- Spencer JP, Ria-Evans C, William RJ. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J Bio Chem. 2003;278:34783–34793. doi: 10.1074/jbc.M305063200
- Pallauf K, Rimbach G. Autophagy, polyphenols and healthy ageing. Ageing Res Rev. 2013;12:237–252. doi: 10.1016/j.arr.2012.03.008
- Sharma A, Kaur M, Katnoria J, et al. Polyphenols in food: cancer prevention and apoptosis induction. Curr Med Chem. 2018;25:4740–4757. doi: 10.2174/0929867324666171006144208
- Munin A, Edwards-Levy F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics. 2011;3:793–829. doi: 10.3390/pharmaceutics3040793
- Fang Z, Bhandri B. Encapsulation of polyphenols – a review. Trends Food Sci Technol. 2010;21:510–523. doi: 10.1016/j.tifs.2010.08.003
- Duda-Chodak A, Tarko T, Satora P, et al. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr. 2015;54:325–341. doi: 10.1007/s00394-015-0852-y
- Parkar SG, Trower TM, Stevenson DE. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe. 2013;23:12–19. doi: 10.1016/j.anaerobe.2013.07.009
- Svegliati-Baron G, Patricio B, Liuci G, et al. Gut-pancreas-liver axis as a target for treatment of NAFLD/NASH. Int J Mol Sci. 2020;21(16):5820. doi: 10.3390/ijms21165820
- Albillos A, Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003
- Fung TC. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis. 2020;136:104714. doi: 10.1016/j.nbd.2019.104714
- Mayer EA, Nance K, Chen S. The gut-brain-axis. Annu Rev Med. 2022;73:439–453. doi: 10.1146/annurev-med-042320-014032
- Bliss ES, Whiteside E. The gut-brain axis, the human gut microbiota and their integration in the development of obesity. Front Physiol. 2018;9:900. doi: 10.3389/fphys.2018.00900
- Buhman H, Roux CW, Bueter MN. The gut-brain axis in obesity. Best Pract Res Clin Gastroenterol. 2014;28:559–571. doi: 10.1016/j.bpg.2014.07.003
- Lotstedt B, Strazar M, Xavier RJ, et al. Spatial host-microbiome sequencing. bioRxiv. 2022. doi:10.1038/s41587-023-01988-1
- Li VSW. Modelling intestinal inflammation and infection using ‘mini-gut’ organoids. Nat Rev Gastroenterol Hepatol. 2021;18:89–90. doi: 10.1038/s41575-020-00391-4
- Kraljević Pavelić S, Simović Medica J, Gumbarević D, et al. Critical review on zeolite clinoptilolite safety and medical applications in vivo. Front Pharmacol. 2018;9:1350. doi: 10.3389/fphar.2018.01350
- Gescher A. Polyphenolic phytochemicals versus non-steroidal anti-inflammatory drugs: which are better cancer chemopreventive agents? J Chemother. 2004;16:3–6. doi: 10.1179/joc.2004.16.Supplement-1.3
- Derakhshankhah H, Jafari S, Sarvari S, et al. Biomedical application of zeolitic nanoparticles with an emphasis on medical interventions. Int J Nanomed. 2020;15:363–386. doi: 10.2147/IJN.S234573
- Sankarasubramanian J, Ahmad R, Avuthu N, et al. Gut microbiota and metabolic specificity in ulcerative colitis and Crohn's disease. Front Med. 2020;7:606298. doi: 10.3389/fmed.2020.606298
- Lu H, Xiang L, Cui X, et al. Molecular weight dependence of synthetic glycopolymers on flocculation and dewatering of fine particles. Langmuir. 2016;32:11615–11622. doi: 10.1021/acs.langmuir.6b03072
- Nacini T, Khaleel Basha S, Sadiq AMM, et al. Development and characterization of alginate / chitosan nanoparticulate system for hydrophobic drug encapsulation. J Drug Deliv Sci Technol. 2019;52:65–72. doi: 10.1016/j.jddst.2019.04.002
- Jakobek L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015;175:556–567. doi: 10.1016/j.foodchem.2014.12.013
- Kanner J. Polyphenols by generating H2O2, affect cell redox signaling, inhibit PTPs and activate Nrf2 axis for adaptation and cell surviving: in vitro, in vivo and human health. Antioxidants. 2020;9(9):797. doi: 10.3390/antiox9090797
- Bunesova V, Lacroix C, Schwab C. Mucin cross-feeding of infant bifidobacteria and eubacterium hallii. Microb Ecol. 2018;75:228–238. doi: 10.1007/s00248-017-1037-4
- Greiner TU, Backhed F. Microbial regulation of GLP-1 and L-cell biology. Mol Metab. 2016;5:753–758. doi: 10.1016/j.molmet.2016.05.012
- Gerard C, Vidal H. Impact of gut microbiota on host glycemic control. Front Endocrinol. 2019;10:29. doi: 10.3389/fendo.2019.00029
- Claus SP. Will gut microbiota help design the next generation of GLP-1-based therapies for type 2 diabetes? Cell Metab. 2017;26:6–7. doi: 10.1016/j.cmet.2017.06.009
- Jackson DN, Theiss A. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes. 2020;11:285–304. doi: 10.1080/19490976.2019.1592421
- Wojciechowska O, Costabile A, Kujawska M. The gut microbiome meets nanomaterials: exposure and interplay with graphene nanoparticles. Nanoscale Adv. 2023;5(23):6349–6364. doi: 10.1039/D3NA00696D
- Cui X, Wang X, Chang X, et al. A new capacity of gut microbiota: fermentation of engineered inorganic carbon nanomaterials into endogenous organic metabolites. Proc Natl Acad Sci U S A. 2023;120(20):e2218739120. doi: 10.1073/pnas.2218739120
- Ahmed S, Bhattacharya T, Annu A, editors. Handbook of nanotechnology in nutraceuticals. CRC Press; Taylor &Francis; 2023.
- Cas MD, Ghidoni R. Dietary curcumin: correlation between bioavailability and health potential. Nutrients. 2019;11:2147. doi: 10.3390/nu11092147
- Huminiecki L. Evidence for multilevel chemopreventive activities of natural phenols from functional genomic studies of curcumin, resveratrol, genistein, quercetin, and luteolin. Int J Mol Sci. 2022;23:14957. doi: 10.3390/ijms232314957
- Liang J, Yan H, Wang X, et al. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017;231:19–24. doi: 10.1016/j.foodchem.2017.02.106
- Zhang X, Wang C, Qi Z, et al. Pea protein based nanocarriers for lipophilic polyphenols: spectroscopic analysis, characterization, chemical stability, antioxidant and molecular docking. Food Res Int. 2022;160:111713. doi: 10.1016/j.foodres.2022.111713
- Dong R, Huang Z, Ma W, et al. Fabrication of nanocomplexes for anthocyanins delivery by ovalbumin and differently dense sulphate half-ester polysaccharides nanocarriers: enhanced stability, bio-accessibility, and antioxidant properties. Food Chem. 2024;432:137263. doi: 10.1016/j.foodchem.2023.137263
- Maqsoudlou A, Assadpour E, Mohebodini H, et al. Improving the efficiency of natural antioxidant compounds via different nanocarriers. Adv Colloid Interface Sci. 2020;278:102122. doi: 10.1016/j.cis.2020.102122
- Viana-Mendieta P, Sanchez ML, Benavides J. Rational selection of bioactive principles for wound healing applications: growth factors and antioxidants. Int Wound J. 2022;19:100–113. doi: 10.1111/iwj.13602
- Chen AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:e2900.
- Shobal G, Joseph T, Majeed M, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Panta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450
- Wang R, Sun W, Liu J, et al. Preparation of curcumin nanocrystalline injection and evaluation of its in vivo and in vitro properties. J China Pharm Univ. 2022;6:54–59.
- Valentini G, Parize AL. Investigation of the interaction between curcumin and hydroxypropyl methylcellulose acetate succinate in solid and solution media. J Mol Liq. 2023;376:121446. doi: 10.1016/j.molliq.2023.121446
- Morimoto T, Sunagawa Y, Katanasaka Y, et al. Drinkable preparation of theracurmin exhibits high absorption efficiency—a single-dose, double-blind, 4-way crossover study. Biol Pharm Bull. 2013;36:1708–1714. doi: 10.1248/bpb.b13-00150
- Wang F, Yang Y, Ju X, et al. Polyelectrolyte complex nanoparticles from chitosan and acylated rape seed cruciferrin protein for curcumin delivery. J Agric Food Chem. 2018;66:2689–2693.
- Schibbor C, Kocher A, Benham D, et al. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol Nutr Food Res. 2014;58:516–527. doi: 10.1002/mnfr.201300724
- Peng S, Li Z, Zou L, et al. Enhancement of curcumin bioavailability by encapsulation in sophorolipid-coated nanoparticles: an in vitro and in vivo study. J Agric Food Chem. 2018;66:1488–1497. doi: 10.1021/acs.jafc.7b05478
- Gota VS, Maru GB, Soni TG, et al. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem. 2010;58:2095–2099. doi: 10.1021/jf9024807
- Jude S, Amaraj A, Kunnumakkara AB, et al. Development of validated methods and quantification of curcuminoids and curcumin metabolites and their pharmacokinetic study of oral administration of complete natural turmeric formulation (Cureit™) in human plasma via UPLC/ESI-Q-TOF-MS spectrometry. Molecules. 2018;23:2415. doi: 10.3390/molecules23102415
- Antony B, Merina B, Lyer V, et al. A pilot cross-over study to evaluate human oral bioavailability of BCM-95 CG (BiocurcumaxTM): a novel bioenhanced preparation of curcumin. Indian J Pharm Sci. 2008;70(4):445–449. doi: 10.4103/0250-474X.44591
- Pasupathy R, Pandian P, Selvamuthukumar S. Nanobubbles: a novel targeted drug delivery system. Braz J Pharm Sci. 2022;58:e19608. doi: 10.1590/s2175-97902022e19604
- Lu S, Zhao P, Deng Y, et al. Mechanistic insights and therapeutic delivery through micro/nanobubble-assisted ultrasound. Pharmaceutics. 2022;14(3):480. doi: 10.3390/pharmaceutics14030480
- Wu M, Zhao H, Guo L, et al. Ultrasound-mediated nanobubble destruction (UMND) facilitates the delivery of A10-3.2 aptamer targeted and siRNA-loaded cationic nanobubbles for therapy of prostate cancer. Drug Deliv. 2018;25:226–240. doi: 10.1080/10717544.2017.1422300
- Yan Y, Chen Y, Liu Z, et al. Brain delivery of curcumin through low-intensity ultrasound-induced blood–brain barrier opening via lipid-PLGA nanobubbles. Int J Nanomed. 2021;16:7433–7447. doi: 10.2147/IJN.S327737
- Abdulmalek S, Nasef M, Awad D, et al. Protective effect of natural antioxidant, curcumin nanoparticles, and zinc oxide nanoparticles against type 2 diabetes-promoted hippocampal neurotoxicity in rats. Pharmaceutics. 2021;13(11):1937. doi: 10.3390/pharmaceutics13111937
- Zha W, Bai Y, Xu L, et al. Curcumin attenuates testicular injury in rats with streptozotocin-induced diabetes. Biomed Res Int. 2018;2018:7468019.
- Shengnuo F, Yuqiu Z, Xuan L, et al. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer's disease. Drug Deliv. 2018;25(1):1091–1102. doi: 10.1080/10717544.2018.1461955
- Othman MS, Hafez MM, Abdel AE. The potential role of zinc oxide nanoparticles in MicroRNAs dysregulation in STZ-induced type 2 diabetes in rats. Biol Trace Elem Res. 2020;197(2):606–618. doi: 10.1007/s12011-019-02012-x
- Swati CA, Rinku DU, Kishore MP. In vitro studies on the pleotropic antidiabetic effects of zinc oxide nanoparticles. Nanomedicine (Lond). 2016;11(13):1671–1687. doi: 10.2217/nnm-2016-0119
- Farooqi AA, Pinheiro M, Granja A, et al. EGCG mediated targeting of deregulated signaling pathways and non-coding RNAs in different cancers: focus on JAK/STAT, Wnt/β-catenin, TGF/SMAD, NOTCH, SHH/GLI, and TRAIL mediated signaling pathways. Cancers (Basel). 2020;12:951. doi: 10.3390/cancers12040951
- Holczer M, Besze B, Zambo V, et al. Epigallocatechin-3-gallate (EGCG) promotes autophagy dependent survival via influencing the balance of mTOR -AMPK pathways upon endoplasmic reticulum stress. Oxid Med Cell Longevity. 2018;2018. Article ID 6721530.
- Van Aller GS, Carson JD, Tang W, et al. Epigallocatechin gallate (EGCG), a major component of green tea, is a dual phosphoinositide-3-kinase/mTOR inhibitor. Biochem Biophys Res Commun. 2011;406(2):194–199. doi: 10.1016/j.bbrc.2011.02.010
- Huang CH, Tsai SJ, Wang YJ, et al. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK in p53 positive and negative human hepatoma cells. Mol Nutr Food Res. 2009;53:1156–1165. doi: 10.1002/mnfr.200800592
- Teng H, Chen L. Polyphenols and bioavailability: an update. Crit Rev Food Sci Nutr. 2019;59:2040–2051. doi: 10.1080/10408398.2018.1437023
- Liang J, Yan H, Yang HJ, et al. Synthesis and controlled – release properties of chitosan/ β-lactoglobulin nanoparticles as carriers for oral administration of epigallocatchin gallate. Food Sci Biotechnol. 2016;25:1585–1590.
- Yang R, Tian J, Liu Y, et al. One-step fabrication of phytoferritin-chitosan-epigallocatechin shell-core nanoparticles by thermal treatment. Food Hydrocolloids. 2018;80:24–32. doi: 10.1016/j.foodhyd.2018.01.014
- Hsieh DS, Lu HC, Chen CC, et al. The preparation and characterization of gold-conjugated polyphenol nanoparticles as a novel delivery system. Int J Nanomed. 2012;7:1623–1633.
- Hsieh DS, Wang H, Tan S-W, et al. The treatment of bladder cancer in a mouse model by epigallocatechin-3-gallate-gold nanoparticles. Biomaterials. 2011;32:7633–7640. doi: 10.1016/j.biomaterials.2011.06.073
- Haghi A, Raussi H, Hashemzadeh H, et al. Designing a high-performance smart drug delivery system for the synergetic co-absorption of DOX and EGCG on ZIF-8. RSC Adv. 2020;10:44533–44544. doi: 10.1039/D0RA08123J
- Yu J, Yang H, Li K, et al. Development of epigallocatechin-3-gallate-encapsulated nanohydroxyapatite/mesoporous silica for therapeutic management of dentin surface. ACS Appl Mater Interfaces. 2017;9:25796–25807. doi: 10.1021/acsami.7b06597
- Manea AM, Vasile BS, Meghea A. Antioxidant and antimicrobial activities of green tea extract loaded into nanostructured lipid carriers. C R Chim. 2014;17:331–341. doi: 10.1016/j.crci.2013.07.015
- Frias I, Neves AR, Pinheiro M, et al. Design, development and characterization of lipid nanocarriers-based epigallocatechin-gallate delivery system for preventive and therapeutic supplementation. Drug Des Devel Ther. 2016;10:3519–3528. doi: 10.2147/DDDT.S109589
- Bancil AS, Sandall AM, Ross M, et al. Food additive emulsifiers and their impact on gut microbiome, permeability and inflammation: mechanistic insights in inflammatory bowel disease. J Crohn's Colitis. 2021;15(6):1068–1079. doi: 10.1093/ecco-jcc/jjaa254
- Nielsen CK, Kjems J, Mygind T, et al. Effects of TWEEN 80 on growth and biofilm formation in laboratory media. Front Microbiol. 2016;7:1878. doi: 10.3389/fmicb.2016.01878
- Bhushani JA, Karthik P, Anandharamakrishnan C. Nanoemulsion based delivery system for improved bioaccessibility and Caco-2 cell monolayer permeability of green tea catechins. Food Hydrocolloids. 2016;56:372–382. doi: 10.1016/j.foodhyd.2015.12.035
- Tsai YJ, Chen BH. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effects on prostate cancer cells PC-3. Int J Nanomedicine. 2016;11:1907–1926.
- Ding J, Liang T, Min Q, et al. “Stealth and fully-laden” drug carriers: self-assembled nanogels encapsulated with epigallocatechin gallate and sirna for drug-resistant breast cancer therapy. ACS Appl Mater Interfaces. 2018;10:9938–9948. doi: 10.1021/acsami.7b19577
- Piran F, Khoshkov Z, Hosseini SE, et al. Controlling the antioxidant activity of green tea extract through encapsulation in chitosan-citrate nanogel. J Food Qual. 2020;10(12). Article ID 7935420.
- Wang H, Zhang H, Gao Z, et al. The mechanism of berberine alleviating metabolic disorder based on gut microbiome. Front Cell Infect Microbiol. 2022;12:854885. doi: 10.3389/fcimb.2022.854885
- Cho AS, Jeon SM, Kim MJ, et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol. 2010;48:937–943. doi: 10.1016/j.fct.2010.01.003
- Niu B, Chen H, Wu W, et al. Co-encapsulation of chlorogenic acid and cinnamalaldehyde essential oil in pickering emulsion stabilized by chitosan nanoparticles. Food Chem. 2022;14:100312.
- DiSanto MC, D'Antoni CC, Dominguez-Rubio AP, et al. Chitosan-tripolyphosphate nanoparticles designed to encapsulate polyphenolic compounds for biomedical and pharmaceutical applications − a review. Biomed Pharmacother. 2021;142:111970. doi: 10.1016/j.biopha.2021.111970
- Goncalves B, Moeenfard M, Rocha F, et al. Microencapsulation of a natural antioxidant from coffee—chlorogenic acid (3-caffeoylquinic acid). Food Bioprocess Technol. 2017;10:1521–1530. doi: 10.1007/s11947-017-1919-y
- Feng Y, Sun C, Yuan Y, et al. Enhanced oral bioavailability and in vivo antioxidant activity of chlorogenic acid via liposomal formulation. Int J Pharm. 2016;501:342–349. doi: 10.1016/j.ijpharm.2016.01.081
- Csaki KF. Synthetic surfactant food additives can cause intestinal barrier dysfunction. Med Hypotheses. 2011;76:676–681. doi: 10.1016/j.mehy.2011.01.030
- Halmos EP, Mack A, Gibson PK. Review article: emulsifiers in the food supply and implications for gastrointestinal disease. Aliment Pharmacol Ther. 2019;49:41–50. doi: 10.1111/apt.15045
- Sikalidis AK, Kelleher AH, Kristo AS. Mediterranean diet. Encyclopedia. 2021;1:371–387. doi: 10.3390/encyclopedia1020031
- Li Q, Huang Y, Du Y, et al. Food-grade olive oil pickering emulsions stabilized by starch/β-cyclodextrin complex nanoparticles: improved storage stability and regulatory effects on gut microbiota. LWT. 2022;155:112950. doi: 10.1016/j.lwt.2021.112950
- Nieto G, Lorenzo JM. Use of olive oil as fat replacer in meat emulsions. Curr Opin Food Sci. 2021;40:179–186. doi: 10.1016/j.cofs.2021.04.007
- Bidooki SH, Alejo T, Sánchez-Marco J, et al. Squalene loaded nanoparticles effectively protect hepatic AML12 cell lines against oxidative and endoplasmic reticulum stress in a TXNDC5-dependent way. Antioxidants. 2022;11:581. doi: 10.3390/antiox11030581
- Valentino A, Conte R, De Luca I, et al. Thermo-responsive gel containing hydroxytyrosol-chitosan nanoparticles (Hyt@tgel) counteracts the increase of osteoarthritis biomarkers in human chondrocytes. Antioxidants. 2022;11:1210. doi: 10.3390/antiox11061210
- Huguet-Casquero A, Moreno-Sastre M, López-Méndez TB, et al. Encapsulation of oleuropein in nanostructured lipid carriers: biocompatibility and antioxidant efficacy in lung epithelial cells. Pharmaceutics. 2020;12:429. doi: 10.3390/pharmaceutics12050429
- Guo X, Huang Z, Chen J, et al. Synergistic delivery of resveratrol and ultrasmall copper-based nanoparticles by aptamer-functionalized ultrasound nanobubbles for the treatment of nonalcoholic fatty liver disease. Front Physiol. 2022;13:2022.950141.
- Mastinu A, Kumar A, Maccarinelli G, et al. Zeolite clinoptilolite: therapeutic virtues of an ancient mineral. Molecules. 2019;24:1517. doi: 10.3390/molecules24081517
- Kraljević Pavelić S, Micek V, Bobinac D, et al. Treatment of osteoporosis with a modified zeolite shows beneficial effects in an osteoporotic rat model and a human clinical trial. Exp Biol Med (Maywood). 2021;246:529–537. doi: 10.1177/1535370220968752
- Kraljević Pavelić S, Krpan D, Žuvić M, et al. Clinical parameters in osteoporosis patients supplemented with PMA-zeolite at the end of 5-year double-blinded clinical trial. Front Med. 2022;9:870962. doi: 10.3389/fmed.2022.870962
- Hao J, Stavljenić Milašin I, Batu Eken Z, et al. Effects of zeolite as a drug delivery system on cancer therapy: a systematic review. Molecules. 2021;26(20):6196. doi: 10.3390/molecules26206196
- Jiang B, Yang Z, Shi H, et al. Potentiation of curcumin-loaded zeolite Y nanoparticles/PCL-gelatin electrospun nanofibers for postsurgical glioblastoma treatment. J Drug Deliv Sci Technol. 2023;80:104105. doi: 10.1016/j.jddst.2022.104105
- Liu J, Liu S, Wu Y, et al. Curcumin doped zeolitic imidazolate framework nanoplatforms as multifunctional nanocarriers for tumor chemo/immunotherapy. Biomater Sci. 2022;10:2384–2393. doi: 10.1039/D2BM00149G
- Chen X, Tong R, Liu B, et al. Duo of (-)-epigallocatechin-3-gallate and doxorubicin loaded by polydopamine coating ZIF-8 in the regulation of autophagy for chemo-photothermal synergistic therapy. Biomater Sci. 2020;8:1380–1393. doi: 10.1039/C9BM01614G
- Tamanai-Shacoori Z, Chandad F, Rébillard A, et al. Silver-zeolite combined to polyphenol-rich extracts of Ascophyllum nodosum: potential active role in prevention of periodontal diseases. PLoS One. 2014;9:e105475. doi: 10.1371/journal.pone.0105475
- Yaneva Z, Ivanova D, Popov N. Clinoptilolite microparticles as carriers of catechin-rich acacia catechu extracts: microencapsulation and in vitro release study. Molecules. 2021;26:1655. doi: 10.3390/molecules26061655
- Tondar M, Parsa MJ, Yousefpour Y, et al. Feasibility of clinoptilolite application as a microporous carrier for pH-controlled oral delivery of aspirin. Acta Chim Slov. 2014;61:688–693.
- Kraljević Pavelić S, Micek V, Filošević A, et al. Novel, oxygenated clinoptilolite material efficiently removes aluminium from aluminium chloride-intoxicated rats in vivo. Microp Mesop Mater. 2017;249:146–156. doi: 10.1016/j.micromeso.2017.04.062
- Pogorelov AG, Panait AI, Gulin AA, et al. Natural clinoptilolite nanoparticles coated with phosphatidylcholine. Dokl Biochem Biophys. 2022;505:156–159. doi: 10.1134/S160767292204007X
- Kim C, Shim J, Han S, et al. The skin-permeation-enhancing effect of phosphatidylcholine: caffeine as a model active ingredient. J Cosmet Sci. 2002;53(6):363–374.