ABSTRACT
Cisplatin is widely employed in clinical oncology as an anticancer chemotherapy drug in clinical practice and is known for its severe ototoxic side effects. Prior research indicates that the accumulation of reactive oxygen species (ROS) plays a pivotal role in cisplatin's inner ear toxicity. Hesperidin is a flavanone glycoside extracted from citrus fruits that has anti-inflammatory and antioxidant effects. Nonetheless, the specific pharmacological actions of hesperidin in alleviating cisplatin-induced ototoxicity remain elusive. The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is a critical mediator of the cellular oxidative stress response, is influenced by hesperidin. Activation of Nrf2 was shown to have a protective effect against cisplatin-induced ototoxicity. The potential of hesperidin to stimulate Nrf2 in attenuating cisplatin's adverse effects on the inner ear warrants further investigation. This study employs both in vivo and in vitro models of cisplatin ototoxicity to explore this possibility. Our results reveal that hesperidin mitigates cisplatin-induced ototoxicity by activating the Nrf2/NQO1 pathway in sensory hair cells, thereby reducing ROS accumulation, preventing hair cell apoptosis, and alleviating hearing loss.
1. Introduction
Cisplatin is one of the most commonly used anticancer drugs in current clinical practice, and it is widely applied in the treatment of various solid tumors, such as bladder cancer, ovarian cancer, head and neck squamous cell carcinoma, and non-small cell lung cancer [Citation1]. The ototoxicity of cisplatin primarily manifests as tinnitus and hearing loss, with hearing loss being bilateral, irreversible, and sensorineural in nature [Citation2,Citation3]. Approximately 40–80% of tumor patients receiving cisplatin treatment experience permanent hearing loss, which severely impacts the quality of life of cancer survivors [Citation4]. Cisplatin ototoxicity also significantly affects the speech development and social functioning of children [Citation5,Citation6]. However, there are currently no effective drugs available in clinical practice to prevent or ameliorate cisplatin-induced ototoxicity. Therefore, drugs that effectively inhibit cisplatin ototoxicity need to be developed.
Reactive oxygen species (ROS) accumulation in sensory hair cells is one of the major mechanisms involved in cisplatin-induced ototoxicity [Citation7,Citation8]. Excessive ROS can directly or indirectly damage proteins, lipids, and nucleic acids within cells, activating caspase 3 in cells and leading to apoptosis [Citation9]. Antioxidants such as sodium thiosulfate (STS), glutathione (GSH), and N-acetylcysteine (NAC) have been shown to have therapeutic effects on alleviating cisplatin-induced oxidative stress and hair cell apoptosis by clearing ROS [Citation10].
Nrf2 is a key factor in the cellular oxidative stress response and is regulated by Keap1. It interacts with antioxidant response elements (AREs) to regulate the expression of antioxidant proteins [Citation11–13]. Under normal conditions, Nrf2 is located in the cytoplasm, and its stability is maintained by the Keap1 protein. When excessive production of oxygen free radicals occurs, the Keap1 protein releases Nrf2 through the release of zinc ions, site mutations and fragmentation of Nrf2. Nrf2 is then translocated into the nucleus, where it binds to the ARE sequence and activates downstream gene transcription in the ARE region. Such activation initiates the antioxidant stress response, which in turn reduces the damaging effect of oxidative stress.
Hesperidin is a natural compound belonging to a class of compounds called flavonoids and is primarily found in the peels of citrus fruits. Hesperidin is an effective natural antioxidant that can help neutralize free radicals and reduce oxidative stress-induced damage to cells. Research has shown that pretreatment with hesperidin can alleviate cisplatin-induced kidney damage and improve kidney function. Multiple studies have demonstrated that hesperidin can inhibit the toxicity of cisplatin on major organs, such as the heart, liver, and kidneys, and have suggested that hesperidin can upregulate Nrf2 [Citation14–16]. Kara et al. reported that hesperidin alleviated the ototoxicity of cisplatin in a rat model [Citation17]. However, the mechanism underlying the alleviation of cisplatin-induced ototoxicity by hesperidin remains elusive. Therefore, the purpose of this study was to investigate the protective effects of hesperidin on cisplatin-induced ototoxicity and its underlying mechanisms. Our findings may provide evidence for new therapies for cisplatin ototoxicity.
2. Materials and methods
2.1. Animals and drug administration
Healthy adult male C57BL/6J mice (8 weeks old) were obtained from the Experimental Animal Center of Sun Yat-sen University (Guangzhou, China). Fifteen male mice were sequentially assigned numbers and then randomly divided into three groups using a random number generator (the control group, cisplatin group, and hesperidin + cisplatin group). Cisplatin-induced ototoxicity was studied by injecting 10 μl of cisplatin (1 mg/ml) into the middle ear through the tympanic membrane, while the control group received 10 μl of saline. Such a cisplatin ototoxicity model has previously been reported [Citation18]. Two hours prior to transtympanic membrane injection, an intraperitoneal injection of 100 mg/kg hesperidin was administered to the hesperidin + cisplatin group, while the cisplatin group and control group received an equivalent volume of saline containing 1% DMSO (vehicle control). All animal maintenance and experiments were conducted according to the recommendations of the Institutional Animal Care and Use Committee (IACUC) at Sun Yat-sen University (SYSU-IACUC-2023-000892).
2.2. Cochlear explants
Cochleae were dissected from P3 C57BL/6j mice and cultured on glass coverslips coated with Cell-Tak (BD Biosciences, 354240, USA) supplemented with 1 mL of DMEM/F12 growth medium (Gibco Life Technologies, 11330–032, USA), B-27 supplement (Gibco Life Technologies, 17504–44, USA), N-2 supplement (Gibco Life Technologies, 17502–048, USA), and ampicillin (50 g/ml, A5354-10ML, Sangon Biotech, China) at 37 °C in a 5% CO2/95% air atmosphere. After being cultured overnight, the cochlear explants were pretreated with hesperidin (20 µM) for 2 h and then cotreated with 40 µM cisplatin for 24 h. The samples were subsequently washed with PBS and cultured in cisplatin-free medium for another 12 h.
2.3. Cell culture
HEI-OC1 cells are a mouse inner ear hair cell-derived cell line that has been isolated from a vestibular schwannoma. The HEI-OC1 cell line was derived by Dr. F. Kalinec at UCLA Health. HEI-OC1 cells were cultured in DMEM supplemented with 10% FBS (Gibco, 10099141C, USA) and ampicillin (Sangon Biotech, Shanghai) at 33 °C with 10% CO2 [Citation19]. The cells were subcultured using 0.25% trypsin/EDTA (Gibco, USA) when they reached 80% confluency. Cisplatin (MCE, HY-17394, USA) was added to the culture medium at a final concentration of 40 µM and incubated for 24 h to disrupt the HEI-OC1 cells, as previously reported [Citation20].
2.4. Measurement of auditory brainstem response (ABR)
All mice were anesthetized using a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). Auditory brainstem response was measured using Tucker-Davis Technologies (TDT System III, Alachua, FL, USA) before and after the 14-day treatment course. Subdermal needle electrodes were inserted at the vertex (active), under the left ear (reference), and under the right ear (ground). The auditory stimuli were recorded in response to ten millisecond (ms) tone bursts with a 1 ms rise/fall time at frequencies of 8, 16, and 32 kHz. The average response to 1000 stimuli was obtained by reducing the sound intensity at 10 dB intervals from 100 dB SPL, and the ABR threshold was defined as the lowest stimulation decibel level that produced a replicable waveform response [Citation21,Citation22].
2.5. Immunofluorescence analysis
Cochlear explants were fixed with 4% paraformaldehyde (Biosharp, BL539A, China) for 30 min at room temperature and washed with 0.01 M phosphate-buffered saline (PBS) 3 times. After being permeabilized with 1% Triton X-100 (Solarbio Life Sciences, T8200, China) for 30 min and blocked with 10% bovine serum albumin (BSA) (Biofroxx, 4240GR100, Germany) for 30 min at room temperature, the samples were then incubated with rabbit polyclonal anti-myosin VIIa (Proteus Biosciences, 25-6790, China) for 12 h (4 °C) at a dilution of 1:500. Afterward, the samples were washed 3 times with PBS and incubated with secondary antibodies (Cell Signaling Technology, 8889, USA) at a dilution of 1:200 for 2 h at room temperature. Finally, the cochlear explants were washed 3 times with PBS, mounted with DAPI (Abcam, ab285390, USA), and imaged using an LSM 710 confocal microscope (Zeiss, Oberkochen, Germany). A detailed video protocol can be found in our previously published article [Citation23].
2.6. TUNEL assay
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) (Elabscience, E-CK-A320, China) staining was conducted to measure cell apoptosis according to the manufacturer’s instructions. Briefly, cochlear explants were fixed with 4% PFA for 30 min, permeabilized with 1% Triton X-100 for 30 min and subsequently incubated with TUNEL working solution for 2 h at 37 °C. DAPI and myosin VIIa were used to label the nucleus and hair cells, respectively. Then, the samples were observed with an LSM 710 confocal microscope (Zeiss, Oberkochen, Germany).
2.7. Flow cytometry
An Annexin V-FITC/PI Apoptosis Kit (Elabscience, E-CK-A211, China) was used to analyze cell apoptosis. HEI-OC1 cells in different plates were trypsinized, collected by centrifugation at 3000 rpm for 5 min and then washed twice with PBS. The cells were resuspended in 1 × binding buffer at a density of 1 × 106 cells/ml, and then 5 µl of PI and 5 µl of Annexin V-FITC were added to the samples. After being incubated for 20 min at room temperature in the dark, the cells were analyzed by flow cytometry as soon as possible, and all experiments were repeated at least 3 times.
A ROS detection kit (Beyotime, S0033S, China) was used to measure the ROS levels according to the manufacturer's instructions. HEI-OC1 cells were incubated with culture medium containing DCFH-DA at a dilution of 1:1000 at 37 °C in the dark for 20 min, followed by two washes with serum-free culture medium. HEI-OC1 cells in different plates were digested with trypsin and collected by centrifugation at 3000 rpm for 5 min. Finally, the HEI-OC1 cells were washed twice with PBS and analyzed by flow cytometry (BD FACSVerse, USA).
2.8. CCK-8 assay
The cells were trypsinized with 0.25% trypsin/EDTA, collected by centrifugation at 1000 rpm for 5 min, resuspended in culture medium, and plated in 96-well plates at a density of 5,000 cells/well with 3 replicates. After 24 h of incubation, the culture medium was replaced with medium containing different concentrations of cisplatin. After different incubation times (1 h to 2 h) with a CCK-8 Kit (APEXBIO, K1018, USA), the absorbance was determined at 490 nm using a microplate reader.
2.9. Western blotting
Cochlear explants were extracted and lysed in RIPA buffer (Sigma-Aldrich, R0278, Germany) containing a protease inhibitor cocktail (Roche, 11836170001, USA) at 4 °C for 30 min. Next, the protein concentration was measured using a BCA protein quantification kit (CWBIO, CW0014S, China), and the samples were then diluted to equal concentrations and volumes. Equal amounts of protein were loaded and separated on 10% or 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gels and subsequently transferred to PVDF membranes (Merck Millipore, USA), after which they were blocked with 5% nonfat dry milk in TBST (Sangon Biotech, Shanghai) for 1 h at room temperature. Immunoblotting was performed with rabbit monoclonal anti-Nrf2 (1:1000, Proteintech, 80593-1-RR, China), rabbit monoclonal anti-NQO1 (1:1000, Abcam, ab80588, USA), rabbit monoclonal anti-cleaved caspase-3 (1:1000, Cell Signaling Technology, 9661, USA), rabbit monoclonal anti-cleaved PARP (1:1000, Cell Signaling Technology, 9661, USA) and rabbit monoclonal anti-β-actin (1:5000, Affinity, AF7018, USA) antibodies. After being incubated with primary antibodies at 4 °C overnight, the membranes were washed with TBST three times and then incubated with the corresponding secondary antibodies (1:5000, ABclonal, AS014, China) for 2 h at room temperature. Finally, the membranes were examined using an Omni-ECL™ Femto Light Chemiluminescence Kit (EpiZyme, SQ201, China), imaged on a ChemiDoc XRS imaging system (Bio-Rad, USA), and analyzed using ImageJ Software (ver. 1.46r, National Institutes of Health, USA). Each experiment was repeated at least 3 times.
2.10. Statistical analysis
All the data are presented as the means ± SDs, and all the experiments were repeated at least 3 times. Statistical analyses were conducted using Microsoft Excel and GraphPad Prism 7 software. We used an independent t test to analyze the statistical significance between 2 groups and one-way ANOVA followed by a Dunnett multiple comparisons test when comparing more than 2 groups. A p value less than 0.05 was considered to indicate statistical significance. For the whole-organ explant culture experiments, n represents the number of independent cochleae, and for all HEI-OC1 cell culture experiments, n represents the number of independent culture replicates.
3. Results
3.1. Hesperidin alleviates cisplatin-induced auditory threshold shift and hair cell loss in mice
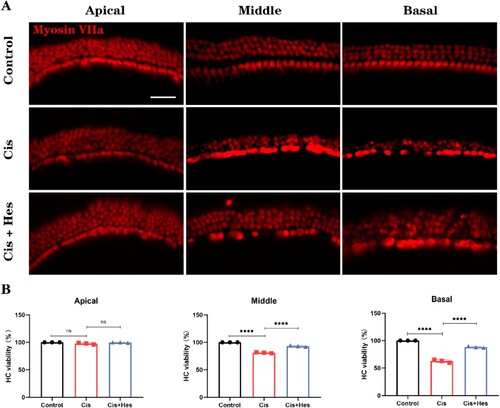
To investigate the protective effect of hesperidin against cisplatin-induced ototoxicity, we tested the auditory brainstem response (ABR) in mice under different treatment conditions. The ABR results demonstrated a significant threshold shift in the cisplatin group compared to the control group, indicating severe hearing loss caused by cisplatin. Compared with those in the cisplatin group, there was a noticeable decrease in the ABR threshold shift at 32 kHz in the hesperidin-pretreated group. The statistical analysis revealed no significant variances between the cisplatin group and the cisplatin + hesperidin group at frequencies of 8 kHz and 16 kHz, possibly attributed to the substantial standard deviations in the data from these frequencies. This result suggested that hesperidin pretreatment can attenuate cisplatin-induced ototoxicity (A). To quantify the hair cells in the cochleae of the mice, we performed immunofluorescence staining using an antibody against myosin VIIa. Hesperidin treatment significantly alleviated cisplatin-induced hair cell loss from the middle to the base turn (B,C). Moreover, we established an in vitro cochlear explant culture model, in which the explants were divided into control, cisplatin, and hesperidin + cisplatin groups (pretreated with 20 μM hesperidin for 2 h followed by cotreatment with 40 μM cisplatin for 24 h). Consistent with the in vivo results, the cisplatin group exhibited significant hair cell loss in the middle and base turns, while treatment with hesperidin significantly attenuated cisplatin-induced explant hair cell loss (A–C).
Figure 1. Hesperidin mitigated cisplatin-induced auditory threshold shift and hair cell loss in C57BL/6J mice. (A) Hesperidin treatment attenuated the increase in auditory thresholds induced by cisplatin (n = 5). (B) Representative images of cochlear basal membrane preparations depicting the differences in hair cell loss across the apex, middle, and base regions of each group (scale bar: 10 µm, n = 5). (C) Quantification of hair cell loss in each group compared to that in the control group (n = 5). For all experiments, ns: nonsignificant, *p < 0.05, **p < 0.01, ****p < 0.0001.
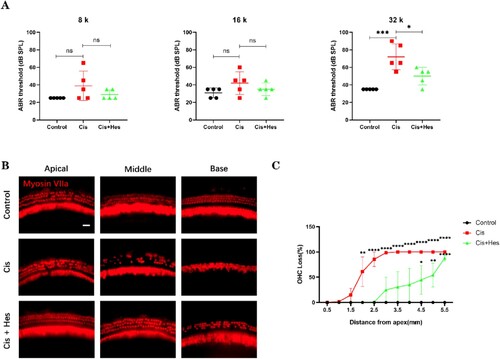
Figure 2. Hesperidin treatment alleviated the loss of cochlear explant hair cells. (A) Cochlear explants from mice were isolated and cultured as described in the Materials and Methods. The cells were treated with 40 μM cisplatin for 24 h or pretreated with 20 μM hesperidin for 2 h followed by the addition of 40 μM cisplatin for 24 h. The cells were then stained with anti-myosin VIIa (scale bar: 20 μm, n = 3). (B) The surviving outer hair cells were counted in a subset of 20 inner hair cells within the three different regions of the cochlea (apex, middle, and base). ns: nonsignificant, *p < 0.05, ***p < 0.001, ****p < 0.0001.
3.2. Hesperidin attenuates cisplatin-induced hair cell apoptosis
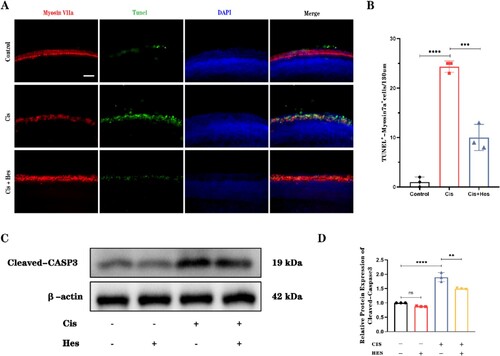
To further validate the protective effect of hesperidin, we used TUNEL staining and Western blot analysis of cleaved-caspase-3 protein levels to analyze the quantity of apoptotic hair cells in the different groups. In the cisplatin group, there was a significant increase in the number of TUNEL-positive hair cells within the basal turn, indicating increased apoptosis. However, in the group treated with a combination of cisplatin and hesperidin, the number of TUNEL-positive cochlear hair cells significantly decreased (A,B). Western blot analysis also demonstrated that hesperidin treatment decreased the expression of cleaved caspase-3, a well-documented apoptotic marker (C,D), while hesperidin treatment alone had no obvious effect on the level of cleaved caspase-3. Overall, these data indicate that hesperidin can protect sensory epithelium hair cells from cisplatin-induced apoptosis.
Figure 3. Hesperidin inhibits cisplatin-induced apoptosis in cochlear hair cells. (A) Immunofluorescence analysis of TUNEL staining demonstrated the presence of apoptotic hair cells in the basal turn of the cochlea in the different groups (scale bar: 20 μm, n = 3). (B) Quantitative analysis of the number of TUNEL-positive hair cells as shown in (A). (C) Western blot results showing the expression of cleaved caspase-3 in different groups. β-Actin served as a loading control. (D) Quantitative analysis of the data shown in (C). ns: nonsignificant, **p < 0.01, ***p < 0.001, ****p < 0.0001.
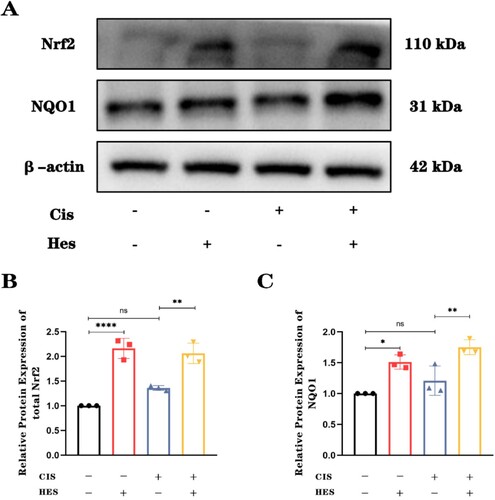
3.3. Hesperidin reduces cisplatin-induced hair cell damage by activating the Nrf2/NQO1 pathway
It has been reported that hesperidin exhibits strong antioxidant properties due to its ability to regulate Nrf2, while promoting Nrf2 activation was shown to have a protective effect against cisplatin ototoxicity [Citation24,Citation25]. To determine whether hesperidin regulates oxidative stress in cochlear hair cells via the Nrf2 pathway to combat cisplatin-induced ototoxicity, we cultured cochlear explants and extracted proteins for Western blot analysis of the expression levels of Nrf2 and its downstream antioxidant protein NQO1. The results indicated that, compared to the control group and the cisplatin-only group, hesperidin treatment significantly increased the expression level of Nrf2 in total explant protein. Furthermore, the expression of the downstream antioxidant protein NQO1 also increased when cochlear tissue was treated with hesperidin (A–C). These findings suggest that hesperidin can counteract cisplatin-induced damage through the Nrf2/NQO1 pathway.
Figure 4. Hesperidin activates the Nrf2/NQO1 pathway to counteract cisplatin-induced damage. (A) Western blot results showing the expression of Nrf2 and downstream NQO1 in the different groups. n = 3. (B) and (C) Quantitative analysis of the Western blot results for Nrf2 and NQO1 showing the activation of Nrf2/NQO1 by hesperidin. ns: nonsignificant, *p < 0.05, **p < 0.01, ****p < 0.0001.
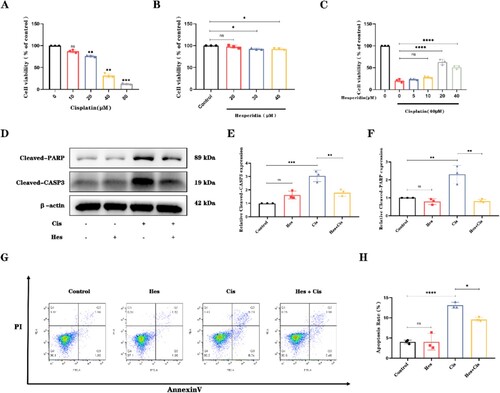
3.4. Hesperidin protects HEI-OC1 cells from cisplatin-induced apoptosis
After treating HEI-OC1 cells with different concentrations of cisplatin for 24 h, we observed a 60% reduction in cell viability at a concentration of 40 µM cisplatin. To better observe cell damage and the protective effects of hesperidin, an appropriate level of damage is needed. Therefore, subsequent cell experiments were conducted under these conditions (A). To avoid potential cellular toxicity from excessive drug concentrations, we treated HEI-OC1 cells with different concentrations of hesperidin. We found that hesperidin treatment at concentrations greater than 20 µM mildly decreased cell viability. (B). Excessive hesperidin may affect cell survival. Therefore, we used various concentrations of hesperidin ranging from 5 to 40 µM in HEI-OC1 cells treated with 40 µM cisplatin. The CCK-8 assay results showed that 20 µM hesperidin exhibited the greatest protective effect against cisplatin-induced damage (C). Under the same conditions, Western blot analysis revealed a significant decrease in the expression of cleaved caspase-3 and cleaved PARP after hesperidin pretreatment (D–F), while 20 µM hesperidin alone caused no significant change in the levels of cleaved caspase-3 and cleaved PARP, indicating the safety of hesperidin at this concentration. Furthermore, flow cytometry confirmed that hesperidin significantly inhibited cisplatin-induced apoptosis in HEI-OC1 cells (G–H). In conclusion, our results demonstrate that hesperidin alleviates cisplatin-induced apoptosis in HEI-OC1 cells.
Figure 5. Hesperidin attenuates apoptosis in cisplatin-treated HEI-OC1 cells. (A) CCK-8 assay results demonstrating the viability of HEI-OC1 cells after treatment with different doses of cisplatin for 24 h. n = 3. (B) Viability of HEI-OC1 cells after treatment with different doses of hesperidin for 24 h. n = 3. (C) Viability of HEI-OC1 cells after cotreatment with different doses of hesperidin and cisplatin. n = 3. (D) Western blot results showing the expression of cleaved caspase-3 (n = 3) and cleaved PARP (n = 3) in the different groups. (E) and (F) Quantitative analysis of the Western blot results shown in (D). (G) Flow cytometry results showing the percentage of apoptotic cells in the different groups. n = 3. (H) Quantitative analysis of the flow cytometry results from (G). ns: nonsignificant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
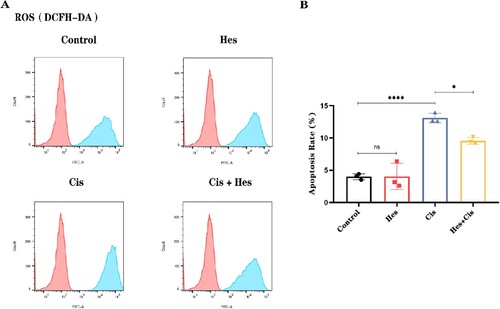
3.5. Hesperidin reduces ROS levels in HEI-OC1 cells following cisplatin-induced damage
Multiple studies have reported that reactive oxygen species (ROS) are among the main molecules by which cisplatin damages sensory hair cells. Hesperidin, in contrast, exhibits strong antioxidant properties [Citation26]. To confirm whether hesperidin suppresses ROS levels induced by cisplatin, we used the ROS detection probe DCFH-DA (2′,7′-Dichlorodihydrofluorescein diacetate) to measure ROS levels in HEI-OC1 cells. Hesperidin treatment alone did not change the ROS levels in HEI-OC1 cells. Compared with those in the control group, the ROS levels in the HEI-OC1 cells increased significantly after 24 h of cisplatin treatment. Compared with cisplatin treatment alone, hesperidin pretreatment for 2 h and cotreatment with cisplatin significantly reduced ROS levels. (A,B). The flow cytometry results indicated that pretreatment with hesperidin significantly abolished the ROS-enhancing effect of cisplatin stimulation in HEI-OC1 cells.
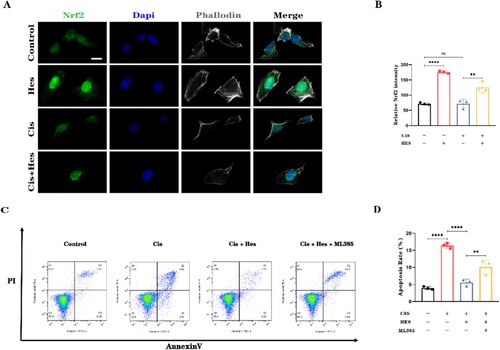
3.6. Hesperidin promotes the nuclear translocation of Nrf2 in HEI-OC1 cells
To further investigate the antioxidant mechanism by which hesperidin protects against cisplatin-induced damage, we utilized immunofluorescence to observe the nuclear translocation and activation of Nrf2 in HEI-OC1 cells. Compared with that in the control group, immunolabeling for Nrf2 in the nuclei of HEI-OC1 cells was significantly greater after hesperidin treatment alone. There was no difference in the Nrf2 fluorescence level between the cisplatin group and the control group. The expression of Nrf2 in the nucleus after treatment with hesperidin combined with cisplatin was greater than that after treatment with cisplatin alone. (A,B). To confirm that hesperidin primarily exerts its antioxidant effects through the Nrf2 pathway, we added the Nrf2 inhibitor ML385 in addition to hesperidin. Flow cytometry analysis of apoptosis in HEI-OC1 cells indicated that the protective effect of hesperidin was significantly diminished when Nrf2 was inhibited by ML385. This resulted in an increase in the percentage of apoptotic HEI-OC1 cells (C,D). These findings suggest that hesperidin primarily exerts its protective effects against cisplatin-induced damage in HEI-OC1 cells through the Nrf2 pathway.
Figure 7. Hesperidin activates the nuclear translocation of Nrf2 in HEI-OC1 cells. (A) Representative immunofluorescence images showing the translocation of Nrf2 to the nucleus in HEI-OC1 cells (scale bar: 10 μm, n = 3). (B) Quantitative analysis of the cell fluorescence intensity in (A). (C) Flow cytometry results demonstrating that the Nrf2 inhibitor ML385 inhibits the protective effect of hesperidin. (D) Quantitative analysis of the flow cytometry results in (C). **p < 0.01, ****p < 0.0001.
4. Discussion
Our in vivo results demonstrated that hesperidin has a significant protective effect on cisplatin-induced sensorineural hearing loss. The in vitro tissue and cell results showed that hesperidin inhibits cell apoptosis and alleviates oxidative stress through the Nrf2 pathway, which ultimately reduces cisplatin-induced hair cell damage.
Regarding the cisplatin ototoxicity model, numerous studies have used systemic administration of cisplatin to induce hearing loss [Citation27–29], while others have reported using transtympanic injection of cisplatin to simulate cisplatin-induced inner ear damage [Citation18]. Although systemic administration of cisplatin better aligns with the clinical setting, the low survival rate and poor stability of mice due to the significant toxic side effects of cisplatin remain major challenges in this area of animal research [Citation30]. In contrast, transtympanic injection of cisplatin, which causes localized inner ear damage, does not significantly impact mouse survival and results in a more stable damage model [Citation18,Citation31,Citation32]. Therefore, in this study, to better investigate the protective effect of hesperidin against cisplatin ototoxicity, we used intratympanic injection to induce cisplatin-induced inner ear damage.
Sensory hair cells are the main sensory organs in the inner ear and play a crucial role in converting sound signals into neural impulses. Moreover, sensory hair cells are nonregenerative cells, which means that the hearing loss caused by cisplatin-induced hair cell damage is often irreversible [Citation33]. Our in vivo experiments and cochlear explant culture results demonstrated that the application of hesperidin significantly alleviated cisplatin-induced hair cell loss, thereby protecting to hearing function. However, we did not detect possible pathological changes in other regions of the inner ear, such as the spiral ganglion and stria vasculari, which are also targets damaged by cisplatin. In future studies, we will aim to determine the overall change in the inner ear during hesperidin administration.
Cisplatin-induced ototoxicity primarily manifests as increased levels of cellular apoptosis. In our study, the results of TUNEL fluorescence staining and the protein expression level of activated caspase-3 were significantly increased in the cisplatin group. However, when hesperidin was administered in combination with cisplatin, the number of TUNEL-positive cells and the level of cleaved caspase-3 decreased significantly, indicating that hesperidin strongly protected against cisplatin-induced damage.
The transcription factor Nrf2 is negatively regulated by the protein Kelch-like ECH-associated protein 1 (KEAP1), which anchors Nrf2 in the cytoplasm and facilitates its rapid degradation. However, when cells are exposed to oxidative stress, inflammation, or other stimuli, Nrf2 is released from KEAP1 inhibition and translocates to the nucleus. In the nucleus, Nrf2 binds to the antioxidant response element (ARE) and promotes the expression of antioxidant genes, such as NQO1 [Citation34]. Our research results suggest that hesperidin promotes the expression of Nrf2 and downstream antioxidant proteins in hair cells. This subsequently enhances the antioxidant stress response capability of hair cells and reduces apoptosis. Immunofluorescence staining of HEI-OC1 cells revealed that hesperidin promoted the nuclear translocation and activation of Nrf2, thereby promoting the expression of antioxidant genes.
Reactive oxygen species (ROS) are common mechanisms underlying cisplatin-induced ototoxicity [Citation35,Citation36]. ROS can directly cause oxidative damage to DNA, leading to the activation of DNA damage signals and triggering cell apoptosis. Multiple studies have shown that inhibiting reactive oxygen species can reduce the damage to hair cells [Citation37–39]. Antioxidants are known to be effective protective agents against cisplatin-induced ototoxicity [Citation40]. Our research demonstrated that hesperidin effectively decreased ROS levels, thereby preventing cisplatin-induced cell apoptosis. This finding is consistent with previous findings, suggesting that hesperidin primarily inhibits cisplatin damage through its antioxidant effects.
Research indicates that cisplatin induces an increase in mitochondrial ROS levels, thereby triggering apoptosis in tumor cells [Citation41,Citation42]. However, our findings suggest that hesperidin reduces the level of ROS, raising the question of whether hesperidin may also attenuate the therapeutic effect of cisplatin on tumors. Multiple studies have shown that hesperidin can not only alleviate the toxic side effects caused by cisplatin but also enhance its antitumor potency [Citation43,Citation44]. Kong et al. reported that hesperidin can reverse cisplatin resistance in tumor cells by modulating the nuclear factor-κB signaling pathway [Citation45]. Overall, hesperidin does not reduce the antitumor efficacy of cisplatin while mitigating its toxic and adverse effects on vital organs. Therefore, hesperidin is a potential adjuvant drug for cisplatin-based tumor therapy.
5. Conclusions
Our study revealed that hesperidin can suppress cisplatin-induced hearing loss and reduce apoptosis and damage in hair cells. Additionally, we established that hesperidin primarily enhances the antioxidant capacity of hair cells and HEI-OC1 cells through the Nrf2 pathway, thereby mitigating cisplatin-induced oxidative damage. Our research further elucidated the protective effect of hesperidin against cisplatin ototoxicity and its potential underlying mechanisms.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the present study appear in the submitted article.
Additional information
Funding
References
- Qi L, Luo Q, Zhang Y, et al. Advances in toxicological research of the anticancer drug cisplatin. Chem Res Toxicol. 2019;32:1469–1486. doi:10.1021/acs.chemrestox.9b00204
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi:10.1038/sj.onc.1206933
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi:10.1038/nrd1691
- Breglio AM, Rusheen AE, Shide ED, et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun. 2017;8:1654. doi:10.1038/s41467-017-01837-1
- Meijer AJM, Li KH, Brooks B, et al. The cumulative incidence of cisplatin-induced hearing loss in young children is higher and develops at an early stage during therapy compared with older children based on 2052 audiological assessments. Cancer. 2022;128:169–179. doi:10.1002/cncr.33848
- Fetoni AR, Ruggiero A, Lucidi D, et al. Audiological monitoring in children treated with platinum chemotherapy. Audiol Neurootol. 2016;21:203–211. doi:10.1159/000442435
- Rybak LP, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Semin Hear. 2019;40:197–204. doi:10.1055/s-0039-1684048
- Sheth S, Mukherjea D, Rybak LP, et al. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front Cell Neurosci. 2017;11:338. doi:10.3389/fncel.2017.00338
- García-Berrocal JR, Nevado J, Ramírez-Camacho R, et al. The anticancer drug cisplatin induces an intrinsic apoptotic pathway inside the inner ear. Br J Pharmacol. 2007;152:1012–1020. doi:10.1038/sj.bjp.0707405
- Tan WJT, Song L. Role of mitochondrial dysfunction and oxidative stress in sensorineural hearing loss. Hear Res. 2023;434:108783. doi:10.1016/j.heares.2023.108783
- Lu MC, Ji JA, Jiang ZY, et al. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med Res Rev. 2016;36:924–963. doi:10.1002/med.21396
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi:10.1146/annurev.pharmtox.46.120604.141046
- Wu F, Hu R, Huang X, et al. CFTR potentiator ivacaftor protects against noise-induced hair cell loss by increasing Nrf2 and reducing oxidative stress. Biomed Pharmacother. 2023;166:115399. doi:10.1016/j.biopha.2023.115399
- Aboraya DM, El Baz A, Risha EF, et al. Hesperidin ameliorates cisplatin induced hepatotoxicity and attenuates oxidative damage, cell apoptosis, and inflammation in rats. Saudi J Biol Sci. 2022;29:3157–3166. doi:10.1016/j.sjbs.2022.01.052
- Jia Y, Guo H, Cheng X, et al. Hesperidin protects against cisplatin-induced cardiotoxicity in mice by regulating the p62-Keap1-Nrf2 pathway. Food Funct. 2022;13:4205–4215. doi:10.1039/D2FO00298A
- Chen X, Wei W, Li Y, et al. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem Biol Interact. 2019;308:269–278. doi:10.1016/j.cbi.2019.05.040
- Kara M, Türkön H, Karaca T, et al. Evaluation of the protective effects of hesperetin against cisplatin-induced ototoxicity in a rat animal model. Int J Pediatr Otorhinolaryngol. 2016;85:12–18. doi:10.1016/j.ijporl.2016.03.019
- He J, Yin S, Wang J, et al. Effectiveness of different approaches for establishing cisplatin-induced cochlear lesions in mice. Acta Otolaryngol. 2009;129:1359–1367. doi:10.3109/00016480902856604
- He W, Wu F, Xiong H, et al. Promoting TFEB nuclear localization with curcumin analog C1 attenuates sensory hair cell injury and delays age-related hearing loss in C57BL/6 mice. Neurotoxicology. 2023;95:218–231. doi:10.1016/j.neuro.2023.02.004
- Park C, Thein P, Kalinec G, et al. HEI-OC1 cells as a model for investigating prestin function. Hear Res. 2016;335:9–17. doi:10.1016/j.heares.2016.02.001
- Wu F, Hill K, Fang Q, et al. Traumatic-noise-induced hair cell death and hearing loss is mediated by activation of CaMKKβ. Cell Mol Life Sci. 2022;79:249. doi:10.1007/s00018-022-04268-4
- Lai R, Fang Q, Wu F, et al. Prevention of noise-induced hearing loss by calpain inhibitor MDL-28170 is associated with upregulation of PI3 K/Akt survival signaling pathway. Front Cell Neurosci. 2023;17:1199656. doi:10.3389/fncel.2023.1199656
- Fang QJ, Wu F, Chai R, et al. Cochlear surface preparation in the adult mouse. J Vis Exp. 2019;153.
- Li D, Zhao H, Xu P, et al. Polydatin activates the Nrf2/HO-1 signaling pathway to protect cisplatin-induced hearing loss in guinea pigs. Front Pharmacol. 2022;13:887833. doi:10.3389/fphar.2022.887833
- Nan B, Zhao Z, Jiang K, et al. Astaxanthine attenuates cisplatin ototoxicity in vitro and protects against cisplatin-induced hearing loss in vivo. Acta Pharm Sin B. 2022;12:167–181. doi:10.1016/j.apsb.2021.07.002
- Cao R, Zhao Y, Zhou Z, et al. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. J Sci Food Agric. 2018;98:2422–2427. doi:10.1002/jsfa.8734
- Wang H, Lin H, Kang W, et al. miR-34a/DRP-1-mediated mitophagy participated in cisplatin-induced ototoxicity via increasing oxidative stress. BMC Pharmacol Toxicol. 2023;24:16. doi:10.1186/s40360-023-00654-1
- Gu J, Wang X, Chen Y, et al. An enhanced antioxidant strategy of astaxanthin encapsulated in ROS-responsive nanoparticles for combating cisplatin-induced ototoxicity. J Nanobiotechnology. 2022;20:268. doi:10.1186/s12951-022-01485-8
- He Y, Zheng Z, Liu C, et al. Inhibiting DNA methylation alleviates cisplatin-induced hearing loss by decreasing oxidative stress-induced mitochondria-dependent apoptosis via the LRP1–PI3K/AKT pathway. Acta Pharm Sin B. 2022;12:1305–1321. doi:10.1016/j.apsb.2021.11.002
- Fernandez K, Wafa T, Fitzgerald TS, et al. An optimized, clinically relevant mouse model of cisplatin-induced ototoxicity. Hear Res. 2019;375:66–74. doi:10.1016/j.heares.2019.02.006
- Xia L, Chen Z, Yin S. Ototoxicity of cisplatin administered to guinea pigs via the round window membrane. J Toxicol Sci. 2012;37:823–830. doi:10.2131/jts.37.157
- Nacher-Soler G, Lenglet S, Coelho M, et al. Local cisplatin delivery in mouse reliably models sensorineural ototoxicity without systemic adverse effects. Front Cell Neurosci. 2021;15:701783. doi:10.3389/fncel.2021.701783
- Qi J, Huang W, Lu Y, et al. Stem cell-based hair cell regeneration and therapy in the inner ear. Neurosci Bull. 2023;40:113–126.
- Weng B, Zhang X, Chu X, et al. Nrf2-Keap1-ARE-NQO1 signaling attenuates hyperoxia-induced lung cell injury by inhibiting apoptosis. Mol Med Rep. 2021;23.
- Gentilin E, Simoni E, Candito M, et al. Cisplatin-induced ototoxicity: updates on molecular targets. Trends Mol Med. 2019;25:1123–1132. doi:10.1016/j.molmed.2019.08.002
- Tang Q, Wang X, Jin H, et al. Cisplatin-induced ototoxicity: updates on molecular mechanisms and otoprotective strategies. Eur J Pharm Biopharm. 2021;163:60–71. doi:10.1016/j.ejpb.2021.03.008
- Gao Y, Wu F, He W, et al. Reactive oxygen species-related disruptions to cochlear hair cell and stria vascularis consequently leading to radiation-induced sensorineural hearing loss. Antioxid Redox Signal. 2024;40:470–491. doi:10.1089/ars.2022.0161
- Wu F, Xiong H, Sha S. Noise-induced loss of sensory hair cells is mediated by ROS/AMPKα pathway. Redox Biol. 2020;29:101406. doi:10.1016/j.redox.2019.101406
- Kim YR, Baek JI, Kim SH, et al. Therapeutic potential of the mitochondria-targeted antioxidant MitoQ in mitochondrial-ROS induced sensorineural hearing loss caused by Idh2 deficiency. Redox Biol. 2019;20:544–555. doi:10.1016/j.redox.2018.11.013
- Fujimoto C, Yamasoba T. Mitochondria-targeted antioxidants for treatment of hearing loss: a systematic review. Antioxidants (Basel). 2019;8:109.
- Marullo R, Werner E, Degtyareva N, et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One. 2013;8:e81162. doi:10.1371/journal.pone.0081162
- He G, He G, Zhou R, et al. Enhancement of cisplatin-induced colon cancer cells apoptosis by shikonin, a natural inducer of ROS in vitro and in vivo. Biochem Biophys Res Commun. 2016;469:1075–1082. doi:10.1016/j.bbrc.2015.12.100
- Omar HA, Mohamed WR, Arafa el SA, et al. Hesperidin alleviates cisplatin-induced hepatotoxicity in rats without inhibiting its antitumor activity. Pharmacol Rep. 2016;68:349–356. doi:10.1016/j.pharep.2015.09.007
- Saleh N, Allam T, Korany RMS, et al. Protective and therapeutic efficacy of hesperidin versus cisplatin against Ehrlich ascites carcinoma-induced renal damage in mice. Pharmaceuticals. 2022;15. doi:10.3390/ph15030294
- Kong W, Ling X, Chen Y, et al. Hesperetin reverses P-glycoprotein-mediated cisplatin resistance in DDP-resistant human lung cancer cells via modulation of the nuclear factor-κB signaling pathway. Int J Mol Med. 2020;45:1213–1224.