ABSTRACT
A systematic literature review was performed to identify all peer-reviewed literature quantifying the association between short-term exposures of particulate matter <2.5 microns (PM2.5), nitrogen dioxide (NO2), and sulfur dioxide (SO2) and COPD-related emergency department (ED) visits, hospital admissions (HA), and mortality. These results were then pooled for each pollutant through meta-analyses with a random effects model. Subgroup meta-analyses were explored to study the effects of selected lag/averaging times and health outcomes. A total of 37 studies satisfied our inclusion criteria, contributing to a total of approximately 1,115,000 COPD-related acute events (950,000 HAs, 80,000 EDs, and 130,000 deaths) to our meta-estimates. An increase in PM2.5 of 10 ug/m3 was associated with a 2.5% (95% CI: 1.6–3.4%) increased risk of COPD-related ED and HA, an increase of 10 ug/m3 in NO2 was associated with a 4.2% (2.5–6.0%) increase, and an increase of 10 ug/m3 in SO2 was associated with a 2.1% (0.7–3.5%) increase. The strength of these pooled effect estimates, however, varied depending on the selected lag/averaging time between exposure and outcome. Similar pooled effects were estimated for each pollutant and COPD-related mortality. These results suggest an ongoing threat to the health of COPD patients from both outdoor particulates and gaseous pollutants. Ambient outdoor concentrations of PM2.5, NO2, and SO2 were significantly and positively associated with both COPD-related morbidity and mortality.
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by progressive irreversible airflow limitation and chronic inflammation of the lungs, is an increasingly prevalent disease in both developed and developing countries Citation(1). It is currently the fourth leading cause of death globally Citation(2,3). The disease represents an important economic burden for individual patients and healthcare systems Citation(4), with estimated direct costs of $29.5 billion and indirect costs of $20.4 billion in the United States Citation(1). These costs are largely due to exacerbation of COPD, which in severe cases can result in emergency department (ED) visits, hospital admissions (HA), and death Citation(5). One potential trigger for such exacerbations is short-term exposures to outdoor air pollution Citation(1). In the past two decades, numerous epidemiological studies have investigated the short-term effects of outdoor air pollution on this sensitive population by studying COPD-related morbidity (as ED and HA) and mortality Citation(6). Most of these studies report significant positive associations for exposures to particulate matter (PM), with conflicting evidence for the other United States Environmental Protection Agency (USEPA) criteria gaseous air pollutants, including nitrogen dioxide (NO2) and sulfur dioxide (SO2) Citation(6).
Although meta-analysis Citation(7) has been widely used to combine the study results quantifying the association between short-term exposures to outdoor air pollution and overall respiratory disease-related ED-HA and mortality, only four have estimated pooled effects among COPD patients Citation(8–11). Two of these looked specifically at PM10 exposures Citation(8,9) and one focused solely on studies completed in China Citation(10). There has yet to be a systematic review of all the existing literature and meta-analysis for two pollutants currently of great public health concern—NO2 and SO2. The lack of pooled risk estimates for these gaseous pollutants limits the ability to fully understand the impact that outdoor air pollution may have on COPD patients. In this study, a systematic literature review and meta-analysis were carried out to synthesize risk estimates for COPD-related morbidity and mortality outcomes due to short-term exposures (up to a maximum of 7 days) to PM2.5, SO2, and NO2. Subgroup analyses were used to evaluate the implications that selected lag/averaging times between exposure and outcome had on pooled effect estimates, as well as to study the differences in pooled effect estimates for various acute COPD-related outcomes.
Methods
Search strategy
A comprehensive systematic literature review Citation(12) was conducted in PubMed and Medline databases to identify relevant peer-reviewed articles. The following Medical Subject Heading (MeSH) criteria Citation(13) were used in PubMed: (“Pulmonary Disease, Chronic Obstructive/epidemiology”[Mesh]) AND ((“Air Pollution/adverse effects”[Mesh]) OR (“Air Pollutants/adverse effects”[Mesh]) OR (“Sulfur Dioxide”[Mesh]) OR (“Nitrogen Dioxide”[Mesh]) OR (“Particulate Matter”[Mesh])). The following key word criteria were used in Medline: (“Air Pollution” OR “Sulfur Dioxide” OR “Nitrogen Dioxide” OR “Particulate Matter”) AND (COPD OR “Chronic Obstructive Pulmonary Disease”) AND (Hospital* OR Emergency OR Mortality). Additional filters were added to both search strings and databases to limit results to studies published in English between the years 1995 and 2015. In addition, the titles of all references from all eligible studies captured in the PubMed/Medline searches, as well as those referenced in USEPA Integrated Science Assessment (ISA) documents for each pollutant Citation(14–16), were reviewed.
Titles and abstracts for all identified articles were screened by two researchers (RD and DK). Any study that investigated the association between short-term outdoor air pollution exposures and COPD-related ED, HA, or mortality was retained for full-text review. Of these studies eligible for full-text review, any study that provided quantitative estimates for the association between short-term exposures to PM2.5, SO2, and NO2 and COPD-related ED, HA, or mortality with measures of uncertainty (p-values or confidence intervals [CIs]) was included and relevant information was extracted into an Excel database. One study Citation(17) was excluded as it re-evaluated data included in an eligible study published on an earlier date Citation(18). We also excluded one study that did not provide sufficient information to estimate 95% CIs Citation(19) and another that did not explore or control for confounding Citation(20).
Many studies investigated and reported the results for different lag/averaging times, which is problematic for meta-analyses, where researchers must select one estimate of the magnitude of the association between exposure and disease from each individual study to inform the meta-effect estimate. Some studies identified in the air pollution–COPD literature specified an exposure window a priori, while others investigated numerous lag/averaging times and either reported all results or only those with the largest or most significant effect estimate. Choosing to report one effect estimate rather than another because of effect size or statistical significance could introduce bias into the meta-effect estimate Citation(21). As there was little consistency in the exposure metrics presented among the eligible studies and limited information regarding their biological mechanisms, results from several exposure categories were extracted from each study: 1) single-day lags, up to a maximum of two days, 2) multi-day averages or distributed lags, up to a maximum of seven days, and 3) the strongest effects across all available lag and averaging times. When multiple estimates were available for any of these categories, the strongest result within that category was selected.
A majority of studies estimated exposures for these lag/averaging times using 24-hour daily average concentrations. When results were provided for multiple daily metrics (such as 1-hour daily maxima and 24-hour daily averages), only results based on 24-hour daily averages were included Citation(22,23), with the exception of one study that only provided results using 1-hour daily maxima Citation(24). A number of studies estimated results across various cities Citation(22,25–29). Some of these studies calculated the effects using raw data from all cities, while others calculated effect estimates for each city and then combined the results in a meta-analysis model. Wherever possible, we included pooled multi-city effects. Only two studies reported the effects stratified by season, and the stratified season-specific estimates were included Citation(30,31). When studies reported results for different age groups, effect estimates from elderly populations (ages 65+) were selected Citation(32,33).
Statistical analyses
As studies presented results for different units of concentration, a series of conversions were completed prior to pooling individual effects through meta-analysis. All results reported in parts per billion (ppb) were first converted to ug/m3, assuming standard pressure and temperature. Risk estimates were then further standardized to represent the effects associated with an increase of 10 ug/m3 in the concentration in PM2.5, SO2, and/or NO2. An increase of 10 ug/m3 was selected for all pollutants as it was the most commonly used unit of analysis across the studies included in our pooled effect estimates.
Pooled summary effects were estimated with Comprehensive Meta-Analysis software (version 2.0) for each pollutant and outcome combination. Summary effects were calculated using the weighted mean of individual effects, with weights equal to the inverse of each study's variance Citation(34). In order to account for between-study variability, a random-effects model was chosen a priori. The appropriateness of this decision was confirmed by evaluating heterogeneity statistics. Forest plots were developed in Microsoft Excel for each pollutant using the exposure category that provided the strongest summary effects (represented by the highest pooled relative risk and discussed further below). In these plots, the size of the symbols represents the relative weight of that study when computing summary effects Citation(34,35). Additional subgroup analyses were completed to assess the differences by health outcome. In this study, a minimum of three studies were required to calculate a pooled effect estimate.
Heterogeneity was examined using standard Q and I2 tests Citation(36–38). In the Q statistic tests, the null hypothesis of homogenous effect sizes Citation(36) with a p < 0.10 suggested substantial heterogeneity between studies. The I2 statistic quantified the percent of total variability in effect sizes due to variability between studies, rather than within-study sampling error Citation(36–38). Consequently, a higher I2 suggests greater heterogeneity between studies.
Summary effect estimates are expressed as relative risks (RRs) for 10 ug/m3 increases in pollutant concentration. The RR was selected because it is an intuitive commonly used measure in public health literature.
Results
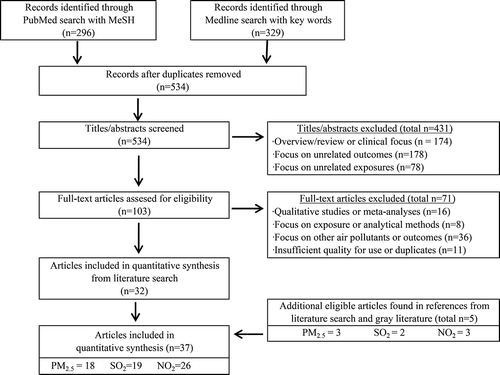
The initial literature searches completed in PubMed and Medline databases identified 296 and 329 citations, respectively (). After duplicates were removed, a total of 534 articles were left for title and abstract review. After screening titles and abstracts to eliminate articles that were overviews/reviews of the existing literature, clinical or animal studies, studies focused on unrelated exposures (such as occupational, indoor, or tobacco smoke exposures), or studies evaluating unrelated outcomes (such as COPD onset, lung cancer, asthma, pulmonary function testing, or self-reported respiratory symptoms), 103 articles were considered potentially eligible and their full text was obtained and reviewed. Of these studies, an additional 71 were excluded as they did not present quantitative effect estimates or provide sufficient detail to estimate 95% CIs, were a re-analysis of data already captured in an eligible article published at an earlier date, focused solely on exposure assessment or analytical methods, or evaluated other outdoor air pollutants not included in this review (such as PM10, PM speciation, ozone, or proximity to traffic). Five additional studies were identified in the references of eligible articles and USEPA ISA documents Citation(14–16), resulting in a total of 37 studies for meta-analysis ().
Of the 37 eligible studies, eight were case crossover and 29 were time series studies. Most of the time series studies were analyzed via Poisson regression with generalized additive models, while the case-crossover studies were analyzed with conditional logistic regression. Nine studies focused on COPD-related ED, 17 on COPD-related HA, and 11 on COPD-related mortality. Effect estimates were available in 18 studies for PM2.5, 25 studies for NO2, and 19 studies for SO2. Nearly all of the studies controlled for seasonality and weather, while approximately half of the studies controlled for regional trends of influenza. Most of the time series studies controlled for long-term trends with smoothing splines and several also controlled for the day of week and holidays. Six of the case-crossover studies used a time-stratified control-sampling strategy, while two followed bidirectional control sampling Citation(63).
Air pollution and COPD-related morbidity
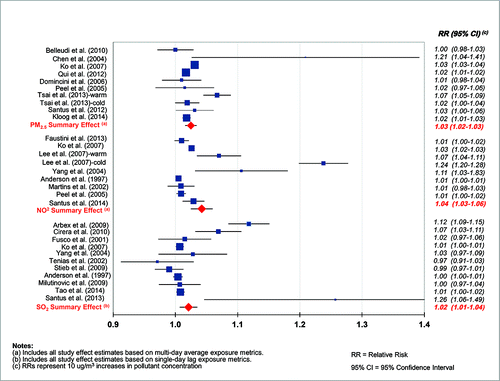
All the three pollutants were positively associated with COPD-related morbidity (as ED or HA), with excess risks ranging from 2% to 4% per 10 ug/m3 increase in concentration (). For PM2.5, we estimated an RR of 1.025 (95% CI 1.016–1.034) per 10 ug/m3 in concentration using multi-day averages (I2 = 74, Q = 22.6, p<0.001, and n = 9 studies). When using single-day lags, we found a slightly weaker but still positive association with COPD-related morbidity (RR = 1.014, 95% CI 1.005–1.024) (). For NO2, we estimated an RR of 1.042 (95% CI 1.025–1.060) per 10 ug/m3 in concentration using multi-day averages (I2 = 96, p-value for Q test <0.001, and n = 9 studies). Similar to PM2.5, we found a slightly weaker but positive association for NO2 when using single-day lags (RR = 1.020, 95% CI 1.006–1.031). For SO2, we estimated an RR of 1.021 (95% CI 1.007–1.035) per 10 ug/m3 increase in concentration using single-day lags (I2 = 87, p-value for Q test <0.001, and n = 11 studies). In contrast to PM2.5 and NO2, we found a weaker albeit still significantly positive association for SO2 when using multi-day averages (RR = 1.012, 95% CI 1.000–1.023).
Table 1. Summary of studies included in meta-analysis.
Table 2. Comparison of pooled effect estimates by exposure metric.
In general, we found slightly stronger effect estimates for COPD-related morbidity (ED and HA) when using multi-day averages for both PM2.5 and NO2. For SO2, the reverse pattern was observed. The use of the strongest effect estimate available in each study produced similar results for each pollutant, suggesting that authors may preferentially report the strongest effects. Using the strongest effect estimates per study, we estimated an RR of 1.02 (95% CI 1.00–1.04) for PM2.5, an RR of 1.03 (95% CI 1.02–1.04) for NO2, and an RR of 1.02 (95% CI 1.01–1.03) for SO2. Data are not shown.
Subgroup analyses: Morbidity and mortality
Differences between morbidity (including EDs and HAs) and mortality outcomes were explored using the exposure category that produced the strongest pooled effects. Pooled effects for PM2.5 were two-fold higher for mortality (RR = 1.048, 95% CI 1.019–1.078, based on five studies) than for morbidity, measured as ED or HA (RR = 1.025, 95% CI 1.016–1.034, based on ten studies). Conversely, stronger effects were calculated for NO2 when evaluating morbidity (RR = 1.042, 95 CI 1.025–1.060, based on nine studies) than for mortality (RR = 1.030, 95% CI 1.016–1.045, based on six studies). However, CIs were wide and overlapping. We did not identify enough mortality studies to investigate SO2 in this way.
As there were not enough studies to compare ED with HA for each pollutant using the exposure metric that resulted in the greatest combined effect, these outcomes were compared using the strongest lag and averaging time across all studies. For SO2, we found stronger effects for ED (RR = 1.041, 95% CI 1.004–1.0879, and n = 8 studies) than HA (RR = 1.010, 95% CI 1.002–1.019, and n = 7 studies). For NO2, however, the pattern was reversed; ED showed a weaker effect (RR = 1.010, 95% CI 1.002–1.018, and n = 7 studies) than HA (RR = 1.045, 95% CI 1.029–1.061). For PM2.5, we estimated similar effects for both outcomes; an RR of 1.023 (95% CI 1.002–1.043 and n = 5 studies) for ED and an RR of 1.019 (95% CI 0.998–1.041 and n = 10 studies) for HA.
Discussion
This meta-analysis is the first to our knowledge to quantify the association between short-term exposures to NO2 and SO2 and COPD-related morbidity, with the exception of one meta-analysis that focused specifically on studies among Chinese populations Citation(10). Positive associations were observed for each of these gaseous pollutants, with significant excess risks estimated between 2 and 4% per 10 ug/m3 increase in concentration. We also estimated a significant 1.4–2.5% increased risk in COPD-related ED and HA per 10 ug/m3 increase in PM2.5 (depending on selected exposure metric), which is slightly weaker but within the same range as that reported in a recent meta-analysis for PM2.5 Citation(11). Li and colleagues estimated a 3% (95% CI 2–5%, 15 studies, I2 = 88%) increase in risk for COPD-related morbidity per 10 ug/m3 increase in PM2.5 Citation(11). This is slightly stronger than our estimate and likely due to the selection of the strongest effect estimate across all available lag and averaging times for each individual study. As noted above, we believe that this method may introduce bias; a better approach would be to use consistent lags or averaging times for all studies combined into a pooled effect estimate. We estimated pooled effects separately for exposures based on single-day lags and multiple-day averages and found that the strength of summary effects varied by 50% or more depending on which exposure metric was used. We hypothesize that this important source of variability may be a function of a pollutant's day-to-day variability and the biological mechanism. Of the four meta-analyses found in the recent literature that specifically focus on acute effects among COPD patients from short-term exposures to outdoor air pollution Citation(8–11), none of them investigated the differences due to the use of different exposure metrics. Our results highlight the sensitivity of pooled effect estimates to the choice of lag/averaging time.
Effects of PM2.5 and NO2 were stronger using multi-day averages compared with single-day estimates. The reverse pattern was observed for SO2; the effect was stronger when exposure was measured as a single-day lag rather than an average of several days.
One possible reason for the observed differences in effect estimates by exposure metric is the pollutants' biological mechanisms. The mechanisms by which airway inflammation are exacerbated following short-term exposures to outdoor air pollution are not yet fully understood, although there are several reasonable hypotheses. PM exposures cause increased airway hyper-responsiveness in rodents and production of reactive oxygen and inflammatory factors in alveolar macrophages in humans Citation(9). Longer lag/averaging times are biologically plausible for PM compared with gaseous pollutants considering the proposed effect of particles on allergic sensitization and lung immune defenses, which have been observed in controlled human exposure and experimental animal studies Citation(16). NO2 exposures can exacerbate existing respiratory disease by impairing the functions of epithelial cells and alveolar macrophages, contributing to airway inflammation Citation(14). Similar to PM, this process may be cumulative over days and therefore a longer time period would be more relevant than the shorter period captured by single-day lags. SO2, on the other hand, is a highly reactive gas with a high degree of day-to-day variability Citation(15). Bronchoconstriction in healthy adult males has been observed after short-term exposures to ambient levels of SO2 Citation(64), as well as in numerous animal studies Citation(15). SO2 is also a well-known respiratory irritant, with acute respiratory symptoms reported immediately upon exposure to elevated concentrations in controlled human studies Citation(15).
Mortality from COPD was about twice as strongly associated with PM2.5 than morbidity (ED and HA) from COPD (RR = 1.050, 95% CI 1.015–1.087 for mortality and RR = 1.026, 95% CI 1.014–1.038 for morbidity). Li and colleagues recently reported similar effects from short-term exposures of PM2.5 on COPD-related morbidity and mortality Citation(11). Zhu and colleagues, in a meta-analysis of Chinese studies, reported the reverse pattern for PM10 and acute COPD outcomes Citation(8). There are a number of reasons why this may have occurred. Firstly, PM10 and PM2.5 are different particle size fractions containing diverse chemical components and moderated by meteorology, topography, and human behavior in different ways Citation(16). Secondly, the PM2.5 studies included in this meta-analysis are more recent (by nearly a decade) than the PM10 studies evaluated Citation(8). The stronger association that we found for PM2.5 and COPD-related mortality, as compared with COPD-related ED-HA, may reflect improvements in disease management over the past decade, whereby patients are increasingly better at avoiding certain triggers and taking care of themselves. This could result in decreased risk of ED-HA through time, while the risk remains high for more severe outcomes like mortality. This discrepancy may also be due to differences in the study populations or geographic regions represented in our studies. For example, a recent meta-analysis quantifying the association between PM and mortality reported higher risk of mortality among elderly and those with a lower socioeconomic status compared with younger, wealthier, and more educated populations Citation(65). Finally, this difference may be due to differences in the rationale for selecting individual effect estimates from eligible journal articles by Zhu and colleagues and as we have done in this paper.
This study also found the stronger effects of SO2 on COPD-related ED than HA. These results are consistent with a recent study that compared ED and HA data from air pollution time series studies for various diseases (i.e., respiratory disease, cardiovascular disease, and pneumonia.) Citation(66). Researchers estimated slightly higher risk ratios for respiratory disease-related ED than HA and attributed this to differences in the types of patients typically experiencing these visits; patients for ED were often younger, from poorer areas, and with less severe illness Citation(66). Researchers also mentioned that HAs typically include scheduled visits, where the timing is unlikely to be caused by air pollution, which could mislead and/or dampen results Citation(66). We did not, however, find the same trend for exposures to PM2.5 and NO2.
Limitations
Heterogeneity and bias are two important limitations to discuss in the context of this meta-analysis. While we investigated several importance sources of heterogeneity through stratum-specific pooled effects with a random-effects model, there was still likely to have been substantial variation between studies. This is reflected in high estimates of between-study variance (as represented by I2, shown in ), most of which were greater than 80%. Due to sample size limitations, we were not able to investigate the important differences in study design, geography, air chemistry, meteorology, and population health characteristics. We were also not able to investigate the impacts of different exposure metrics on the effect estimates for mortality due to the limited number of available studies.
Due to the limited number of studies available within strata and the large number of results presented in each article, we were also not able to formally evaluate publication bias in a meaningful way. If positive studies were more likely to have been published, these results may have been biased away from the null. Bias could also occur within published studies if authors only chose to present the strongest effect estimates. We tried to avoid this by mostly including studies that focused specifically on COPD and therefore explored/presented results from various lag/averaging times for this particular population. We intentionally kept our search criteria in PubMed and Medline quite specific to capture studies specifically focused on COPD outcomes and exclude the larger scope time series studies that investigate all causes of ED, HA, and mortality but often only present results with the strongest effect estimates and/or highest level of statistical significance. Inclusion of estimates from such studies could be misleading and bias results away from the null hypothesis.
Finally, it is important to remember that meta-estimates of the effects of pollutants on COPD necessarily represent single-pollutant models, while of course the COPD populations are breathing urban air containing all these pollutants and several other pollutants. Thus, these estimates represent simplifications of the true impacts of urban air pollution on existing COPD.
Conclusion
A comprehensive meta-analysis can help researchers recognize and understand inconsistencies among studies, especially where available studies report varying associations for the same exposure and health outcome. In this study, we found consistently positive associations between PM2.5, NO2, and SO2 and COPD-related morbidity and mortality. Although there was important variability among study results, they varied within a relatively narrow range. Excess risks were estimated at approximately 2–3% (per 10 ug/m3), regardless of pollutant, exposure metric, or COPD outcome. Looking specifically at COPD-related morbidity, 23 of the 25 individual effect estimates were positive and 70% had 95% CIs excluding the null (). This is a strong body of evidence for outdoor concentrations of particulates (PM2.5) and gaseous pollutants (NO2 and SO2) as important risk factors for COPD.
This study identified some of the key challenges associated with synthesizing diverse air pollution literature and the implications that certain study design decisions have on meta-analyses. More specifically, this study identified the sensitivity of these findings to the lag/averaging times for pollutants used in the air pollution–COPD literature. There are no agreed upon standards for how exposure data should be summarized for epidemiological studies. In the absence of strong biological evidence, it would be difficult to set such standards. In the meantime, researchers are urged to clearly define and present exposure–response estimates using several alternative exposure metrics so that meta-analysts can investigate the effects of alternative metrics, as we have reported here.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding
This research was supported in part by a grant from the U.S. National Institute for Environmental Health Sciences R21-ES017849 and a training grant from the U.S. National Institute for Occupational Safety and Health T01-OH008424.
References
- Global Initiative for Chronic Lung Disease (GOLD). Global Strategy for Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2015.
- World Health Organization. The Global Burden of Disease. 2004 Update; World Health Organization: Geneva, Switzerland. 2008.
- World Health Organization. Chronic Obstructive Pulmonary Disease (COPD). [Internet]. Available from: http://www.who.int/respiratory/copd/en/(Accessed on May 5, 2015).
- Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J 2006; 27:188–207.
- Burt L, Corbridge S. COPD Exacerbations. Evidence-based guidelines for identification, assessment, and management… second in a two-part series. Am J Nursing 2013; 113(2):34–45.
- Ko F, Hui D. Air pollution and chronic obstructive pulmonary disease. Respirology 2012; 17:395–401.
- Glass G. Primary, secondary and meta-analysis of research. Education Res 1975; 5(10):3–8.
- Zhu R, Chen Y, Wu S, Deng F, Liu Y, Yao W. The relationship between particulate matter (PM10) and hospitalizations and mortality of chronic obstructive pulmonary disease: A meta-analysis. COPD 2013; 10(3):307–315.
- Song Q, Christiani D, Wang X, Ren J. The global contribution of outdoor air pollution to the incidence, prevalence, mortality and hospital admission for chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int J Environ Res Public Health 2014; 11:11822–11832.
- Lai H, Tsang H, Wong C. Meta-analysis of adverse health effects due to air pollution in Chinese populations. BMC Public Health 2013; 13(1):1–12.
- Li MH, Fan LC, Mao B, Yang JW, Choi AM, Cao WJ, et al. Short term exposures to ambient fine particulate matter (PM2.5) increases hospitalizations and mortality of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Chest 2015; 149(2):447–458.
- Pai M, McCulloch M, Gorman JD, Pai N, Enanoria W, Kennedy G, et al. Systematic reviews and meta-analyses. Natl Med J India 2004; 17(2):86–95.
- National Library of Medicine (NLM). Fact Sheet: Medical Subject Headings (MeSH). [Internet]. 2013. Available from: http://www.nlm.nih.gov/pubs/factsheets/mesh.html.
- USEPA. Integrated Science Assessment for Oxides of Nitrogen - Health Criteria. EPA/600/R-08/071, National Center for Environmental Assessment – RTP Division, Research Triangle Park, NC. 2008. July.
- USEPA. Integrated Science Assessment for Sulfur Oxides - Health Criteria. EPA/600/R-08/047F, National Center for Environmental Assessment – RTP Division, Research Triangle Park, NC. 2008. September.
- USEPA. Integrated Science Assessment for Particulate Matter, EPA/600/R-08/139F. National Center for Environmental Assessment - RTP Division, Research Triangle Park, NC. 2009. December.
- Kan H, Chen B. Air pollution and daily mortality in Shanghai: a time-series study. Arch Environ Health 2003; 58(6):360–367.
- Kan H, Chen B. A case-crossover analysis of air pollution and daily mortality in Shanghai. J Occup Health 2003; 45(2):119–124.
- Moolgavkar SH. Air pollution and hospital admissions for chronic obstructive pulmonary disease in three metropolitan areas in the United States. Inhal Toxicol 2000; 12(4):75–90.
- Hapcioglu B, Issever H, Kocyigit E, Disci R, Vatansever S, Ozdilli K. The Effect of air pollution and metrological parameters on chronic obstructive pulmonary disease at an Istanbul hospital. Indoor Built Environ 2006; 15:147–153.
- Smith TJ, Kriebel D. A Biologic Approach to Environmental Assessment and Epidemiology. Oxford University Press, Oxford. 2010.
- Andersen HR, Spix C, Medina S, Schouten JP, Castellsague J, Rossi G, et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: Results from the APHEA project. Eur Respir J 1997; 10(5):1064–1071.
- Tenías JM, Ballester F, Pérez-Hoyos S, Rivera ML. Air pollution and hospital emergency room admissions for chronic obstructive pulmonary disease in Valencia, Spain. Arch Environ Health 2002; 57(1):41–47.
- Martins CL, Latorre Mdo R, Saldiva PH, Braga AL. Air pollution and emergency room visits due to chronic lower respiratory diseases in the elderly: An ecological time-series study in São Paulo, Brazil. J Occup Environ Med 2002; 44(7):622–627.
- Slaughter JC, Kim E, Sheppard L, Sullivan JH, Larson TV, Claiborn C. Association between particulate matter and emergency room visits, hospital admissions and mortality in Spokane, Washington. J Expo Anal Environ Epidemiol 2005; 15(2):153–159.
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. J Am Med 2006; 295(10)L:1127–1134.
- Stieb DM, Szyszkowicz M, Rowe BH, Leech JA. Air pollution and emergency department visits for cardiac and respiratory conditions: A multi-city time-series analysis. Environ Health 2009; 8(25).
- Meng X, Wang C, Cao D, Wong CH, Kan H. Short-term effect of ambient air pollution on COPD mortality in four Chinese cities. Atmos Environt 2013; 77:149–154.
- Samoli E, Stafoggia M, Rodopoulou S, Ostro B, Alessandrini E, Basagana X, et al. Which specific causes of death are associated with short term exposure to fine and coarse particles in Southern Europe? Results from the MED-PARTICLES project. Environ Inter 2014; 67:54–61.
- Lee IM, Tsai SS, Chang CC, Ho CK, Yang CY. Air pollution and hospital admissions for chronic obstructive pulmonary disease in a tropical city: Kaohsiung, Taiwan. Inhal Toxicol 2007; 19(5):393–398.
- Tsai SS, Chang CC, Yang CY. Fine particulate air pollution and hospital admissions for chronic obstructive pulmonary disease: a case-crossover study in Taipei. Int J Environ Res Public Health 2013; 10(1):6015–6026.
- Halonen JI, Lanki T, Yli-Tuomi T, Kulmala M, Tiittanen P, Pekkanen J. Urban air pollution, and asthma and COPD hospital emergency room visits. Thorax 2008; 63(7):635–641.
- Fischer P, Hoek G, Brunekreef B, Verhoeff A, van Wijnen J. Air pollution and mortality in the Netherlands: are the elderly more at risk? Eur Respir J Suppl 2003; 40:34S–38S.
- Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to Meta-Analysis, West Sussex, UK: John and Wiley Sons, Ltd., 2009.
- Schriger D, Altman D, Vetter JA, Heafner T, Moher D. Forest plots in reports of systematic reviews: a cross-sectional study reviewing current practice. Int J Epidemiol 2010; 39(2): 421–429.
- Huedo-Medina T, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? CHIP Documents. Paper 19. 2006. [Internet]. Available at: http://digitalcommons.uconn.edu/chip_docs_19.
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statist Med 2002; 21(11):1539–1558.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. John Wiley and Sons. 2008.
- Arbex MA, de Souza Conceicao GM, Cendon SP, Arbex FF, Lopes AC, Moyses EP, et al. Urban air pollution and chronic obstructive pulmonary disease-related emergency department visits. J Epidemiol Community Health 2009; 63(1):777–783.
- Belleudi V, Faustini A, Stafoggia M, Cattani G, Marconi A, Perucci CA, et al. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology 2010; 21(3):414–423.
- Chen Y, Yang Q, Krewski D, Shi Y, Burnett RT, McGrail K. Influence of relatively low levels of particulate air pollution on hospitalization for COPD in elderly people. Inhal Toxicol 2004; 16(1):21–25.
- Cirera L, Garcia-Marcos L, Gimenez J, Moreno-Grau S, Tobia A, Perez-Frenandez V, et al. Daily effects of air pollutants and pollen types on asthma and COPD hospital emergency visits in the industrial and Mediterranean Spanish city of Cartagena. Allergol Immunopathol 2012; 40(4):231–237.
- Faustini A, Stafoggia M, Cappai G, Forastiere F. Short-term effects of air pollution in a cohort of patients with chronic obstructive pulmonary disease. Epidemiology 2012; 23(6):861–879.
- Faustini A, Stafoggia M, Colais P, Berti G, Bisanti L, Cadum E, et al. Air pollution and multiple acute respiratory outcomes. Eur Respir J 2013; 42(2):304–313.
- Fusco D, Forastiere F, Michelozzi P, Spadea T, Ostro B, Arca M, et al. Air pollution and hospital admissions for respiratory conditions in Rome, Italy. Eur Respir J 2001; 17(6):1143–1150.
- Garcia-Aymerich J, Tobías A, Anto JM, Sunyer J. Air pollution and mortality in a cohort of patients with chronic obstructive pulmonary disease: A time series analysis. J Epidemiol Community Health 2000; 54(1):73–74.
- Janssen NA, Fischer P, Marra M, Ameling C, Cassee FR. Short-term effects of PM2.5, PM10 and PM2.5–10 on daily mortality in The Netherlands. Sci Total Environ 2013; 463–464:20–26.
- Kloog I, Nordio F, Zanobetti A, Coull BA, Koutrakis P, Schwartz JD. Short term effects of particle exposure on hospital admissions in the Mid-Atlantic states: a population estimate. PLoS One 2014; 9(2).
- Ko FW, TamW Wong TW, Chan D, Tung Al, Lai C, et al. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax 2007; 62(9):780–785.
- Milurinovic S, Nikic D, Stosic L, Stankovic A, Bogdanovic D. Short-term association between air pollution and emergency room admissions for chronic obstructive pulmonary disease in Nis, Serbia. Cent Eur J Public Health 2009; 17(1):8–13.
- Morgan G, Corbett S, Wlodarczyk J. Air pollution and hospital admissions in Sydney, Australia, 1990 to 1994. Am J Public Health 1998; 88(12):1761–1766.
- Neuberger A, Rabczenko D, Moshammer H. Extended effects of air pollution on cardiopulmonary mortality in Vienna. Atmos Environ 2007; 41(38):8549–8556.
- Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, et al. Ambient air pollution and respiratory emergency department visits. Epidemiology 2005; 16(2):164–174.
- Qiu H, Yu IT, Tian L, Wang X, Tse LA, Tam W, et al. Effects of coarse particulate matter on emergency hospital admissions for respiratory diseases: a time-series analysis in Hong Kong. Environ Health Perspect 2012; 120(4):572–576.
- Santus P, RussoA Madonini E, Allegra L, Blasi F, Centanni S, et al. How air pollution influences clinical management of respiratory diseases. A case-crossover study in Milan. Respir Res 2012; 13:95.
- Sauerzapf V, Jones AP, Cross J. Environmental factors and hospitalization for chronic obstructive pulmonary disease in a rural county of England. J Epidemiol Community Health 2009; 63(4):324–328.
- Sunyer J, Basagaña X. Particles, and not gases, are associated with the risk of death in patients with chronic obstructive pulmonary disease. Int J Epidemiol 2001; 30(5):1138–1140.
- Tao Y, Mi S, Zhou S, Wang S, Xie X. Air pollution and hospital admissions for respiratory diseases in Lanzhou, China. Environ Pollut 2014; 185:196–201.
- To T, Feldman L, Simatovic J, Gershon A, Dell S, Su J, et al. Health risk of air pollution on people living with major chronic diseases: a Canadian population-based study. BMJ Open 2015; 5(9):e009075–e009075.
- Valdez A, Zanobetti A, Halonen J, Cifuentes L, Morata D, Schwartz J. Elemental concentrations of ambient particles and cause specific mortality in Santiago, Chili: a time series study. Environ Health 2012; 11(82).
- Wong T, Ram W, Yu T, Wong A. Associations between daily mortalities from respiratory and cardiovascular disease and air pollution in Hong Kong, China. Occup Environ Med 2002; 59(1):30–35.
- Yang Q, Chen Y, Krewski D, Burnett RT, Shi Y, McGrail KM. Effect of short-term exposure to low levels of gaseous pollutants on chronic obstructive pulmonary disease hospitalizations. Environ Res 2005; 99(1):99–105.
- Whitaker H, Hocine M, Farrington P. On case-crossover methods for environmental time series data. Environmetrics 2006; 18(2):157–171.
- Devalia JL, Rusznak C, Herdman MJ, Trigg CJ, Tarraf H, Davies RJ. Effect of nitrogen dioxide and sulfur dioxide on airway response of mild asthmatic patients to allergen inhalation. Lancet 1994; 344(8938):1668–1671.
- Bell M, Zanobetti A, Domincini F. Evidence on the vulnerability and susceptibility to health risks associated with short-term exposure to particulate matter: a systematic review and meta-analysis. Am J Epidemiol 2013; 178(6):865–876.
- Winquist A, Klein M, Tolbert P, Flanders WD, Hess J, Sarnat SE. Comparison of emergency department and hospital admissions data for air pollution time-series studies. Environ Health 2012; 11(70).