Abstract
A series of Mn(II), Fe(III) and Zn(II) complexes have been synthesized with Schiff bases derived from isatin and 3-substituted-4-amino-5-mercapto-1,2,4-triazole. The elemental, spectroscopic (Infrared, nuclear magnetic resonance, ultraviolet-visible, fast atom bombardment-mass, fluorescence and electrochemistry) and magnetic studies suggested that the metal complexes possess octahedral geometry. The Schiff bases and their metal complexes exhibit fluorescent properties. The antimicrobial studies of Schiff bases and their metal complexes against various bacterial (Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus subtilis) and fungal (Aspergillus niger, and Penicillium chrysogenum) species by the minimum inhibitory concentration method revealed that the metal complexes possess more healing antibacterial activities than the Schiff bases. DNA cleavage property of Mn(II), Fe(III) and Zn(II) complexes revealed the important role of metal ion in the biological system.
1. Introduction
Isatin (1H-indole-2,3-dione) and its derivatives possess wide spectrum of biological activities [Citation1–6] and are extensively used as a versatile reagent in organic synthesis [Citation7–10]. Antibacterial and antifungal activities of Schiff and mannich bases derived from isatin and thiolyl-thiosemicarbazide have also been described [Citation5]. The various spectral studies of Co(II), Ni(II) and Cu(II) complexes of Schiff bases derived from isatin, 1-methylisatin and N(4)-substituted thiosemicarbazide have been reported [Citation11,Citation12]. Copper(II), chromium(III), manganese(II), cobalt(II), nickel(II), zinc(II), cadmium(II) and lead(II) chelates with tetradentate Schiff base of isatin and o-phenylenediamine were isolated [Citation13]. Also, chemistry of 1,2,4-triazole derivatives [Citation14,Citation15] and metal complexes of 1,2,4-triazole Schiff bases has been the subject of considerable interest [Citation16–19].
We have reported the synthesis, characterization and biological studies of Co(II), Ni(II) and Cu(II) complexes with Schiff bases derived from 3-substituted-4-amino-5-mercapto-1,2,4-triazole and isatin [Citation20]. Thus, in continuation with our earlier work we report here the synthesis and characterization of a new series of Mn(II), Fe(III) and Zn(II) metal complexes of these Schiff bases. The electron transfer process of the metal complexes is investigated. In the light of wide spectrum of biological activity, the Schiff bases and their metal complexes were screened for their in vitro antibacterial, antifungal and also for their DNA cleavage activity.
2. Experimental
2.1 Physical measurements
All the chemicals used were of reagent grade. Carbon, hydrogen and nitrogen were estimated by using Elemental Analyzer Carlo Erba EA1108 analyzer. The infra red (IR) spectra of the metal complexes were recorded on a HITACHI-270 IR spectrophotometer in the 4000–350 cm−1 region. The electronic spectra of the complexes were recorded at room temperature in high pressure liquid chromatography (HPLC)-grade dimethyl formamide (DMF) and dimethyl sulphoxide (DMSO) solvent on a VARIAN CARY 50-BIO UV-spectrophotometer in the region of 200–1100 nm. The 1H-nuclear magnetic resonance (NMR) spectra of Zn(II) complexes were recorded in d6-DMSO on a BRUKER 300 MHz spectrometer at room temperature using tetramethylsilane as an internal reference. Fast atom bombardment (FAB)-mass spectra were recorded on a JEOL SX 102/DA-6000 mass spectrometer/data system using Argon/Xenon (6 kV, 10 A) as the FAB gas. The accelerating voltage was 10 kV and the spectra were recorded at room temperature and m-nitrobenzyl alcohol was used as the matrix. The mass spectrometer was operated in the positive ion mode. The fluorescence studies of Schiff bases and their metal complexes were recorded on a HITACHI F-7000 Fluorescence Spectrophotometer (made in Japan). The solutions of 10−3 M concentration were prepared in HPLC-grade DMSO solvent and the experiment was carried out at room temperature. Electrochemical behavior of the Mn(II) and Fe(III) complexes was studied on a CHI1110A-Electrochemical analyzer (made in USA) in HPLC-grade DMF containing 0.05 M n-Bu4NClO4 as the supporting electrolyte. The three-electrode system consisted of glassy carbon (working), platinum wire (counter) and Ag/AgCl (reference) electrodes. Molar conductivity measurements were recorded on ELICO-CM-82 T conductivity bridge with a cell having cell constant 0.51 and magnetic moment was carried out by using Faraday balance.
2.2 Synthesis
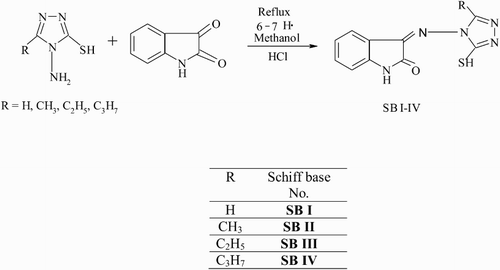
3-Substituted-4-amino-5-mercapto-1,2,4-triazole was synthesized according to the literature [Citation21–23]. Isatin was recrystallized before using. The Schiff bases have been synthesized according to the reported method [Citation20] which is schematically presented in .
2.2.1 Synthesis of Mn(II), Fe(III) and Zn(II) complexes [Citation1–12]
Methanolic solution (35 mL) of Schiff base (2 mmol) was mixed with methanolic solution (15 mL) of (1 mmol). Then, to the reaction mixture 2 mmol of sodium acetate was added and refluxed for 3 h. The separated complex was filtered, washed thoroughly with water, methanol and dried in vacuum over fused CaCl2. Yield of all the metal complexes lie in the range of 71–73%.
2.3 Biological studies
2.3.1 In vitro antibacterial and antifungal studies
The biological activities of synthesized Schiff bases and their Mn(II), Fe(III) and Zn(II) complexes have been studied for their antibacterial and antifungal activities by the agar and potato dextrose agar diffusion method, respectively. The antibacterial and antifungal activities were done at 100, 200 and 500 μg mL−1 concentrations in DMF solvent by using four bacteria (Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus subtilis) and two fungi (Aspergillus niger and Penicillium chrysogenum) strains by the minimum inhibitory concentration (MIC) method [Citation24]. These bacterial strains were incubated for 24 h at 37°C and fungi strains were incubated for 48 h at 37°C. Standard antibacterial (Streptomycin) and antifungal drugs (Nyastatin) were used for comparison under similar conditions. Activity was determined by measuring the diameter of the zone showing complete inhibition (mm).
2.3.2 DNA cleavage experiment
2.3.2.1. Preparation of culture media
Nutrient broth [peptone, 10; Yeast extract, 5; NaCl, 10; in (g L−1)] was used for culturing of S. aureus and potato dextrose broth [potato, 250; dextrose, 20; in (g L−1)] was used for the culture of A. niger. The 50 mL media were prepared and autoclaved for 15 min at 121°C under 15 lb pressure. The autoclaved media were inoculated with the seed culture. Staphylococcus aureus was incubated for 24 h and A. niger for 48 h at 37°C.
2.3.2.2. Isolation of DNA
The fresh bacterial culture (1.5 mL) is centrifuged to obtain the pellet which is then dissolved in 0.5 mL of lysis buffer (100 mM tris pH 8.0, 50 mM EDTA, 10% SDS). To this 0.5 mL of saturated phenol was added and incubated at 55°C for 10 min. Then centrifuged at 10,000 rpm for 10 min and to the supernatant, equal volume of chloroform: isoamyl alcohol (24: 1) and 1/20th volume of 3 M sodium acetate (pH 4.8) was added. Then, centrifuged at 10,000 rpm for 10 min and to the supernatant, three volumes of chilled absolute alcohol were added. The precipitated DNA was separated by centrifugation and the pellet was dried and dissolved in tris acetate EDTA (TAE) buffer (10 mM tris pH 8.0, 1 mM EDTA) and stored in cold condition.
2.3.2.3. Agarose gel electrophoresis
Cleavage products were analyzed by the agarose gel electrophoresis method. Test samples (1 mg mL−1) were prepared in DMF. The samples (25 μg) were added to the isolated DNA of S. aureus and A. niger. The samples were incubated for 2 h at 37°C and then 20 μL of DNA sample (mixed with bromophenol blue dye at 1: 1 ratio) was loaded carefully into the electrophoresis chamber wells along with standard DNA marker containing TAE buffer (4.84 g Tris base, pH 8.0, 0.5 M EDTA/1 L) and finally loaded on agarose gel and passed the constant 50 V of electricity for around 30 min. The gel was removed and stained with 10.0 μg mL−1 ethidium bromide for 10–15 min and the bands observed under the vilberlourmate gel documentation system and photographed to determine the extent of DNA cleavage. Then the results are compared with standard DNA marker.
2.4 Anthelmintic activity (in vitro)
Anthelmintic assay was done by the method given in the literature with minor modifications [Citation25,Citation26]. The assay was performed on adult Indian earthworms (Pheretima posthuma), due to its anatomical and physiological resemblance with the intestinal roundworm parasite of human beings. Earthworms (P. posthuma), collected from moist soil and washed with normal saline to remove all fecal matter, were used for the anthelmintic study. The earthworms of 3–4 cm in length and 0.1–0.2 cm in width were used for the experimental protocol. The compounds were subjected to anthelmintic activity studies against earthworms at 2 and 10 mg mL−1 by using Albendazole as a standard drug. The paralyzing and death times were noted and their mean was calculated for triplicate sets. The death time was ascertained by placing the earthworms in warm water (50°C) which stimulated the movement, if the worm was alive.
3. Results and discussion
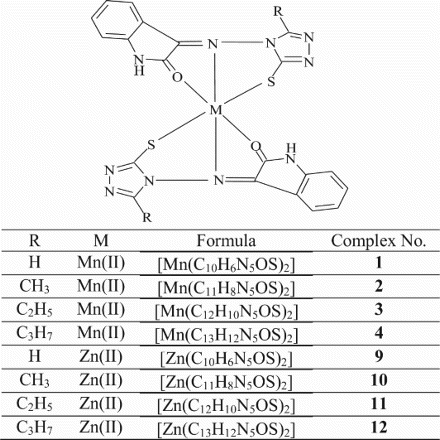
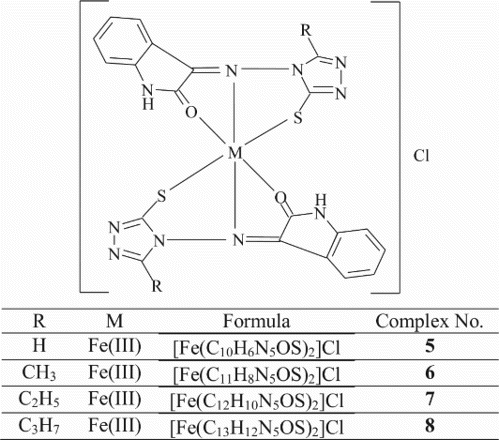
All the Mn(II), Fe(III) and Zn(II) complexes are stable and non-hygroscopic in nature. The complexes are insoluble in common organic solvents but soluble in DMF and DMSO. The elemental analyses show that the Mn(II) and Zn(II) complexes have 1: 2 stoichiometry of the ML2 type where as Fe(III) complexes possess 1: 2 stoichiometry of the type [ML2]Cl; L stands for a deprotonated ligand and exhibits thiol-thione tautomerism. The molar conductance values of Mn(II) and Zn(II) complexes are too low to account for any dissociation of the complexes in DMF, indicating the non-electrolytic nature; in case of Fe(III) complexes, high molar conductance values suggest that the complexes are electrolytic in nature ().
Table 1. Elemental analyses, molar conductance and magnetic moment data of metal complexes.
3.1 IR spectral studies
The prominent IR frequencies of the metal complexes are presented in . In comparison with the spectra of the Schiff bases, all the Mn(II), Fe(III) and Zn(II) metal complexes exhibited the band of ν(C˭O) in the region 1724–1714 cm−1 showing the shift to lower wave numbers indicating that the carbonyl oxygen is coordinated to the metal ion. This is due to the transfer of charge from oxygen to metal(II/III) center. Murukan et al. [Citation27] have reported the similar behavior of the carbonyl oxygen of isatin. The characteristic band in the region 1596–1603 cm−1 of ν (C˭N) showing the shift to lower wave number indicates that the nitrogen is coordinated to the metal ion. The absence of a band around ∼2700 cm−1 in all the metal complexes indicates deprotonation of the thiol group suggesting that the metal ion is coordinated through the sulfur atom, which is further supported by a band around ∼750 cm−1 in the metal complexes due to ν(C‒S). Thus, the new bands in the region of 480–456 and 371–351 cm−1 in the spectra of the complexes are assigned to stretching frequencies of (M‒N) and (M‒S) bond formation, respectively [Citation28]. The unaltered position of a band due to ν(NH) in all the metal complexes compared with Schiff bases indicates that the nitrogen atom of isatin is not involved in coordination.
Table 2. The important infrared frequencies (in cm−1) of metal complexes.
3.2 NMR studies
The spectral data of 1H and 13C NMR of all the Schiff bases are given in . In the 1H NMR spectrum of the Schiff base-II, the signal at 11.7 ppm (s, 1H, NH) is assigned to NH proton of isatin and another signal observed at 10.4 ppm (s, 1H, SH) [Citation20] is due to S‒H proton. In addition to this, the multiplet signals around 6.3–7.2 ppm (m, 4H, Ar-H) are ascribed to aromatic protons. The singlet observed at 2.7 ppm (s, 3H, CH3) is due to CH3 group. 13C NMR spectrum of Schiff base-II exhibited signal 139.5 and 162 ppm assigned to (C˭N) and (C˭O), respectively. Isatin and triazole moieties exhibited peaks in the region 155.2–131.1 and 185–172, respectively.
Table 3. The important 1H NMR and 13C NMR data of Schiff bases.
3.3 Electronic spectral and magnetic studies
Electronic spectra of all the Mn(II) complexes display weak absorption bands around 18575 (ν1), 23642 (ν2), 27716 (ν3) and 38872 cm−1 (ν4) characteristic of octahedral geometry corresponding to ,
(4D),
,
transitions, respectively. The complexes showed the magnetic moment value in the range of 5.7–5.9 BM which is within the range of octahedral geometry for Mn(II) complexes.
The electronic spectra of the Fe(III) complexes showed strong bands around 17,932 and 13,122 cm−1. It was not possible to identify the type of the d–d transition. This is due to a strong charge-transfer (CT) band tailing from the UV-region to the visible region. The magnetic moment of the Fe(III) complex was measured and the value lies in the range of 4.98–5.21 BM, which is lower than the magnetic moment of the high-spin octahedral arrangement which amounts close to 5.92 BM On the other hand, the experimental value 4.98–5.21 BM is too far higher than the low-spin octahedral,
arrangement, where its magnetic moment value is 2.3 BM Generally, a tentative interpretation expects the structure of Fe(III) to be an octahedral geometry [Citation29].
The electronic spectra of the Zn(II) complexes exhibited only a high-intensity band in the region 28,275–29,380 cm−1 and are assigned to a ligand → metal CT. The Zn(II) complexes are diamagnetic.
3.4 FAB-mass spectral studies
The discussion of FAB-mass spectra of the Schiff base-III, IV and Mn(II) complex (3) (figures S1, S2 and S3, respectively) are given in the supplementary file.
3.5 Fluorescence spectroscopy
The emission spectra of the Schiff bases and its complexes were studied in DMSO. The emission spectra of Schiff base SB II and its Mn(II) (2), Fe(III) (6) and Zn(II) (10) complexes are discussed here.
The SB II exhibits an excitation maximum wavelength at 343 nm which decreases and shifts to blue region upon complex formation with Mn(II) and Fe(III) complexes. This shift is 24 nm for Mn(II) and 7 nm for Fe(III) complexes. But maximum excitation wavelength in the case of Zn(II) complex of SB II was red shifted.
The SB II is characterized by an emission maximum at 406 nm which is shifted upon binding to metal ions. The emission spectra of the Mn(II), Fe(III) and Zn(II) complexes are characterized by an emission band around 470, 410 and 461 nm in DMSO. The red shift of the value of the complexes compared with the Schiff base may be due to the deprotonation of the thiol group. The fluorescence emission intensity of the SB II increases depending on the complex formation with metal ions. This indicates that the Schiff base and its complexes are fluorescent in nature.
3.6 Electrochemistry
The electrochemical properties of metal complexes, particularly those with sulfur donor atoms, have been studied in order to monitor spectral and structural changes accompanying electron transfer [Citation30].
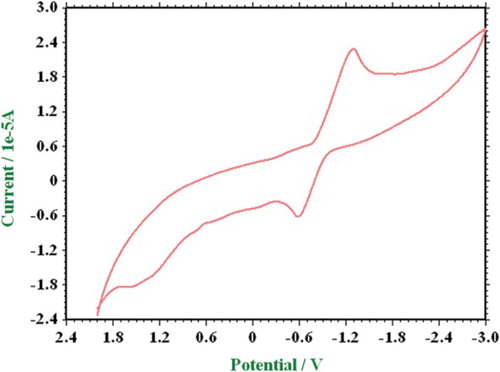
The redox property of the Mn(II) complex was studied in the potential range of +2.4 to −3.0 V. Cyclic voltammogram of Mn(II) (2) complex is shown in . The Mn(II) complex is electroactive with respect to the metal center and exhibited one redox process associated with a single-electron transfer at room temperature [Citation31,Citation32]. The cathodic and anodic peak heights are equal, and vary as the square root of the scan rate; Epc and Epa are virtually independent of the scan rate. A well-defined quasi-reversible one-electron cyclic response was observed at V with a corresponding oxidation peak at
V at a scan rate of 50 mV s−1. The electrochemical behavior shows moderately high reduction potentials.
value for the redox couple is 0.6766 V.
value is higher for the complex due to the difference between the original complex and the reduced species.
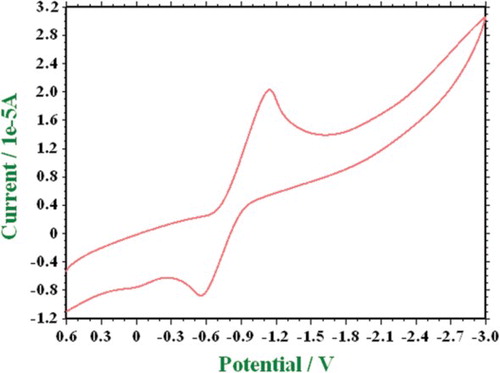
The representative cyclic voltammogram of Fe(III) (6) complex is shown in . A cyclic voltammogram of Fe(III) displays one reduction peak, at V with an associated oxidation peak at
V corresponding to the Fe(III)/Fe(II) couple at a scan rate of 0.05 V s−1. The value of
is 0.5192 and increases with an increase in scan rate giving evidence for quasi-reversible nature associated with one electron reduction.
4. Biological studies
4.1 In vitro antibacterial and antifungal studies
The Co(II) and Cu(II) complexes of these Schiff bases exhibited good antibacterial activity whereas Ni(II) complexes were found to be inactive [Citation33]. In the present case, all the Mn(II), Fe(III)and Zn(II) complexes had shown high antibacterial activity against S. aureus, B. subtilis and P. auregenosa (). In particular complexes 1, 2, 3, 6, 7, 9, 10, 11 and 12 showed higher activity than the respective Schiff bases. Thus, the Mn(II), Fe(III) and Zn(II) complexes show good selectivity over Co(II), Ni(II) and Cu(II) complexes. The higher activity of the metal complexes compared with Schiff bases may be due to the change in structure due to coordination, which make the metal complexes act as more powerful and potent bactereostatic agents, thus inhibiting the growth of the microorganisms [Citation34–36]. In case of antifungal activity, the Mn(II), Fe(III) and Zn(II) complexes were found to be inactive or moderately active. Thus the results revealed that the newly synthesized compounds possess high antibacterial activity than the antifungal nature.
Table 4. Antimicrobial results of metal complexes.
The MIC of some selected compounds, which showed significant activity against selected bacterial and fungi species, were determined. The MIC results of these compounds indicated that these compounds were the most active in inhibiting the growth of the tested organisms at a 10 μg mL−1 concentration.
4.2 Anthelmintic activity studies
The anthelmintic activity of the Schiff bases and their metal complexes was tested on earthwarms (P. posthuma). The Schiff bases exhibited high activity which increased upon complexation with Mn(II), Fe(III) and Zn(II) ions (). The results showed that the metal complexes exhibited higher anthelmintic activity compared with Schiff bases. Among the Mn(II), Fe(III) and Zn(II) complexes, the Mn(II) complexes showed more activity.
Table 5. Anthelmintic activity results of metal complexes.
4.3 DNA cleavage studies
The representative Mn(II) (2), Fe(III) (6) and Zn(II) (10) complexes were studied for their DNA cleavage activity by the agarose gel electrophoresis method.
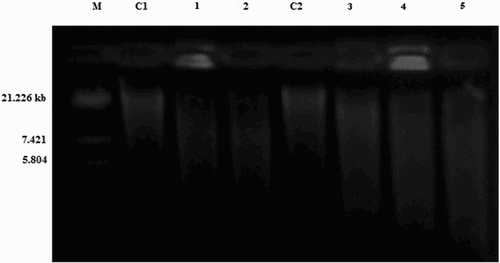
The results of oxidative DNA cleavage experiments are presented in . Control experiments clearly revealed that untreated DNA does not show any cleavage (; Lane C1 and C2), whereas all the metal complexes have exhibited cleavage activity on DNA. The difference in the migration was observed in Lanes 1 and 2 of Mn(II) and Zn(II) complexes, respectively, compared with the control DNA, Lane C1 of S. aureus. Similarly in case of Lanes 3, 4 and 5 of Fe(III), Mn(II) and Zn(II) complexes, respectively, the migration was observed, whereas the untreated control DNA, Lane C2 of A. niger has not shown migration. This shows that the untreated control DNA alone does not show any apparent cleavage, whereas Mn(II), Fe(III) and Zn(II) complexes show. However, the nature of reactive intermediates involved in the DNA cleavage by the complexes has not been clear. The results indicated the important role of metal ions in these isolated DNA cleavage reactions. From these results we can infer that the Mn(II), Fe(III) and Zn(II) complexes act as a potent nuclease agent. As the compound was observed to cleave the DNA, it can be concluded that the compound inhibits the growth of the pathogenic organism by cleaving the genome.
Figure 3. DNA cleavage of Mn(II) (2), Fe(III) (6) and Zn(II) (10) complexes. M, standard molecular weight marker; C1, control DNA of S. aureus; Lane 1, S. aureus DNA treated with Mn(II); Lane 2, S. aureus DNA treated with Zn(II); C2, control DNA of A. niger; Lane 3, A. niger DNA treated with Fe(III); Lane 4, A. niger DNA treated with Mn(II); Lane 5, A. niger DNA treated with Zn(II).
5. Conclusion
In this study, the Mn(II), Fe(III) and Zn(II) complexes with isatin–triazole Schiff bases were synthesized. On the basis of spectroscopic techniques, octahedral geometry ( and ) for all the complexes have been proposed wherein the Schiff bases act as versatile tridentate chelating agents. The electrochemical studies of the Mn(II) and Fe(III) metal complexes investigated in DMF revealed the most significant redox process of one electron transfer. In vitro antibacterial and antifungal activity studies against representative bacterial and fungal strains suggest that the metal complexes possess higher antimicrobial activity than the Schiff bases and metal complexes possess high antibacterial activity than the antifungal activity. The activity is high particularly against S. aureus, B. subtilis and P. auregenosa. Mn(II) and Zn(II) complexes exhibited the DNA cleavage property against the DNA isolated from the S. aureus bacterial species whereas the Mn(II), Fe(III) and Zn(II) complexes cleave the DNA isolated from A. niger. Among the Mn(II), Fe(III) and Zn(II) complexes, the complexes of Mn(II) showed more antheminitic activity and the remaining Fe(III), Zn(II) complexes and Schiff bases are less active. Thus, the biological studies revealed the important role of metal ions in the biological systems.
Supplementary data
Supplemental data for this article can be accessed doi:10.1080/2164232X.2014.884939
Supplementary Material
Download MS Word (975.5 KB)Acknowledgements
The authors sincerely express their thanks to research Supervisor Dr S.A. Patil, Senior Professor, Department of Chemistry, Karnataka University, Dharwad, Karnataka for his moral support and encouragement. Also, thank to Management of CMRJT, Principal & Dr B. Narashimhamurty, CMRIT, Bangalore, Karnataka, India. Special thanks to Prof. Ragunathareddy, Head, Department of Toxicology, BIONEEDS Laboratory, Tumakur, Karnataka, for his help in Biological experiments.
References
- R.W. Daisley, V.K. Shah. J. Pharm. Sci., 73, 407 (1984). doi: 10.1002/jps.2600730333
- S.N. Pandeya, D. Sriram, E.D.E. Clercq, C. Pannecouque, M. Witvrouw. Indian J. Pharm. Sci., 60, 207 (1998).
- S.N. Pandeya, J.R. Dimmock. Pharmazie, 48, 659 (1993).
- C. Giselle, A.M.C. Ferreira. J. Braz. Chem. Soc., 17, 1473 (2006). doi: 10.1590/S0103-50532006000800003
- S.N. Pandeya, D. Sriram, G. Nath, E. De Clercq. Eur. J. Pharm. Sci., 9, 25 (1999). doi: 10.1016/S0928-0987(99)00038-X
- S.K. Sridhar, S.N. Pandeya, J.P. Stables, A. Ramesh. Eur. J. Pharm. Sci., 16, 129 (2002). doi: 10.1016/S0928-0987(02)00077-5
- B. Jiang, J.M. Smallheer, G. Amaral-Ly, M.A. Wuonola. J. Org. Chem., 59, 6823 (1994). doi: 10.1021/jo00101a051
- J.C. Torres, S.J. Garden, A.C. Pinto, F.S.Q. da Silva, N. Boechat. Tetrahedron, 55, 1881 (1999). doi: 10.1016/S0040-4020(98)01229-0
- J. Bergman, C. Stalhandske. Tetrahedron Lett., 35, 5279 (1994). doi: 10.1016/S0040-4039(00)77084-5
- D.A. Walsh, H.W. Moran, D.A. Shamblee, W.J. WelsteadJr., J.C. Nolan, L.F. Sancilio, G. Graff. J. Med. Chem., 33, 2296 (1990). doi: 10.1021/jm00170a039
- D.X. West, A.K. El-Sawaf, G.A. Bain. Trans. Met. Chem., 23, 1 (1997). doi: 10.1023/A:1006901328527
- G.A. Bain, D.X. West, J. Krejci, J. Valdes-Martínez, S. Hernandez-Ortega, R.A. Toscano. Polyhedron, 16, 855 (1997). doi: 10.1016/S0277-5387(96)00323-3
- A.M.A. Hassaan, M.A. Khalifa. Monatsch. Chem., 124, 803 (1993). doi: 10.1007/BF00816402
- S. Sun, H. Lou, Y. Gao, P. Fan, B. Ma, W. Ge, X. Wang. J. Pharm. Biomed. Anal., 34, 1117 (2004). doi: 10.1016/j.jpba.2003.11.013
- V. Geert, S. Karel, M. Guy Van den, B. Lieven, P. Jef, E.B. Marcus. Int. J. Pharm., 251, 165 (2003).
- A.N. Heleen, G.H. Jaap, H. Ronald, R. Jan, L.S. Theo, J.S. Derk, G.V. Johannes. Inorg. Chem., 30, 48 (1991). doi: 10.1021/ic00001a010
- J.P. Cornelissen, J.H. Van Diemen, L.R. Groeneveld, J.G. Haasnoot, A.L. Spek, J. Reedijk. Inorg. Chem., 31, 198 (1992). doi: 10.1021/ic00028a014
- P.G. Avaji, P.S. Badami, S.A. Patil. J. Coord. Chem., 61, 1884 (2008). doi: 10.1080/00958970701771210
- L. Mishra, M.K. Said. Indian J. Chem., 35, 304 (1996).
- A.D. Kulkarni, S.A. Patil, P.S. Badami. J. Sulfur Chem., 30, 145 (2009). doi: 10.1080/17415990802663133
- K.S. Dhaka, J. Mohan, V.K. Chadha, H.K. Pujari. Ind. J. Chem., 12, 288 (1974).
- M.H. Palmer, C. Dines. J. Mol. Struct., 705, 177 (2004). doi: 10.1016/j.molstruc.2004.07.001
- S.A. Patil, B.M. Badiger, S.M. Kudari, V.H. Kulkarni. Trans. Met. Chem., 8, 238 (1983). doi: 10.1007/BF00620701
- A.K. Sadana, Y. Mirza, K.R. Aneja, O. Prakash. Eur. J. Med. Chem., 38, 533 (2003). doi: 10.1016/S0223-5234(03)00061-8
- L. C. Garg, C.K. Atal. Indian J Pharm. Sci., 59, 240 (1963).
- R.D. Vidyarthi. A Text Book of Zoology, 14th Edn, S. Chand and Co workers, New Delhi (1967).
- B. Murukan, B. Sindhu Kumari, M. Kochukittan. J. Coord. Chem., 60, 1607 (2007). doi: 10.1080/00958970601099167
- K. Nakamoto. Infrared Spectra of Inorganic and Coordination Compounds, Wiley-Interscience, New York (1970).
- J.R. Ferrero. Low-Frequency Vibrations of Inorganic and Coordination Compounds, John Wiley, New York (1971).
- F.A. Cotton, G. Wilkinson. Advanced Inorganic Chemistry, A Comprehensive Text, 4th Edn, John Wiley and Sons, New York (1986).
- A.M. Bond, R.L. Martin. Coord. Chem. Rev., 54, 23 (1984). doi: 10.1016/0010-8545(84)85017-1
- M. Donzello, D. Dini, G. Arcangelo, C. Ercolani, R. Zhan, Z. Ou, P.A. Stuzhiz, K.M. Kadish. J. Am. Chem. Soc., 125, 14190 (2003). doi: 10.1021/ja0344361
- B.W. Rossister, J.F. Hamilton. Physical Method of Chemisty, 2nd Edn, Wiley, New York (1985).
- A. Kulkarni, P.G. Avaji, G.B. Bagihalli, P.S. Badami, S.A. Patil. J. Coord. Chem., 62, 481 (2009). doi: 10.1080/00958970802226387
- G.B. Bagihalli, P.S. Badami, S.A. Patil. J. Enz. Med. Chem., 24, 381 (2009). doi: 10.1080/14756360802187901
- Z.H. Chohan, H. Pervez, A. Rauf, K.M. Khan, C.T. Supuran. J. Enz. Inhib. Med. Chem., 19, 417 (2004). doi: 10.1080/14756360410001710383