ABSTRACT
Carbon aerogels are versatile materials for value-added applications such as supercapacitors, insulation materials, and super absorbers. In this work, a way to produce carbon aerogels from kraft lignin which is an abundant waste product from the pulp and paper industry is presented. This was achieved by forming hydrogels of lignin and formaldehyde which were then subjected to solvent exchange and extraction with supercritical CO2. The resulting aerogels were converted into carbon aerogels by means of pyrolysis. The characterization was performed by gas adsorption and scanning electron microscopy that showed highly porous carbon aerogels with a specific surface area up to almost 600 m2 g−1 which is relevant for technical applications.
Introduction
There is a growing interest in substituting products obtained from fossil fuels, as these materials are running out and associated with the release of greenhouse gases. Especially, lignin is a promising feedstock material due to its abundant occurrence in nature and the fact that it is accumulating as a waste product during pulp and paper production [Citation1,Citation2].
Today most of the lignin is burnt for energy recovery rather than being utilized as material. This is a result of the irregularly macromolecular structure of lignin which comprises various functional groups. Depending on plant sources and pulping processes, large differences in the chemical and physical properties of lignin can occur. Nevertheless, lignin has been investigated in numerous studies as a precursor material for various chemicals and products [Citation3,Citation4]. In the field of polymer chemistry, several applications of lignin have been reported. Amongst them, lignin was either used as an additive or filler material for polymers or chemically functionalized for incorporation into composite materials and thermosets [Citation5,Citation6].
In particular, the production of value-added products from kraft lignin is a worthwhile approach, as this type of lignin originates from the most commonly used pulping process. Furthermore, kraft lignin contains sulfur which can cause problems during chemical processing. One way to utilize sulfurous lignin is the production of aerogels [Citation7,Citation8].
Aerogels are technically attractive materials since their characteristic properties are low density, high pore volume, and high specific surface area[Citation9]. That offers the potential for applications such as energy storage in form of supercapacitors [Citation10], superabsorbents [Citation11], thermal and acoustic insulation materials [Citation12], flame retardants [Citation13], and catalyst supports [Citation14].
The preparation of aerogels from lignin was derived from the synthesis of a resorcinol aerogel which is based on the polycondensation of resorcinol and formaldehyde. In this process, a sol-gel reaction is used to form a hydrogel in which crosslinking takes place to form pores that are filled with an aqueous liquid. The water phase is exchanged with an organic solvent, which is then extracted with supercritical CO2 resulting in an aerogel with gas filled with pores [Citation15,Citation16]. Finally, a carbonization procedure can be applied to increase the specific surface area in the resulting carbon aerogel[Citation17]. During carbonization, the oxygen and hydrogen content in the aerogel is reduced resulting in a highly pure carbon structure[Citation18].
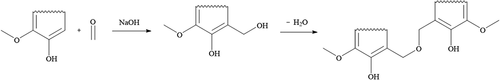
Since the chemical structure of lignin has a phenolic character, it is possible to substitute resorcinol with lignin for aerogel production. The polycondensation of lignin and formaldehyde is based on the chemical reaction between phenol and formaldehyde. One lignin molecule comprises several phenolic hydroxyl groups with unsubstituted ortho-positions where crosslinking via formaldehyde can take place[Citation15]. First, hydroxymethylated derivatives are formed by lignin and formaldehyde. These moieties then undergo a step-growth polymerization resulting in a network of lignin molecules connected by methoxy bridges. Because this work focuses on a softwood lignin containing largely guaiacyl units [Citation19,Citation20], the polycondensation reaction of these lignin components with formaldehyde is depicted in .
Although different attempts for aerogel production from lignin have been reported, these techniques either used additives like phenol [Citation19] and resorcinol [Citation15] or the resulting aerogels exhibited an insufficient specific surface area which is crucial for technical applications[Citation20].
In this study, the commercially available softwood kraft lignin “Indulin AT” was investigated for the production of highly porous carbon aerogels without any addition of phenolic compounds like phenol or resorcinol. The impact of the formaldehyde–lignin ratio on crucial parameters such as the specific surface area and pore size was examined via nitrogen gas adsorption.
Methods and materials
Materials
The softwood kraft lignin “Indulin AT” was purchased from MeadWestvaco, sodium hydroxide from ACROS ORGANICS, aqueous formaldehyde solution (37%) from Carl Roth and ethanol (absolute) from VWR. All reagents were used as received without any further purification.
Preparation of carbon aerogels
The preparation of each carbon aerogel started with the synthesis of a lignin–formaldehyde hydrogel. This was done by base-catalyzed sol-gel condensation reaction between lignin and formaldehyde. The procedure was developed on the basis of lignin-resorcinol-formaldehyde gels [Citation15] by adaption of proper reaction conditions. A lignin stock solution containing 10 wt% Indulin AT was prepared by dissolving the lignin in an aqueous sodium hydroxide solution at a pH of 11. For each hydrogel synthesis, the appropriate amount of lignin stock solution and formaldehyde solution was mixed together under stirring and the pH was adjusted to 11 by adding a 20 wt% sodium hydroxide solution. The mixture was diluted with deionized water to obtain a solution with a lignin-formaldehyde content of 15 wt%. The reaction mixtures were enclosed in glass tubes with screw caps and cured in a heating cabinet at 90°C for 5 days. The resulting hydrogels were cooled to room temperature and immersed in ethanol. Every 24 h the spent ethanol was replaced by a fresh one and repeated 3 times. After this ethanol exchange, the obtained gels were transferred in a high-pressure stainless steel vessel and extracted with supercritical CO2 for 2 h. Subsequently, the resulting aerogels were placed in ceramic containers and put into a high-temperature oven. Under a nitrogen flow of 150 L h−[Citation1], the samples were carbonized using a certain temperature program (heat from 20°C to 500°C at a rate of 1°C min−1; followed by an isothermal step of 500°C for 1 h; subsequently heat from 500°C to 900°C at a rate of 2°C min−1; followed by an isothermal step of 900°C for 2 h and finally, cool to room temperature as fast as possible). In this way, three carbon aerogels were made from solutions with varying formaldehyde-lignin mass ratios of 1, 2, and 3 and a lignin-formaldehyde content of 15 wt%.
Aerogel characterization
The specific surface area and pore size distribution of each aerogel and carbon aerogel was measured via nitrogen gas adsorption at −196°C using an Autosorb-iQ automatic volumetric sorption analyzer from Anton Paar GmbH. Before each measurement, the sample was degassed for 60 min at 110°C under a high vacuum. To determine the specific surface area the Brunauer-Emmet-Teller (BET) method was used. Pore size distribution was investigated with non-local density functional theory (NLDFT).
The morphology of the aerogels and carbon aerogels was analyzed with a Phenom Pro X scanning electron microscope. Before each analysis, the sample was sputtered with gold using an SC7620 Mini Sputter Coater from Quorum Technologies.
Thermogravimetric analysis of the aerogels was performed on a TGA 4000 thermogravimetric analyzer from PerkinElmer. Approximately 5 mg of sample material was heated from 40°C to 950°C at a heating rate of 20°C min−1 under a nitrogen flow of 20 mL min−1.
Results and discussion
Gel preparation
During the 5 days at 90°C, the lignin–formaldehyde reaction mixtures gelled within 48 h and a color change from black to brownish took place. The gelation time of the hydrogels made from pure lignin was 10 to 30 times longer compared to reported gelation times for gels with additional resorcinol. This results from a low reactivity of lignin, as its phenolic groups are sterically hindered [Citation15,Citation19]. After ethanol exchange and drying with supercritical CO2, the resulting aerogels showed a shrinkage of about 20% and the color changed to pale brown. Carbonization of the gels results in black carbon aerogels with approximately 30% shrinkage. In , the lignin–formaldehyde hydrogel (left), aerogel (middle), and carbon aerogel (right) based on a formaldehyde–lignin ratio of 1 (F/L 1) are shown.
Specific surface area
For each gel preparation, the formaldehyde–lignin mass ratio (F/L) of the initial reaction mixture and specific BET surface area of the resulting aerogel and carbon aerogel are depicted in and .
Figure 3. Correlation of initial formaldehyde–lignin mass ratio (F/L) and the specific BET surface area (SBET) for the resulting aerogels
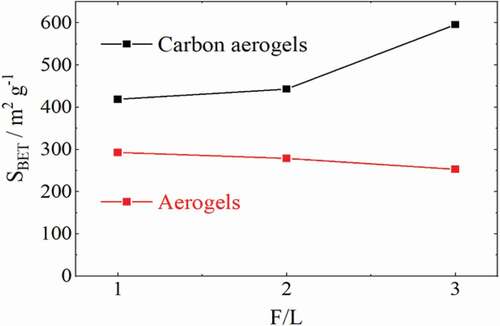
Table 1. Formaldehyde-lignin mass ratio (F/L) of each reaction mixture and specific BET surface area (SBET) of the respective aerogel and carbon aerogel
The obtained values from the BET surface measurements range from 293 to 596 m2 g−1 and indicate a high porosity of the prepared (carbon) aerogels. The porous structure results from a polycondensation reaction of lignin and formaldehyde which causes a network of connected particles with gaps between them. As depicted in , a higher formaldehyde–lignin ratio in the initial mixture leads to a higher specific surface area in the resulting carbon aerogel, while in the aerogel before carbonization it results in slightly lower values. This contradiction might be caused by compounds formed by formaldehyde during hydrogel synthesis that accumulates within the porous structure and initially remain in the aerogel. In the course of carbonization, these products then decompose into gases that promote the formation of additional pores. These compounds might be polyoxymethylene or paraformaldehyde formed by a side reaction of formaldehyde[Citation21]. Another possibility would be the accumulation of formate, which can form as a result of the Cannizzaro reaction[Citation22]. The probability for accumulating formate is lower than for polyoxymethylene, as formate would be soluble in ethanol during the solvent exchange and therefore flushed out of the porous structure.
The reported specific BET surface areas of lignin–formaldehyde aerogels, prepared with additional resorcinol or phenol, range from 100 to 550 m2 g−1.15[Citation19]. Yang et al. [Citation20] reported a maximum BET value of ~100 m2 g−1 for aerogels prepared from lignin and formaldehyde without any additional phenolic compounds. In this work, a carbon aerogel was prepared from lignin that achieved a BET value of 596 m2 g−1.
Pore size distribution
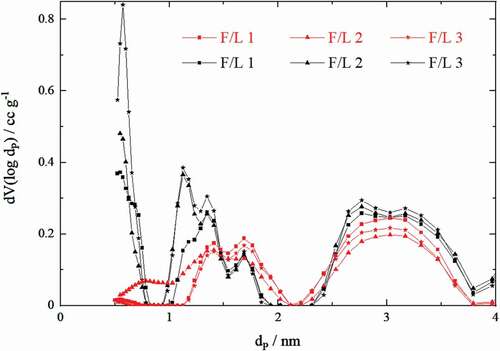
The pore size distributions of the prepared aerogels and carbon aerogels are depicted in .
Figure 4. Pore size distributions of the aerogels (red) and carbon aerogels (black) made from solutions of varying formaldehyde–lignin mass ratios (F/L), where dP is the pore diameter and V the relative pore volume
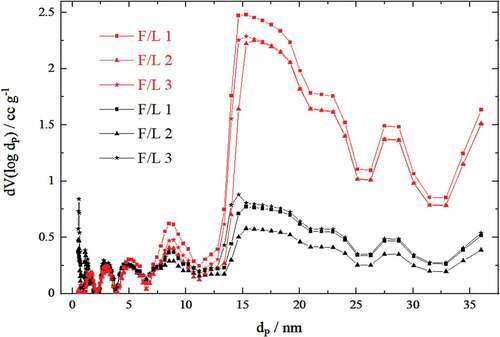
A comparison of the pore size distributions of aerogels and carbon aerogels shows that in the course of aerogel carbonization the proportion of larger pores (larger than 7 nm) is reduced, while the proportion of smaller pores (smaller than 4 nm) increases significantly. This is caused by carbonization, as the volume of the macropores decreases and the volume of micropores increases[Citation18]. For better illustration, a magnification of the pore size distributions in the low nanometer region is depicted in . Within the aerogels, a higher formaldehyde–lignin mass ratio (F/L) leads to a lower occurrence of pores over the entire measuring range. After carbonization, a higher F/L value causes a significant increase of pores smaller than 4 nm in the resulting carbon aerogels. This effect is particularly strong for pores between 0.5 and 1.5 nm. The results indicate that a high fraction of pores in the low nanometer range caused by a high F/L value is crucial for high specific surface areas in the carbon aerogels. Moreover, it seems that a high formaldehyde content leads to the formation of compounds (e.g. polyoxymethylene and formate) that occupy the pores in the aerogels before carbonization resulting in lower relative pore volume values.
Gel morphology
Scanning electron microscopy (SEM) images of aerogels with different formaldehyde–lignin ratios look very similar and no difference in morphology could be detected. Nevertheless, there is an observable difference in the surface structure between an aerogel before and after carbonization. In the SEM image of the aerogel based on a formaldehyde–lignin ratio of 2 (F/L 2) before (left) and after (right) carbonization, is shown.
Figure 6. SEM image of the lignin–formaldehyde aerogel (F/L 2) before (left) and after (right) carbonization
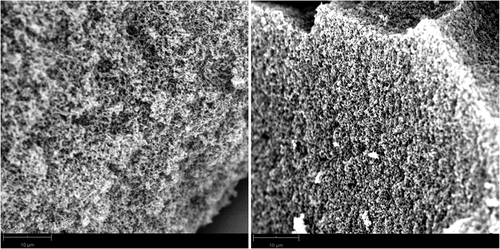
Both SEM images show an aerogel morphology with pores in the range of approximately 100 nm to 1 µm. Compared to the aerogel before carbonization, the carbon aerogel offers more pores that are smaller in size. These findings correspond well to those described for the pore size distributions via gas adsorption.
Thermal decomposition
The aerogels were tested by thermogravimetric analysis (TGA) after drying with supercritical carbon dioxide to characterize their transformation into carbon aerogels during the carbonization process. There was no significant difference for the TGA curves of aerogels with different formaldehyde-lignin ratios. In the TGA curve of the aerogel with a formaldehyde–linin ratio of 1 (F/L 1) is shown.
Figure 7. TGA curve (relative weight Rw plotted against temperature T) and the first derivative of the lignin–formaldehyde aerogel (F/L 1) after drying with supercritical CO2.
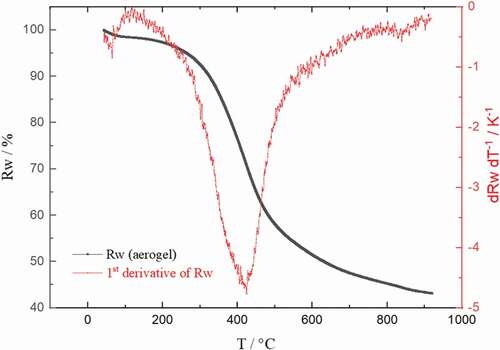
shows that most of the mass loss occurs between 250°C and 500°C and reaches a maximum at 425°C. According to the literature [Citation23], this decomposition results from a loss of oxygen in the form of carbon dioxide. During TGA, the aerogel lost 57% of its initial weight. This correlates well with the weight loss (55%) of the corresponding aerogel during carbonization. Based on these TGA results the parameters for carbonization were chosen. The heating rate for the first step was set to 1°C min−1 from 20°C to 500°C to allow the molecules to rearrange within the material and form new bonds. For the second carbonization step, a temperature of 900°C had been chosen as previous studies reported that the specific surface area beyond 900°C decreases with increasing temperature, but for sufficient electric conductivity at least a temperature of 750°C is required [Citation24,Citation25].
Conclusion
This work focused on the preparation of aerogels and carbon aerogels based on a sol-gel reaction using lignin and formaldehyde. The impact of the formaldehyde–lignin ratio (F/L) in the initial reaction mixture on the resulting aerogels was investigated. The results from SEC and gas adsorption indicated highly porous aerogels with pore sizes between 0.5 nm and 1 µm. It was found that a higher F/L value leads to a higher specific surface area (SBET) in the carbon aerogel. A maximum value of 596 m2 g−1 was achieved with an F/L value of 3. The pore size distributions of the aerogels revealed that a higher F/L value causes a significant increase in the number of pores between 0.5 and 1.5 nm which is crucial for the specific surface area.
Acknowledgments
The authors thank Dr. Sebastian Leitner for his preliminary work in the field of lignin-based aerogels. The assistance provided by DI Dr. Christoph Unterweger and Oliver Katzenberger during the carbonization experiments was greatly appreciated.
Disclosure statement
No potential conflict of interest was reported by the authors. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Data availability statement
Data availability statement Data will be made available upon request to the corresponding author at [email protected]. https://www.wood-kplus.at/en.
Additional information
Funding
Notes on contributors
Gottfried Aufischer
Gottfried Aufischer is a PhD student at the Institute for Chemical Technology of Organic Materials at the Johannes Kepler University Linz working for Wood K plus – Competence Center for Wood Composites & Wood Chemistry. He carried out the preparative work dealing with the production of lignin-formaldehyde aerogels and is currently working on the topic of lignin depolymerization for generating UV absorbers and antioxidants.
Birgit Kamm
Birgit Kamm is a Key researcher at Wood Kplus, Austría and an Honorary Professor Biorefinery Technology and Biobased Products at the Brandenburg University of Technology, BTU Cottbus-Senftenberg. Her research is focused on commercially viable biobased platform chemicals and materials. She is currently working on understanding the structure–property relationships between renewably sourced biobased polymers, biobased polymer additives.
Christian Paulik
Christian Paulik is a Professor at the Johannes Kepler University Linz and Head of the Institute for Chemical Technology of Organic Materials with expertise in conventional and biobased plastics and biotechnology.
References
- Kamm B, Gruber PR, Kamm M. Ullmann’s encyclopedia of industrial chemistry. Vol. 4. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2000. p. 1–38.
- Schorr D, Diouf PN, Stevanovic T. Evaluation of industrial lignins for biocomposites production. Ind Crops Prod. 2014;52:65–73.
- Ponnusamy VK, Nguyen DD, Dharmaraja J, et al. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour Technol. 2019;271:462–472.
- Rinaldi R, Jastrzebski R, Clough MT, et al. Wege zur Verwertung von Lignin: Fortschritte in der Biotechnik, der Bioraffination und der Katalyse. Angew Chem. 2016;128:8296–8354.
- Laurichesse S, Avérous L. Chemical modification of lignins: towards biobased polymers. Prog Polym Sci. 2014;39(7):1266–1290.
- Doherty WOS, Mousavioun P, Fellows CM. Value-adding to cellulosic ethanol: lignin polymers. Ind Crops Prod. 2011;33(2):259–276.
- Gellerstedt G. Softwood kraft lignin: raw material for the future. Ind Crops Prod. 2015;77:845–854.
- Alekhina M, Ershova O, Ebert A, et al. Softwood kraft lignin for value-added applications: fractionation and structural characterization. Ind Crops Prod. 2015;66:220–228.
- Mirzaeian M, Hall PJ. The control of porosity at nano scale in resorcinol formaldehyde carbon aerogels. J Mater Sci. 2009;44(10):2705–2713.
- Dong X-C, Xu H, Wang X-W, et al. 3D graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano. 2012;6(4):3206–3213.
- Gui X, Wei J, Wang K, et al. Carbon nanotube sponges. Adv Mater. 2010;22(5):617–621.
- Buratti C, Moretti E, Belloni E, et al. Development of innovative aerogel based plasters: preliminary thermal and acoustic performance evaluation. Sustainability. 2014;6(9):5839–5852.
- Zuo L, Zhang Y, Zhang L, et al. Polymer/carbon-based hybrid aerogels: preparation, properties and applications. Materials (Basel). 2015;8(10):6806–6848.
- Lee Y, Yoon JS, Suh DJ, et al. 5-hydroxymethylfurfural as a potential monomer for the preparation of carbon aerogel. Mater Chem Phys. 2012;136(2–3):837–844.
- Chen F, Min X, Wang L, et al. Preparation and characterization of organic aerogels from a lignin - resorcinol - formaldehyde copolymer. BioResources. 2011;6(2):1262–1272.
- Pekala RW. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J Mater Sci. 1989;24(9):3221–3227.
- Pekala RW, Farmer JC, Alviso CT, et al. Carbon aerogels for electrochemical applications. J Non-Crystalline Solids. 1998;225:74–80.
- Moreno-Castilla C, Maldonado-Hódar FJ. Carbon aerogels for catalysis applications: an overview. Carbon. 2005;43(3):455–465.
- Grishechko LI, Amaral-Labat G, Szczurek A, et al. Lignin–phenol–formaldehyde aerogels and cryogels. Microporous Mesoporous Mater. 2013;168:19–29.
- Yang BS, Kang K-Y, Jeong M-J. Preparation of lignin-based carbon aerogels as biomaterials for nano-supercapacitor. J Korean Phys Soc. 2017;71(8):478–482.
- Grajales EJ, Alarcón EA, Villa AL. Kinetics of depolymerization of paraformaldehyde obtained by thermogravimetric analysis. Thermochim acta. 2015;609:49–60.
- Martin RJL. The mechanism of the Cannizzaro reaction of Formaldehyde. Aust J Chem. 1954;7:335.
- Patton A, ed. Formaldehyde. Synthesis, applications, and potential health effects. New York: Nova Science Publishers Inc; 2015.
- Zhang SQ, Wang J, Shen J, et al. The investigation of the adsorption character of carbon aerogels. Nanostruct Mater. 1999;11(3):375–381. http://www.sciencedirect.com/science/article/pii/S0965977399000549
- Zanto EJ, Al-Muhtaseb SA, Ritter JA. Sol−gel-derived carbon aerogels and xerogels. Ind Eng Chem Res. 2002;41:3151–3162. https://doi.org/https://doi.org/10.1021/ie020048g