 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Green microalgae in the genus Dunaliella have become increasingly important in biotechnology and industry. The high adaptability of Dunaliella to high salinity, as well as its fast growth and production of several metabolites have triggered interest. The attention of industry relates to its ability to synthesize several high-value compounds, such as β-carotene, lipids, glycerol, vitamins, and proteins. In addition, due to its tolerance to high salinity, contamination is reduced, and it can grow in open systems. Dunaliella salina can accumulate up to 25% dry weight in lipids and is the most efficient natural source of β-carotene. This review highlights the general characteristics of the genus, associated with its history, morphology, reproduction, occurrence, and taxonomy. The metabolic pathways for carotenoid and lipid synthesis are described. Relevant information on the most common strains is provided as well as the most widely used growth systems and conditions, and the expression systems under development. Applications of Dunaliella in several areas of the industry are also highlighted. Thus, this review can serve as a basis for future work and for the development of environmentally friendly, simple, and highly cost-effective production methods.
Introduction
Algae have shown great potential in achieving sustainable and efficient solutions. In addition to their importance in ecosystems, involved in the main biogeochemical cycles (Du, Wang, You, & Zhao, Citation2013), they have also been widely used in industry. Specifically, microalgae are recognized as potential resources for applications in the food industry, for pharmaceutical compounds, as environmental solutions and for the production of specific compounds, among others. These organisms have many advantages, such as low production costs, high photosynthetic efficiency, high resistance, and simple genetics (Chisti, Citation2007; Koh & Ghazoul, Citation2008; León-Bañares, González-Ballester, Galván, & Fernández, Citation2004). The great diversity of substances, such as pigments, lipids or proteins, and mechanisms that are present in microalgae result partly from their wide diversity of origins, with multiple metabolic and physiological strategies (Iglesias et al., Citation2019). Thus, microalgal biotechnology has developed quickly in the last three decades (Spolaore, Joannis-Cassan, Duran, & Isambert, Citation2006).
Our aim here is to report on the genus Dunaliella, a well-known microalga used in many industrial applications. We describe the general characteristics of Dunaliella are described and also address its ecology, occurrence, metabolism, scientific relevance, most used species and strains, growth conditions and growing systems, genetic manipulation and specific biotechnological applications. The number of publications associated with the term [Dunaliella] was analysed in the PubMed database (National Center for Biotechnology Information, US National Library of Medicine), from the year 2000 to 2019. According to , in recent years, scientific interest and consequently the number of publications related to this genus have been increasing.
General description
Dunaliella cells were identified for the first time by Michel F. Dunal (Dunal, Citation1838) in salt pans in the south of France and described as reddish unicellular algae. In 1905, Emanoil C. Teodoresco proposed the genus (Teodoresco, Citation1905). A distinguishing feature of these microalgae is their high resistance to various environmental factors, and there is therefore a high diversity of strains available for the industry. The study of particular strains has increased our understanding of the physiological adaptation mechanisms to high salinity, low pH and a wide range of temperatures (Oren, Citation2014). Many Dunaliella species have been isolated from environments with high salinity and a wide range of concentrations of other chemical components (Yao et al., Citation2016). Dunaliella species have already been grown in salinities from 0.5% to 35%, so they are widely used in the industry, grown in extreme environments (Hadi, Shariati, & Afsharzadeh, Citation2008). Dunaliella‘s adaptation mechanisms to different salt concentrations have been shown to be essentially based on the ability to alter the intracellular concentration of glycerol, a well-known compatible solute (Ghoshal, Mach, Agarwal, Goyal, & Goyal, Citation2002). On the other hand, data also indicate that this genus has an exceptional ability to remove sodium ions, in high salinity environments, through a redox sodium pump (Katz & Pick, Citation2001).
Dunaliella species have also been shown to be resistant to high concentrations of other compounds, such as glycerol, and to high light intensities, e.g., 2000 µmol photons m−2 s−1. Dunaliella acidophila can grow in extremely acidic environments (pH 0–1) and Dunaliella antarctica resists temperatures below zero (Hosseini Tafreshi & Shariati, Citation2009; Pick, Citation1999), demonstrating its great adaptation capacity.
This genus occurs in extreme environments around the world (Oren, Citation2009). It has been isolated from cobwebs in the Atacama Desert, which can be used as a model for studying the evolution of aquatic organisms to land organisms (Cereceda, Larrain, Osses, Farías, & Egaña, Citation2008), in saline soils in the Great Salt Plains, Oklahoma (Buchheim, Kirkwood, Buchheim, Verghese, & Henley, Citation2010) and in three Antarctic lakes (Wright & Burton, Citation1981). D. salina, D. viridis and D. parva are found in the Great Salt Lake, USA (Oren, Citation2014). D. salina is also found in Lake Tyrrell in Australia (Oren, Citation2014), in lakes in Iran (Hadi, Shariati, & Afsharzadeh, Citation2008), and in halite crystals in Death Valley, USA, approximately 10–34 ky and 100 ky old (Schubert, Timofeeff, Lowenstein, & Polle, Citation2010).
Morphologically, Dunaliella cells are biflagellate, can be ovoid, spherical, pyriform, fusiform, or ellipsoidal, and can vary between 5 to 25 μm in length and 3 to 13 μm in width (Hosseini Tafreshi & Shariati, Citation2009). These cells do not have a rigid cell wall, being delimited by a thin and elastic membrane that allows more effective morphological adaptation, according to the osmotic pressure variations of the external environment (Ben-Amotz & Avron, Citation1990; Ben-Amotz, Citation1993). The cells contain a single, central, cup-shaped chloroplast with a central pyranoid surrounded by starch grains (Borowitzka & Siva, Citation2007).
Dunaliella cells multiply by longitudinal division, and sexual reproduction by isogamy can occur, where two cells merge, forming a zygote (Oren, Citation2005). It has also been shown that these cells can lead to the formation of palmelloid or aplanospore cells, if the environmental conditions inhibit growth (Borowitzka & Siva, Citation2007; Polle, Tran, & Ben-Amotz, Citation2009).
Knowing that Dunaliella species are mostly photoautotrophic, the presence of a wide variety of pigments in the cells is expected. In addition to chlorophyll a and b, several carotenoids such as α and β-carotene, neoxanthin, lutein, violaxanthin and zeaxanthin are found (Ben-Amotz & Avron, Citation1989a).
Regarding the taxonomy, the genus Dunaliella has been quite controversial (Polle, Tran, & Ben-Amotz, Citation2009). Dunaliella species belong to the phylum Chlorophyta, class Chlorophyceae, order Chlamydomonadales and family Dunaliellaceae. In the last few years, reassessments of the genus have been carried out, with 29 species being listed, of which 17 are halophiles: D. parva, D. salina, D. pseudosalina, D. ruineniana, D. gracilis, D. bioculata, D. carpatica, D granulata, D. baasbeckingii, D. minuta, D. media, D. minutissima, D. terricola, D. viridis, D. asymmetrica, D. peircei and D. turcomanica. The most researched species are D. salina, D. tertiolecta, D. primolecta, D. viridis, D. bioculata, D. acidophila, D. parva and D. media (Borowitzka & Siva, Citation2007). Dunaliella bardawil is considered to be a subspecies of Dunaliella salina (Gonzalez, Coleman, Gomez, & Montoya, Citation2001).
According to the NCBI database, the genomes of five strains of this genus have already been sequenced: D. salina CCAP 19/18 (GenBank: GCA_002284615.1), in 2017, Dunaliella sp. M2 (GenBank: GCA_004335885.1), Dunaliella sp. RO (GenBank: GCA_004335775.1), Dunaliella sp. WIN1 (GenBank: GCA_004335645.1) and Dunaliella sp. YS1 (GenBank: GCA_004335685.1), in 2019.
Industrially relevant metabolites
Dunaliella cells synthesize a wide range of products, among which carotenoids and lipids are the most relevant. Some processes related to these components and their metabolism will be described.
Carotenoids
Some species, such as D. salina and D. bardawil, have the ability to accumulate high amounts of carotenoids (Ye, Jiang, & Wu, Citation2008). The most common type of carotenoid in Dunaliella cells is β-carotene, but α-carotene, lutein, zeaxanthin, cryptoxanthin and neoxanthin can also be found (Ben-Amotz, Katz, & Avron, Citation1982). Under specific growth conditions, some strains have the capacity to accumulate more than 15% of β-carotene by dry weight (Hosseini Tafreshi & Shariati, Citation2009). The high concentrations of β-carotene give the cells a red colouration (Oren, Citation2005).
Specifically, β-carotene has been a widely used component in the industry; thus, several strategies have been developed to maximize its production. Diverse Dunaliella strains have been used for the production of β-carotene in several countries, such as Australia, the USA and China (Borowitzka, Citation1999). Dunaliella salina is considered to be the most efficient natural source to produce β-carotene (Lamers, Janssen, De Vos, Bino, & Wijffels, Citation2008). For the induction of β-carotene accumulation, Dunaliella cells should be exposed to high light intensities and growth conditions that usually reflect low growth rates, such as extreme temperatures, high salinities, and limited nitrogen (Lamers, Janssen, De Vos, Bino, & Wijffels, Citation2008). β-carotene is a component of photosynthesis and is accumulated in lipid globules in the inter-thylakoid spaces of the chloroplasts in Dunaliella, under stress conditions (Hadi, Shariati, & Afsharzadeh, Citation2008).
The functions of carotenoids have been studied in the previous work. Knowing their conjugated polyene structure, these components play a very important role in the photosynthetic reaction, in terms of energy and oxygen transport (Siefermann-Harms, Joyard, & Douce, Citation1978), also preventing chlorophyll photo-damage (Ben-Amotz & Shaish, Citation1992). On the other hand, carotenoids can also suppress singlet oxygen and free radicals (Ye, Jiang, & Wu, Citation2008). More than 700 types of carotenoids have been described in natural resources (Feltl, Pacakova, Stulik, & Volka, Citation2006). Most of the carotenoids are C40 isoprenoids, that is, composed of eight isoprene units (Naik, Chanemougasoundharam, Khurana, & Kalloo, Citation2003). Specifically, β-carotene can be found in several structures since the configuration of each double bond can occur naturally as trans or cis. The most common isomers are all-trans, 9-cis, 13-cis and 15-cis (Patrick, Citation2000). Dunaliella cellular β-carotene is composed of a mixture of all-trans (42%), 9-cis (41%), 15-cis (10%) and other isomers (6%) (Borowitzka & Borowitzka, Citation1989).
Most of the β-carotene industrial production (approximately 85%) is still carried out by chemical synthesis (Ye, Jiang, & Wu, Citation2008). However, synthetic β-carotene is essentially made up of all-trans isomers, whereas in Dunaliella cells, high amounts of 9-cis isomers can be found, which have a much more relevant antioxidant effect (Shaish et al., Citation2006).
Regarding carotenoid metabolism, studies indicate that it is strongly related to the synthesis of chlorophyll (Fu et al., Citation2013; Lamers et al., Citation2010). The synthesis of each of these components can be competitive or cooperative, knowing that these processes are controlled by related metabolic pathways (Song et al., Citation2018).
The first step in the metabolic pathway for carotenoid synthesis is the synthesis of GGPP (geranylgeranyl pyrophosphate), by the catalyst enzyme GGPS (geranylgeranyl pyrophosphate synthase), from the addition of three IPP molecules (isopentenyl pyrophosphate) to a DMAPP molecule (Dimethylallyl pyrophosphate), via sequential reactions (Hirschberg, Citation2001). GGPP is the precursor to carotenoids (Xu et al., Citation2022).
For GGPP synthesis to be possible, IPP molecules have to be produced. Studies indicate the existence of two relevant metabolic pathways for the synthesis of IPP in plants and algae: the mevalonate (MVA) pathway and the non-mevalonate pathway (1-deoxy-D-xylulose 5-phosphate (DXP) pathway or 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway) (Lange, Rujan, Martin, & Croteau, Citation2000). The mevalonate pathway, described in 1950, starts with acetyl-CoA (Bloch, Citation1992), with the participating enzymes located in the cytosolic compartment. The vast majority of cytoplasmic IPP is synthesized by this pathway (Newman & Chappell, Citation1999). On the other hand, the non-mevalonate pathway, described in 1980, is responsible for the synthesis of most of the IPP present in plastids. It starts with the conjugation of D-glyceraldehyde 3-phosphate (GA3P) and pyruvate via intermediates. The pathway follows this order: 1-deoxy-D-xylulose 5-phosphate (DXP), 2-C-methyl-D-erythritol 4-phosphate (MEP), 4-diphosphocytidyl−2-C-methyl-D-erythritol (DPME), 4-diphosphocytidyl−2-C-methyl-D-erythritol 2-phosphate, 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MEcDP) and 4-hydroxy−2-methylbut−2-enyl pyrophosphate (HMBPP) (Seemann, Tse Sum Bui, Wolff, Miginiac-Maslow, & Rohmer, Citation2006). Finally, IPP, DMAPP and, consequently, GGPP are generated. This pathway has been described in bacteria (Rohmer, Knani, Simonin, Sutter, & Sahm, Citation1993), algae (Schwender, Seemann, Lichtenthaler, & Rohmer, Citation1996) and plants (Lichtenthaler, Schwender, & Müller, Citation1998). Genes, enzymes, and other components participate in the non-mevalonate pathway (Xu et al., Citation2022).
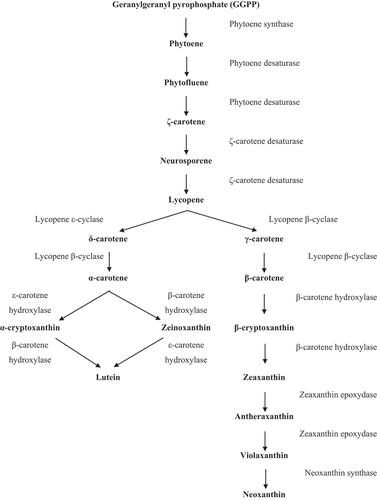
From GGPP, a pathway follows where several carotenoids are synthesized. The synthesis of carotenoids from GGPP is quite common in several organisms. Initially, two GGPP molecules are conjugated to obtain the first colourless carotenoid, phytoene, from the enzyme phytoene synthase (PSY). This enzyme is indicated as an essential element in the synthesis of carotenoids and regulation of carbon flow in this process (Shewmaker, Sheehy, Daley, Colburn, & Ke, Citation1999). Studies indicate that isomerization reactions occur in this step (Ben-Amotz, Lers, & Avron, Citation1988). As soon as phytoene is formed, denaturation reactions occur, obtaining, in this order: phytofluene, ζ-carotene, neurosporene and lycopene. From the formation of lycopene, two pathways for the formation of cyclic or acyclic carotenoids can be followed (Schmidt-Dannert, Citation2000). β-carotene is a cyclic carotenoid, so the most prevalent pathway in Dunaliella cells is the one that promotes the formation of cyclic carotenoids. In the cyclization process, cyclohexene rings are added to one or both ends of the lycopene molecule, from the enzyme lycopene cyclase (LYC), and the formation of δ-, α-, γ- and β-carotene takes place. Two types of the LYC enzyme have already been described, the lycopene β-cyclase (LYC-Β) and lycopene ε-cyclase (LYC-Ε). LYC-Β adds a β ring at one or two ends of the lycopene to form γ-carotene (monocyclic) or β-carotene (dicyclic) molecules. LYC-Ε only has the ability to add an ε ring to one end of the lycopene generating the δ-carotene (monocyclic) (Cunningham et al., Citation1996). For the production of α-carotene, an ε ring (by LYC-E) at one end and a β ring (by LYC-B) at the other must be added to the lycopene. It is possible to conclude that Dunaliella cells have both cyclases, as these cells contain α-carotene and β-carotene. From these two pathways, other components can be generated, such as α-cryptoxanthin, zeinoxanthin and lutein from α-carotene, and β-cryptoxanthin, zeaxanthin, antheraxanthin, violaxanthin and neoxanthin from β-carotene according to . The metabolic processes previously mentioned were described in detail by Ye, Jiang, & Wu (Citation2008).
Figure 2. Metabolic pathway of carotenoid production in Dunaliella (Hirschberg et al., Citation1997; Hirschberg, Citation2001; Sandmann, Citation2001; Ye, Jiang, & Wu, Citation2008).
Lipids
In addition to carotenoids, Dunaliella is a source of lipids, reaching 25% in dry weight (Liu et al., Citation2013). In general, the production of these compounds is also associated with non-favourable growth conditions, such as temperature, light intensities (Gim et al., Citation2014) salinities (Takagi, Karseno, & Yoshida, Citation2006), or with the presence of specific compounds in the medium such as sodium tungstate (Benhima et al., Citation2018)
Lipids are essential components in cell structure and storage, and the variation of the lipid content in microalgae is usually due to a response to changes in the medium composition. Fatty acids (FA) allow the separation of the cellular content from the extracellular medium (Matsumoto, Shioya, & Nagashima, Citation1984). Sterols are among the compounds of greatest interest, usually used to determine the nutritional value of microalgal food products, used as biomarkers of the presence of organic matter in sediments (Volkman et al., Citation1998), and as indicators of cell proliferation, modulating the activity of membrane-bound enzymes (Francavilla, Trotta, & Luque, Citation2010).
Large amounts of fatty acids are found in D. salina, with about 50% of these being mono- or polyunsaturated (Cakmak, Kaya, & Asan-Ozusaglam, Citation2014), principally palmitic (C16: 0), alpha linolenic (C18: 3), and oleic acid (C18: 1). Alpha-linolenic acid is one of the best-known ω−3 fatty acids, associated with benefits in brain development, and in the prevention of cardiovascular diseases (Cakmak, Kaya, & Asan-Ozusaglam, Citation2014).
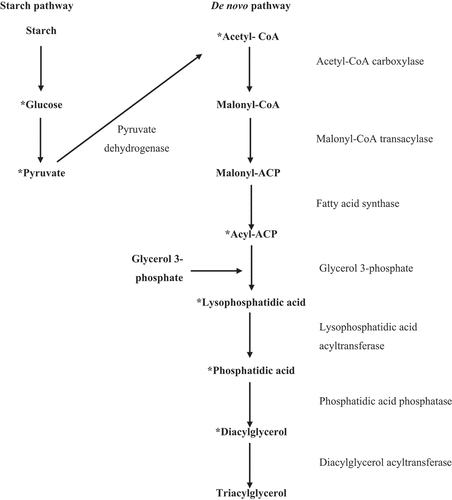
Some studies indicate that in eukaryotic algae, the synthesis of fatty acids is simpler than in plants (Goncalves, Wilkie, Kirst, & Rathinasabapathi, Citation2016). Three carbon pathways have been identified for biosynthesis in chloroplasts: 1- De novo synthesis directly from assimilation by the Calvin cycle, the formation of acetyl-CoA by pyruvate dehydrogenase and its assimilation via fatty acid synthesis cycle () (Coleman & Lee, Citation2004; Ohlrogge & Browse, Citation1995); 2- fatty acid acyl transfer from pre-formed polar lipids, particularly from chloroplast galactoglycerolipids (Lippold et al., Citation2012); 3- Synthesis from the degradation of pre-formed starch () (Pick & Avidan, Citation2017). Pick and Avidan (Citation2017) suggest that, in Dunaliella, about two-thirds of the triacylglycerol come from the third route, one-third from the first, and some residual amounts come from the second route.
Figure 3. The starch and De novo pathway of Triacylglycerol synthesis in green algae. (*the metabolic pathways were simplified to a better understanding) (Coleman & Lee, Citation2004; Lippold et al., Citation2012; Ohlrogge & Browse, Citation1995; Pick & Avidan, Citation2017).
Other products
This genus is also used as a glycerol source. Dunaliella, in hypersaline conditions, produces glycerol that helps to maintain the integrity of the membrane and proteins, with some strains accumulating more than 50% dry weight (Ben-Amotz & Avron, Citation1990), and to maintain osmotic balance. These organisms have the ability to secrete glycerol into the medium that functions as a carbon dioxide scavenger (Chow et al., Citation2013). The mechanisms of glycerol production can vary depending on the growth conditions, with higher production rates when cells are exposed to limited light (Ng, Low, Chow, & Lee, Citation2014) and high salinities. D. salina produces higher glycerol levels than other Dunaliella species (Hadi, Shariati, & Afsharzadeh, Citation2008).
Dunaliella is also used for the production of proteins, which can represent between 50% and 80% of dry biomass (Becker, Citation2007; Sui, Muys, Vermeir, D’Adamo, & Vlaeminck, Citation2019). D. acidophila grows at very low pH and has been used for the production of the enzyme H+-ATPase, which is one of the main proteins in its membrane (Matalin et al., Citation2021; Sekler & Pick, Citation1993). Some strains of D. salina have large amounts of adenosylcobalamin and are identified as a possible source of vitamin B12, accumulating about 50% of dry weight (Kumudha & Sarada, Citation2016). D. tertiolecta is used to synthesize vitamin E, xanthophyll and violaxanthin that have antiproliferative activities (Pasquet et al., Citation2011).
Under particular growth conditions, some species of Dunaliella have antimicrobial activity against several microorganisms (Kocberber Kilic, Erdem, & Donmez, Citation2018). Chang et al. (Citation1993) demonstrated that the crude extract of D. primolecta shows antimicrobial activity against Staphylococcus aureus, Enterobacter aerogenes, Bacillus subtilis and Bacillus cereus. The production of these compounds occurred under osmotic stress (Kocberber Kilic, Erdem, & Donmez, Citation2018). Lutein and ferulic acid were the main compounds responsible for the antimicrobial response (Chang et al., Citation1993).
Commonly used species and strains in the industry
Several species of Dunaliella have been studied and widely used in recent years. D. salina is one of the most used microalgae in the industry. Its most significant feature is its ability to accumulate large amounts of β-carotene and lipids (Yilancioglu et al., Citation2014). In addition, aliphatic and isoprenoid hydrocarbons, sterols, phospholipids and glycolipids have been identified in D. salina. D. salina has been the target of several studies as a physiological model and for growth optimization (Besson & Guiraud, Citation2013; Béchet et al., Citation2018; Khadim et al., Citation2018). Thus, numerous strains of this species have been developed and registered, partly due to their halotolerance, that prevents the growth of most competitors or predators (Ben-Amotz, Shaish, & Avron, Citation1991), as indicated in .
bardawil (considered by some authors as a subspecies of D. salina; Gonzalez, Coleman, Gomez, & Montoya, Citation2001) has been grown mainly for the production of fatty acids.
tertiolecta has also shown great biotechnological potential. This species has been the subject of many studies due to its exceptional ability to accumulate lipids, glycerol and starch (Slocombe et al., Citation2015). It has many advantages, such as high growth rates, efficient use of carbon dioxide and high halotolerance, among others (Chow et al., Citation2013; Katz, Paz, & Pick, Citation2019).
acidophila is one of the most representative extreme acidophilic organisms (Aguilera & Amils, Citation2005). It can grow at a pH below 1.0, maintaining its intracellular pH at 7.0 (Pick, Citation1999).
maritima shows high production of ATPases (Khramov, Matalin, Karpichev, Balnokin, & Popova, Citation2019).
viridis has applications in the aquaculture feed industry (García, Freile-Pelegrín, & Robledo, Citation2007). lists some strains that have been used commercially.
Table 1. Strains used in identified studies, of different species of the genus Dunaliella. (Strain availability: “CCAP” - Culture Collection of Algae and Protozoa; “DF” - the Marine Biological Association; “SAG” - SAG Culture Collection; “LB” - UTEX Culture Collection; “RCC” - Roscoff Culture Collection; “CCMP”- NCMA Bigelow Laboratory for Ocean Sciences; “ATCC”- American Type Culture Collection).
Production methods
The growing biotechnological applications of microalgae have increased interest in the research and development of growth systems and processes associated with industrial production, including a need to make microalgal biorefineries profitable (Monte et al., Citation2020). In the production of Dunaliella, systems and processes that promote the extraction of the largest number of components in the most efficient way possible must be developed (Chew et al., Citation2017). After laboratory evaluations define the potential of a given species, the design of the process, the scale up of the indicated photobioreactors and the development of techniques for the extraction and harvesting of the target components are carried out. This scale-up step is vital for the feasibility of future applications. The main parameters that influence microalgal productivity are mixing rate, culture depth, inoculum volume and the growth conditions (Kroumov et al., Citation2016).
Growth systems
Numerous microalgal growth systems have been developed, such as large open ponds, circular ponds, raceway ponds, cascade ponds, large bags, tanks, heterotrophic fermenters, and several kinds of closed photobioreactors (Borowitzka, Citation1999; Pulz, Citation2001). Within these, two main types can be distinguished: open-grown systems and closed-growth systems. The main distinction between these is the control of the conditions to which the cultures are exposed. In closed systems, the conditions are controlled more efficiently. Open growth systems have been the most widely used in recent years (Vigani et al., Citation2015). Open ponds represent the most economical and conventional system for microalgae growth (Ben-Amotz & Avron, Citation1989a). There are two main types of open growth systems, very large ponds (extensive mode) of up to 250 ha (Borowitzka & Borowitzka, Citation1990), and smaller paddle wheel stirred raceway ponds (Ben-Amotz, Citation1995). For the growth of Dunaliella in open systems, it is favourable to establish structures in locations with a warmer and drier climate, preferably close to locations with a high concentration of NaCl. It is also important that there are no sources of contamination nearby. These systems are advantageous as they are considerably cheaper and the media can be quite selective, given the resistance to high salinity of Dunaliella cells (Borowitzka & Borowitzka, Citation1988). However, there are some disadvantages associated with this type of systems, such as the difficulty of controlling the temperature of the medium and the quality of the light to which the cells are exposed, so the productivity tends to be lower (Ben-Amotz & Avron, Citation1989a).
Photobioreactors are very advantageous systems for controlling culture parameters and are used for the growth of microalgae, cyanobacteria, and plants. There are three basic designs in the development of closed-growth systems: flat plate bioreactors, tubular photobioreactors and ultrathin immobilized configurations (Borowitzka, Citation1999; Pulz, Citation2001; Tredici & Zitelli, Citation1997). Tubular photobioreactors have been shown to have higher biomass productivities than flat plate bioreactors, but the latter have distinct advantages due to low oxygen accumulation, greater surface area in relation to volume, the possibility of applying turbulent flow, lower cost, easy maintenance, and bio-encrustation control (Bergmann, Ripplinger, Beyer, & Trösch, Citation2013). In general, closed systems allow precise control of growth conditions, enhancing the ability to precisely control light quality and the photoperiod (Lamers, Janssen, De Vos, Bino, & Wijffels, Citation2008). The main disadvantage of these systems is the high cost, in relation to open systems.
Growth conditions
Species of the genus Dunaliella have great resilience to external factors. Studies indicate that some species can survive at temperatures from 0°C to 45°C, with optimum growth occurring between 25°C and 35°C (Ben-Amotz, Citation1995; Jin & Polle, Citation2009). Borowitzka & Borowitzka (Citation1987) demonstrated that very low night-time temperatures (such as in Hutt Lagoon, Australia) can slowdown growth, thus decreasing the process yield. On the other hand, temperatures close to 40°C promote carotenoid synthesis, but also decrease the growth rate (Borowitzka & Borowitzka, Citation1989). Temperatures above 40°C can lead to the release of glycerol into the environment, which may be a carbon source for microbial contaminants (Ben-Amotz, Citation1995). The conditions used for the laboratory growth of some species, in some studies, are described in . The average temperature used was 24.7°C, and the most used temperature was 25°C.
Table 2. Growth conditions applied to different species of the genus Dunaliella, in the analysed studies.
Dunaliella species can resist pH values between 1, in the case of D. acidophila, up to 11, but for optimal growth, they require a pH close to 9 (Hosseini Tafreshi & Shariati, Citation2009). In intensive production systems, the pH is kept close to 7.5 as it tends to increase with the fixation process (Ben-Amotz, Citation1995). Considering , the average pH used was 7.6 and the most used pH value was 7.5.
In the studies indicated in , the most used medium was Modified Johnson’s medium (Johnson, Johnson, MacElroy, Speer, & Bruff, Citation1968), followed by F/2 medium (Guillard, Citation1975) and Walne’s medium (Walne, Citation1970). Carbon dioxide and bicarbonate are used as carbon sources for the growth of Dunaliella (usually photoautotrophic). Ben-Amotz & Avron (Citation1989a) indicated the possibility of using sodium bicarbonate as a carbon source. As a source of nitrogen, nitrate is considered the most efficient. Other sources of nitrogen, such as ammonia salts or urea, are not appropriate and can lead to cell death (Borowitzka, Citation1990). To increase biomass and protein synthesis, a favourable availability of nitrogen is necessary (Uriarte, Farías, Hawkins, & Bayne, Citation1993). The source of phosphorus that leads to better results is monopotassium phosphate or monosodium phosphate. For optimal growth, Dunaliella cells also need sulphate and several ions, such as , chelated iron and trace elements (Hosseini Tafreshi & Shariati, Citation2009). For culture conservation, cultures are usually kept on agar media plates or liquid media, subculturing every 1–2 months. Studies also indicate the possibility of preservation at very low temperatures for up to 12 months (Taylor & Fletcher, Citation1998). Colusse, Mendes, Duarte, Carvalho, & Noseda (Citation2020) directly compare the effect on growth, biochemistry, and costs of the most used media in D. salina cultivation. el Agawany, Kaamoush, El-Zeiny, & Ahmed (Citation2021) demonstrated the effects of heavy metals on D. tertiolecta production.
Dunaliella cells usually grow under autotrophic conditions and light is their most common source of energy. Usually, the cell growth increases with photoperiod (Sui, Muys, Vermeir, D’Adamo, & Vlaeminck, Citation2019). Cell growth and carotenoid synthesis clearly depend on the quantity and intensity of the light. Ben-Amotz & Avron (Citation1989b) demonstrated that the synthesis of carotenoids does not depend on the wavelength of the absorbed radiation but depends fundamentally on its irradiance. In the growth tests referenced in , the average of the photoperiod is 18 h, and the most common photoperiod was 24 h. The average photon irradiance is 103 µmol photons m−2 s−1 and the most used irradiance was 80 µmol photons m−2 s−1. Recent studies indicate the possibility of growing D. salina under heterotrophic and mixotrophic conditions for the production of lipids. In this case, the most used carbon sources are glucose, acetate, and glycerol (Capa-Robles, García-Mendoza, & Paniagua-Michel, Citation2021; Chavoshi & Shariati, Citation2019; Gonabadi, Samadlouie, & Shafafi Zenoozian, Citation2022).
Dunaliella cells grow in environments with high concentrations of NaCl. This characteristic is extremely advantageous since most of the contaminating organisms, harmful for Dunaliella, do not resist high salinities (Ben-Amotz & Avron, Citation1989a; Butinar, Sonjak, Zalar, Plemenitaš, & Gunde-Cimerman, Citation2005). The optimal growth of Dunaliella is obtained between concentrations of 58.5 g l−1 (1 M) and 116.9 g l−1 (2 M) of NaCl (Hadi, Shariati, & Afsharzadeh, Citation2008). In the studies indicated in , the average NaCl concentration was 74.6 g l−1, and the most used concentration was 87.7 g l−1 (1.5 M). D. salina increases its synthesis of carotenoids and glycerol, when grown in high salinities (Hadi, Shariati, & Afsharzadeh, Citation2008). These cells respond to the osmotic stress by regulating the flow of carbon between the synthesis of starch in chloroplasts and the production of glycerol in the cytoplasm (Bental, Pick, Avron, & Degani, Citation1990). Cycil et al. (Citation2021) also demonstrated that D. salina grows exposed to low atmospheric pressures.
Harvesting and component extraction
The development of a sustainable and economically viable extraction and harvesting process for microalgal components is a major challenge. The cost represents between 20% and 40% of the total production cost (Mata, Martins, & Caetano, Citation2010). As such, a universal technique cannot be defined; each process depends on the species used, its characteristics and the component to be extracted (Uduman, Qi, Danquah, Forde, & Hoadley, Citation2010). In the case of Dunaliella cells, there are three main characteristics that influence the harvesting and extraction process: the absence of a rigid cell wall, the high salinity of the medium and the low cell densities (Monte et al., Citation2020).
Harvesting of microalgae can occur by centrifugation, filtration, flocculation, and flotation (Garg, Li, Wang, & Schenk, Citation2012). Component extraction is usually done via flocculation by pH modulation (Besson & Guiraud, Citation2013; Vandamme, Foubert, & Muylaert, Citation2013), electrolysis (Wan et al., Citation2015), metallic cations (Pirwitz, Rihko-Struckmann, & Sundmacher, Citation2015), or cationic polymers like chitosan or modified starch (Vandamme, Foubert, Fraeye, Meesschaert, & Muylaert, Citation2012). Carotenoids can be extracted from algal biomass or dry powder, using these methods: extraction in conventional organic solvents (Ruane, Citation1974), extraction of the carotene from the algae in an edible oil (Nonomura, Citation1987), separation of β-carotene isomers via supercritical fluid extraction (Gamlieli-Bonshtein, Korin, & Cohen, Citation2002), or selective extraction by biocompatible organic solvents in two-phase bioreactors (Hejazi et al., Citation2002). These conventional processes are not very efficient, with high costs and energy consumption. In the case of cell harvesting methods, cells are often damaged, as is the case with centrifugation and filtration (Pragya, Pandey, & Sahoo, Citation2013). Monte et al. (Citation2018) described a membrane-harvesting process for D. salina that allows cell pre-concentration, significantly increasing the efficiency of cell harvesting compared to other harvesting processes. Besson, Formosa-Dague, & Guiraud (Citation2019) also described a process of cell harvesting of D. salina by flocculation/flotation that can be applied on an industrial scale. This method allows flocculation without the need for chemical flocculants, thus reducing cell damage and contamination.
Multi-product biorefineries
The concept of “Blue Biotechnology” has recently gained relevance, and consequently, the development of biorefineries that allow the extraction of several products in a single industrial production has also attracted interest (Nishshanka, Anthonio, Nimarshana, Ariyadasa, & Chang, Citation2022). Marine microalgae have been widely included in these processes and methods for multi-product extraction from Dunaliella species have already been described. Francavilla, Kamaterou, Intini, Monteleone, & Zabaniotou (Citation2015) verified the production of a total lipid fraction rich in β-carotene, phytosterols and fatty acids in D. tertiolecta, and evaluated the possibility of extracting, from the residues of the first process, 45% of bio-oil and 29% biochar by pyrolysis. Harvey & Ben-Amotz (Citation2020) demonstrated the production of 9-cis β-carotene in D. salina, and verified that, after the clean extraction of this compound, the residual biomass still included 50% of the lipids present in the cells. In addition, this residual biomass could also be used to produce animal feed. Monte et al. (Citation2020) proposed a method for extracting several components of D. salina. A possible biorefinery has been developed that allows efficient fractional extraction of carotenoids, lipids, glycerol and proteins. This sustainable design allowed the collection of 85% of carotenoids, 94% of polar lipids and 86% of glycerol.
Recombinant gene expression
Dunaliella has been shown to be an advantageous candidate for the synthesis of high-value products. The delay in the use of most eukaryotic algae in these systems is mainly due to the lack of knowledge of specific promoters for the efficient expression of the introduced genes. Until recently, there has not been much research on the genetic transformation and metabolism of Dunaliella. As a consequence of advances in genetic engineering and with the need for higher quality and yield of recombinant proteins, some species such as D. salina, D. tertiolecta and D. parva have aroused great interest (Feng, Li, Xu, & Qi, Citation2014; Ismaiel, El-Ayouty, Said, & Fathey, Citation2018; Lin, Shen, & Lee, Citation2018).
Thus, this genus has been the target of mutagenesis and genetic manipulation to increase the amounts of carotenoids and other high-value products. Recombination technologies allowed heterotrophy by autotrophic algae, thus allowing an increase in biomass per litre and the economic value of the algae (Jin & Melis, Citation2003). Jin & Polle (Citation2009) created strains of D. salina with a mutation that allowed the manipulation of the content of carotenoids, especially zeaxanthin, producing 20 times more zeaxanthin than the wild type (Jin & Melis, Citation2003). Recently, a transgenic strain of D. tertiolecta was characterized, with an increase in medium chain length fatty acids (MCFAs) levels (Lin, Shen, & Lee, Citation2018). This compound is used in the production of insect pheromones (Tillman, Seybold, Jurenka, & Blomquist, Citation1999), detergents (Knaut & Richtler, Citation1985), antibiotics (Laakel et al., Citation1994) and surfactants (Ohlrogge, Citation1994). In D. parva transformation with an external PSY gene (phytoene synthase gene) resulted in carotenoids with greater antioxidant capacity (Ismaiel, El-Ayouty, Said, & Fathey, Citation2018).
The most used systems in recombination are Escherichia coli, yeast, mammalian cells, or transgenic animals. However, all have disadvantages that affect yield and production cost. Expression systems with E. coli and yeasts are low-cost, however, these have a reduced ability to make post-translational modifications (Gonçalves et al., Citation2013; Liu et al., Citation2013). Systems of transgenic animals and mammalian cells are complex and costly and show low yield (Aricescu & Owens, Citation2013; Guan et al., Citation2013). Thus, Dunaliella is a competitive alternative to produce recombinant compounds (Barzegari et al., Citation2010). As it is a microalga that does not have a rigid cell wall (Borowitzka & Siva, Citation2007), the transformation process is easier, requiring less time to scale-up production (Feng, Xue, Liu, & Lu, Citation2009). Also, it is possible to perform the harvest by removing the product of interest and taking advantage of residuals as secondary products (Hejazi, Holwerda, & Wijffels, Citation2004). As a haploid microorganism, the introduced genes are always expressed, whether dominant or recessive (Jones & Sparks, Citation2009). Although reproduction is mainly due to vegetative cell division, sexual reproduction can occur (Borowitzka & Siva, Citation2007). When sexual reproduction occurs between individuals with different inserted genes, the offspring can receive several genes (Primrose, Citation1991); however, it is not desirable in the production of multi-chain antibodies. Furthermore, post-translational processing occurs naturally in eukaryotic organisms, which is an advantage. Also, recombinant proteins can be easily removed with cell lysis procedures (Feng, Li, Xu, & Qi, Citation2014). The great advantage in comparison with other species is that there are no escape mechanisms for genes, making transgenic individuals easy to control and with a low probability of escape of transformed cells into the environment (Barzegari et al., Citation2010).
This genus has been the target of several transformation methods, including electroporation (Sun et al., Citation2005), particle bombardment (Tan, Qin, Zhang, Jiang, & Zhao, Citation2005), glass beads (Feng, Xue, Liu, & Lu, Citation2009), and lithium acetate/polyethylene glycol (PEG) mediated method (Chai, Chen, Xu, & Xu, Citation2013).
Many Dunaliella genes have been isolated, characterized, and transformed (He et al., Citation2020). Some of these genes express DNA photolyases with the function of repairing DNA damage caused by UV radiation (Yi et al., Citation2006), a nitrate transporter gene named DsNRT2Æ1 (He et al., Citation2004), plastid glycerol 3-phosphate dehydrogenases (He et al., Citation2007), x3 fatty acid desaturases (Lyukevich, Mouradyan, & Los, Citation2003), the 5-enolpyruvylshikimate−3-phosphate synthase (Yi et al., Citation2007), and the sodium-dependent phosphate transporter, DvSPT1 (Li et al., Citation2006). In D. maritima, the DmHA2 ATPase gene was isolated as responsible for the production of Na+-ATPase (Khramov, Matalin, Karpichev, Balnokin, & Popova, Citation2019).
The genes responsible for carotenogenesis of a carotenogenic strain were identified, extracted, and used to genetically manipulate the species (Zhu, Jiang, & Chen, Citation2008). However, the genes involved in this pathway and its regulation are not fully understood (Jin & Polle, Citation2009).
Industrial applications
Due to its advantageous characteristics mentioned previously, Dunaliella is one of the most used species in the biotechnological industry, and its applications are observed in diverse sectors, such as carotenoid production, food and pharmaceutical industry, bioactive compounds, bioremediation, bioindicators, biofuel and antifouling.
Carotenoids
Currently, carotenoids are the main product obtained from Dunaliella (Duan et al., Citation2023). In the industry, these components are applied as dyes, and with the growing demand for natural, healthy, and colourful products, carotenoids are highly valued as natural additives for food and cosmetics (Ye, Jiang, & Wu, Citation2008). These compounds are also applied in animal feeding to improve the colour manipulation of ornamental fish (Pulz & Gross, Citation2004) and eggs (Moulton & Burford, Citation1990). According to a Fortune Business Insights report the world market for carotenoids reached US $1.4 billion in 2019 and should reach US $1,8 billion by 2027.
β-carotene can be obtained naturally or synthetically, however, the natural form has greater antioxidant and anti-tumour capabilities (Hu, Lin, Lu, Chou, & Yang, Citation2008). Microalgae are one of the natural sources with the highest production, as well as some vegetables, fruits, and fungi (Dufossé et al., Citation2005). Dunaliella cells accumulate hundreds of times more β-carotene than a carrot cell (Klausner, Citation1986) in addition to being of higher quality.
The therapeutic effects of β-carotene are mainly due to its function as a protector of cells against harmful free radicals and to stimulate the immune system (Ben-Amotz, Citation1996). In addition, β-carotene is known to be oxidized by liver enzymes that produce vitamin A, improving vision and epithelial tissues (Hosseini Tafreshi & Shariati, Citation2009). This component is also effective in controlling cholesterol levels (Törnwall et al., Citation2004), in the prevention and treatment of diseases, such as cataracts (Gupta et al., Citation2003), cardiovascular problems (Shaish et al., Citation2006), and certain cancers (Mayne et al., Citation1994; Michaud et al., Citation2000; van Poppel, Citation1993). A recent study proved the effectiveness of using Dunaliella as a nutraceutical in rats with diet-induced obesity and hepatic lipid accumulation, without affecting food intake (Xu et al., Citation2022; Yamashita et al., Citation2020).
Food and feed industry
Due to the high production of protein, fatty acids, β-carotene, and the lack of cell walls, Dunaliella is an excellent feed for aquaculture and cattle, and a food source for humans (Hosseini Tafreshi & Shariati, Citation2009). Dunaliella products have been identified as important alternatives for ruminant and crustacean feed due to their high mineral and vitamin content, stimulating optimum growth (Madeira et al., Citation2017) and increasing the weight and survival capacity of shrimps (Supamattaya, Kiriratnikom, Boonyaratpalin, & Borowitzka, Citation2005). Furthermore, due to antimicrobial activity against Escherichia coli, Staphylococcus aureus, Candida albicans, and Aspergillus niger, Dunaliella extracts can prevent contamination during the production of food products (Herrero, Jaime, Martín-Álvarez, Cifuentes, & Ibáñez, Citation2006).
There is also great potential for the use of components extracted from Dunaliella species in the development and production of food and feed supplements (Camacho, Macedo, & Malcata, Citation2019). Considering the β-carotene and xanthophylls extracted from these species, relevant antioxidant and anticancer capacities have already been demonstrated (Jayappriyan, Rajkumar, Venkatakrishnan, Nagaraj, & Rengasamy, Citation2013; Singh, Baranwal, & Reddy, Citation2016). The use of Dunaliella for protein production in the food industry is still not very common; however, the great quantity, quality, and nutritional value indicate it as a sustainable source of protein, with an amino acid content similar to soy. Currently, despite its great potential as a protein source for human consumption, it has mainly been used in feed supplements (e.g., feeding ornamental fish, shrimp and birds) (Sui & Vlaeminck, Citation2020). The direct use of Dunaliella biomass in food products is also potentially advantageous, having positive effects on the treatment of cardiovascular diseases and cancer, with anti-inflammatory properties (Silva et al., Citation2021).
It was also verified that the exopolysaccharides of D. salina are potential alternatives for the protection and improvement of agricultural crops, acting as attenuators of biotic and abiotic stress. Positive results have been obtained in tomato plants under osmotic stress (Arroussi et al., Citation2018).
Pharmaceutical industry and bioactive compounds
Dunaliella produces a large number of bioactive compounds, enzymes, and vitamins that are of high value in applications in the pharmaceutical industry. D. salina produces a unique enzyme, dihydroxy acetone reductase, which has already been commercialized (Ben-Amotz & Avron, Citation1990). The extract has antioxidant, antimicrobial, and antiaging activity (Cakmak, Kaya, & Asan-Ozusaglam, Citation2014; Havas et al., Citation2022). D. tertiolecta extracts demonstrated muscle relaxation, antiserotonin, antihypertensive, analgesic, bronchodilator, polysynaptic block, and antioedema activities (Borowitzka, Citation1995; Villar, Laguna, Calleja, & Cadavid, Citation1992). Moreover, D. primolecta contains several substances with antibiotic activity (Chang et al., Citation1993). Pentapharm (Basel, Switzerland) developed a bioactive compound from D. salina that stimulates cell proliferation (Stolz & Obermayer, Citation2005).
In two species of Dunaliella (D. tertiolecta and D. salina) phytosterols (about 1% of dry weight) were identified and isolated (Francavilla, Trotta, & Luque, Citation2010). These compounds are of great value for the pharmaceutical industry as they are precursors of some bioactive molecules and act in the prevention of coronary heart disease through the reduction of cholesterol (Fernandes & Cabral, Citation2007). In addition, these compounds have anti-inflammatory activity and may have anti-cancer and anti-oxidative properties (Francavilla, Trotta, & Luque, Citation2010).
The protective effects of D. salina against fibrosarcoma cells are already known (Raja, Hemaiswarya, & Rengasamy, Citation2007); this species has selective cytotoxic potential in malignant neuroblastoma cells (Atasever-Arslan, Yilancioglu, Bekaroglu, Taskin, & Cetiner, Citation2015). However, in vivo studies are still needed to expand knowledge of the cytotoxic and anti-tumour mechanisms.
Bioremediation
The treatment of wastewater involves several steps, one of which may involve the use of microorganisms to metabolize organic matter. Microalgae can contribute to this process by removing inorganic nitrogen, phosphorus and CO2, products generated by these microorganisms (Talbot & de la Noüe, Citation1993). Some species of Dunaliella can remove 98% of the metals in a closed system after one week of exposure (Dahmen-Ben Moussa et al., Citation2018). D. tertiolecta has heavy metal-binding peptides (phytochelatins) and can be used in bioremediation to remove heavy metals from the environment (Tsuji et al., Citation2002). Thus, it is a candidate for the third treatment phase of saline wastewater due to its capacity to accumulate NH4+ and PO43- and heavy metals, such as copper and arsenic (Takimura, Fuse, Murakami, Kamimura, & Yamaoka, Citation1996; Yamaoka, Takimura, Fuse, & Murakami, Citation1999).
Bioindicators
Dunaliella tertiolecta is a good bioindicator for assessing the ecotoxicity of anthropogenic components in the environment. This species, as well as other algae, is highly sensitive to effluents from urban areas and industries (Lewis, Weber, & Stanley, Citation1998). Sacan & Balcioglu (Citation2006) studied the effects and responses of seaweed when grown in a medium with aluminium and effluent from a pharmaceutical industrial production. D. primolecta was the most advantageous alga as a biomarker for four representative herbicides (Santín-Montanyá, Sandín-España, García Baudín, & Coll-Morales, Citation2007). D. salina has also been used as a model to evaluate the toxicity of a typical mutagenic phenol (Chen, Jiang, & Lin, Citation2007), and the effects of high concentrations of microplastics (Chae, Kim, & An, Citation2019).
Biofuel production
Microalgae are identified as potential sources of biomass for biofuel production due to their high lipid content and rapid growth (Chisti, Citation2007). However, these organisms are still not widely applied due to being economically nonviable at large scale (Benhima et al., Citation2018). However, the interest in applying microalgae in the production of biodiesel is increasing (Huntley & Redalje, Citation2007). Thus, some species of Dunaliella, such as D. tertiolecta, D. primolecta and D. salina, are strong candidates for these applications, as they can accumulate about 40% of their dry weight in the form of lipids and show high growth rates and high CO2 absorption rates (Hosseini Tafreshi & Shariati, Citation2009; Sathya et al., Citation2023).
Antifouling
Biofouling is a worldwide phenomenon that causes enormous damage to submerged structures every year (Acevedo et al., Citation2013). To mitigate this, paints with toxic and biocidal components are commonly used (Rittschof, Lai, Kok, & Teo, Citation2003). However, these compounds have high toxicity for the surrounding environments and are very persistent in time (Thomas & Brooks, Citation2010). Thus, natural and environmentally friendly compounds with antifouling properties have been developed. In this context, D. salina emerges as a potential producer of these compounds (Gao et al., Citation2014). Gao et al. (Citation2014) verified that the extract of D. salina, composed of unsaturated and saturated 16- and 18-carbon fatty acids, has antifouling properties against diatom species and barnacle larvae.
Conclusion
Dunaliella is a genus resistant to several extreme environments, highlighting hypersaline, low pH and low temperature conditions, often reflected in the production of compounds, such as carotenoids, lipids, and glycerol. In addition to these, Dunaliella species synthesize compounds with antimicrobial and antiproliferative characteristics. Among the species of this genus, Dunaliella salina is the most studied and applied in the industry, followed by D. tertiolecta, D. parva, and D. viridis. These species are usually easy to grow, with open growth systems being the most used method in industrial production. When grown in photobioreactors, different conditions are used depending on the purpose of the production.
With the advent of genetic engineering, these species were considered as a biological platform to produce several recombinant products, presenting advantages when compared to other organisms, such as yeast or bacteria. When compared to cell lines of mammals and live animals, some Dunaliella species show rapid growth and higher yield with less risk of contamination. Genetic engineering has also allowed the identification and isolation of genes of interest, thus new strains have been developed with mutations in production pathways of carotenoids and other high-value products.
Dunaliella has several potential applications in the pharmaceutical and cosmetic industries, through bioactive compounds, and the food industry, as it is a rich source of protein and food supplements. These microalgae are also applied in antifouling paints, biofuel production, and bioremediation. Thus, it is expected that the high biotechnological potential associated with this genus, as reviewed here, will be reflected in a growing scientific and economic interest so that more ecological, efficient and innovative industrial solutions can be developed.
Author contributions
M. Barbosa: research question, literature search and writing; L. G. Inácio: research question, literature search and writing; C. Afonso: editing and final draft; P. Maranhão: editing and final draft.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Acevedo, M. S., Puentes, C., Carreño, K., León, J. G., Stupak, M. … Blustein, G. (2013). Antifouling paints based on marine natural products from Colombian Caribbean. International Biodeterioration & Biodegradation, 83, 97–104. doi:10.1016/j.ibiod.2013.05.002
- Aguilera, A., & Amils, R. (2005). Tolerance to cadmium in sp. (Chlorophyta) strains isolated from an extreme acidic environment, the Tinto River (SW, Spain). Aquatic Toxicology, 75, 316–329. doi:10.1016/j.aquatox.2005.09.002
- Aricescu, A. R., & Owens, R. J. (2013). Expression of recombinant glycoproteins in mammalian cells: Towards an integrative approach to structural biology. Current Opinion in Structural Biology, 23, 345–356. doi:10.1016/j.sbi.2013.04.003
- Atasever-Arslan, B., Yilancioglu, K., Bekaroglu, M. G., Taskin, E., & Cetiner, E. A. S. (2015). Cytotoxic effect of extract from Dunaliella salina against SH-SY5Y neuroblastoma cells. General Physiology and Biophysics, 34, 201–207. doi:10.4149/gpb_2014034
- Barzegari, A., Hejazi, M. A., Hosseinzadeh, N., Eslami, S., Mehdizadeh Aghdam, E., & Hejazi, M. S. (2010). Dunaliella as an attractive candidate for molecular farming. Molecular Biology Reports, 37, 3427–3430. doi:10.1007/s11033-009-9933-4
- Béchet, Q., Coulombier, N., Vasseur, C., Lasserre, T., Le Dean, L., & Bernard, O. (2018). Full-scale validation of an algal productivity model including nitrogen limitation. Algal Research, 31, 377–386. doi:10.1016/j.algal.2018.02.010
- Becker, E. W. (2007). Micro-algae as a source of protein. Biotechnology Advances, 25, 207–210. doi:10.1016/j.biotechadv.2006.11.002
- Ben-Amotz, A. (1993). Production of β-carotene and vitamine by the halotolerant algae Dunaliella. In O. Ahaway & Zabrosky (Eds.), Marine biotechnology (pp. 411–417). New- York: Plenum Press. doi:10.1007/978-1-4899-2391-2_11
- Ben-Amotz, A. (1995). New mode of Dunaliella biotechnology: Two-phase growth for β-carotene production. Journal of Applied Phycology, 7, 65–68. doi:10.1007/BF00003552
- Ben-Amotz, A. (1996). Effect of low temperature on the stereoisomer composition of β-carotene in the halotolerant alga Dunaliella bardawil (Chlorophyta). Journal of Phycology, 32, 272–275. doi:10.1111/j.0022-3646.1996.00272.x
- Ben-Amotz, A., & Avron, M. (1989a). The biotechnology of mass culturing of Dunaliella for products of commercial interest. In R. C. Cresswell, T. A. V. Ress, & N. Shah (Eds.), Algal and Cyanobacterial Biotechnology (pp. 90–114). London, England: Longman Scientific and Technical Press.
- Ben-Amotz, A., & Avron, M. (1989b). The wavelength dependence of massive carotene synthesis in Dunaliella bardawil (Chlorophyceae). Journal of Phycology, 25, 175–178. doi:10.1111/j.0022-3646.1989.00175.x
- Ben-Amotz, A., & Avron, M. (1990). The biotechnology of cultivating the halotolerant alga Dunaliella. Trends in Biotechnology, 8, 121–126. doi:10.1016/0167-7799(90)90152-N
- Ben-Amotz, A., Katz, A., & Avron, M. (1982). Accumulation of b-carotene in halotolerant algae: Purification and characterization of b-carotene-rich globules from Dunaliella bardawil (chlorophyceae). Journal of Phycology, 18, 529–537. doi:10.1111/j.1529-8817.1982.tb03219.x
- Ben-Amotz, A., Lers, A., & Avron, M. (1988). Stereoisomers of β-carotene and phytoene in the alga Dunaliella bardawil. Plant Physiology, 86, 1286–1291. doi:10.1104/pp.86.4.1286
- Ben-Amotz, A., & Shaish, A. (1992). B-carotene biosynthesis. In M. Avron & A. Ben-Amotz (Eds.), Dunaliella: Physiology, biochemistry, and biotechnology (pp. 205–216). Boca Raton: CRC press.
- Ben-Amotz, A., Shaish, A., & Avron, M. (1991). The biotechnology of cultivating Dunaliella for production of β-carotene rich algae. Bioresource Technology, 38, 233–235. doi:10.1016/0960-8524(91)90160-L
- Benhima, R., El Arroussi, H., Kadmiri, I. M., El Mernissi, N., Wahby, I. … Bendaou, N. (2018). Nitrate reductase inhibition induces lipid enhancement of Dunaliella tertiolecta for biodiesel production. Scientific World Journal, 2018, 1–8. doi:10.1155/2018/6834725
- Bental, M., Pick, U., Avron, M., & Degani, H. (1990). The role of intracellular orthophosphate in triggering osmoregulation in the alga Dunaliella salina. European Journal of Biochemistry, 188, 117–122. doi:10.1111/j.1432-1033.1990.tb15378.x
- Bergmann, P., Ripplinger, P., Beyer, L., & Trösch, W. (2013). Disposable flat panel airlift photobioreactors. Chemie Ingenieur Technik, 85, 202–205. doi:10.1002/cite.201200132
- Besson, A., Formosa-Dague, C., & Guiraud, P. (2019). Flocculation-flotation harvesting mechanism of Dunaliella salina: From nanoscale interpretation to industrial optimization. Water Research, 155, 352–361. doi:10.1016/j.watres.2019.02.043
- Besson, A., & Guiraud, P. (2013). High-Ph-induced flocculation–flotation of the hypersaline microalga Dunaliella salina. Bioresource Technology, 147, 464–470. doi:10.1016/j.biortech.2013.08.053
- Bloch, K. (1992). Sterol molecule: Structure, biosynthesis, and function. Steroids, 57, 378–383. doi:10.1016/0039-128X(92)90081-J
- Borowitzka, M. A. (1990). The mass culture of Dunaliella salina. Technical Resource Paper, FAO Network of Agriculture Centers in Asia, 63–80.
- Borowitzka, M. A. (1995). Microalgae as sources of pharmaceuticals and other biologically active compounds. Journal of Applied Phycology, 7, 3–15. doi:10.1007/BF00003544
- Borowitzka, M. A. (1999). Commercial production of microalgae: Ponds, tanks, tubes and fermenters. Journal of Biotechnology, 70, 313–321. doi:10.1016/S0168-1656(99)00083-8
- Borowitzka, L. J., & Borowitzka, M. A. (1990). Commercial production of b-carotene by Dunalella salina in open ponds. Bulletin of Marine Science, 47, 244–252.
- Borowitzka, M. A., & Borowitzka, L. J. (1987). Limits to growth and carotenogenesis in laboratory and large-scale outdoors of Dunalella salina. In T. Stadler, J. Mollion, M-C. Verdus, Y. Karamanos, H. Morvan & D. Christiaen (Eds.), Algal biotechnology (pp. 345–402). Essex, UK: Elsevier Applied Science.
- Borowitzka, M. A., & Borowitzka, L. J. (1988). Algal growth media sources of algal culture. In M. A. Borowitzka & L. J. Borowitzka (Eds.), Micro-algal Biotechnology (pp. 465–465). Cambridge, UK: Cambridge University Press.
- Borowitzka, M., & Borowitzka, L. J. (1989). Beta-carotene (provitamin A) with algae. Biotechnology of Vitamins, Pigments and Growth Factors, 1, 15–26.
- Borowitzka, M. A., & Siva, C. J. (2007). The taxonomy of the genus Dunaliella (Chlorophyta, Dunaliellales) with emphasis on the marine and halophilic species. Journal of Applied Phycology, 19, 567–590. doi:10.1007/s10811-007-9171-x
- Buchheim, M. A., Kirkwood, A. E., Buchheim, J. A., Verghese, B., & Henley, W. J. (2010). Hypersaline soil supports a diverse community of Dunaliella (Chlorophyceae). Journal of Phycology, 46, 1038–1047. doi:10.1111/j.1529-8817.2010.00886.x
- Butinar, L., Sonjak, S., Zalar, P., Plemenitaš, A., & Gunde-Cimerman, N. (2005). Melanized halophilic fungi are eukaryotic members of microbial communities in hypersaline waters of solar salterns. Botanica Marina, 48, 73–79. doi:10.1515/BOT.2005.007
- Cakmak, Y. S., Kaya, M., & Asan-Ozusaglam, M. (2014). Biochemical composition and bioactivity screening of various extracts from Dunaliella salina, a green microalga. Excli Journal, 13, 679–690. doi:10.17877/DE290R-6669
- Camacho, F., Macedo, A., & Malcata, F. (2019). Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Marine Drugs, 17, 312. doi:10.3390/md17060312
- Capa-Robles, W., García-Mendoza, E., & Paniagua-Michel, J. D. J. (2021). Enhanced β-carotene and biomass production by induced mixotrophy in Dunaliella salina across a combined strategy of glycerol, salinity, and light. Metabolites, 11, 866. doi:10.3390/metabo11120866
- Çelekli, A., & Dönmez, G. (2006). Effect of pH, light intensity, salt and nitrogen concentrations on growth and β-carotene accumulation by a new isolate of Dunaliella sp. World Journal of Microbiology and Biotechnology, 22, 183–189. doi:10.1007/s11274-005-9017-0
- Cereceda, P., Larrain, H., Osses, P., Farías, M., & Egaña, I. (2008). The climate of the coast and fog zone in the Tarapacá Region, Atacama Desert, Chile. Atmospheric Research, 87, 301–311. doi:10.1016/j.atmosres.2007.11.011
- Chae, Y., Kim, D., & An, Y.-J. (2019). Effects of micro-sized polyethylene spheres on the marine microalga Dunaliella salina: Focusing on the algal cell to plastic particle size ratio. Aquatic Toxicology, 216, 105296. doi:10.1016/j.aquatox.2019.105296
- Chai, X.-J., Chen, H.-X., Xu, W.-Q., & Xu, Y.-W. (2013). Expression of soybean Kunitz trypsin inhibitor gene SKTI in Dunaliella salina. Journal of Applied Phycology, 25, 139–144. doi:10.1007/s10811-012-9847-8
- Chang, T., Ohta, S., Ikegami, N., Miyata, H., Kashimoto, T., & Kondo, M. (1993). Antibiotic substances produced by a marine green alga, Dunaliella primolecta. Bioresource Technology, 44, 149–153. doi:10.1016/0960-8524(93)90189-I
- Chavoshi, Z. Z., & Shariati, M. (2019). Lipid production in Dunaliella salina under autotrophic, heterotrophic, and mixotrophic conditions. Biologia, 74, 1579–1590. doi:10.2478/s11756-019-00336-6
- Chen, J., Jiang, J. G., & Lin, Q. S. (2007). Technical note: Toxicity tests of typical mutagenic phenols on Dunaliella salina. Transactions of the ASABE, 50, 685–688. doi:10.13031/2013.22657
- Chew, K. W., Yap, J. Y., Show, P. L., Suan, N. H., Juan, J. C. … Chang, J.-S. (2017). Microalgae biorefinery: High value products perspectives. Bioresource Technology, 229, 53–62. doi:10.1016/j.biortech.2017.01.006
- Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25, 294–306. doi:10.1016/j.biotechadv.2007.02.001
- Chow, Y. Y. S., Goh, S. J. M., Su, Z., Ng, D. H. P., Lim, C. Y. … Lee, Y. K. (2013). Continual production of glycerol from carbon dioxide by Dunaliella tertiolecta. Bioresource Technology, 136, 550–555. doi:10.1016/j.biortech.2013.03.040
- Coleman, R. A., & Lee, D. P. (2004). Enzymes of triacylglycerol synthesis and their regulation. Progress in Lipid Research, 43, 134–176. doi:10.1016/S0163-7827(03)00051-1
- Colusse, G. A., Mendes, C. R. B., Duarte, M. E. R., Carvalho, J. C. D., & Noseda, M. D. (2020). Effects of different culture media on physiological features and laboratory scale production cost of Dunaliella salina. Biotechnology Reports, 27, e00508. doi:10.1016/j.btre.2020.e00508
- Cunningham, F. X., Pogson, B., Sun, Z., McDonald, K. A., DellaPenna, D., & Gantt, E. (1996). Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. The Plant Cell, 8, 1613–1626. doi:10.1105/tpc.8.9.1613
- Cycil, L. M., Hausrath, E. M., Ming, D. W., Adcock, C. T., Raymond, J., Remias, D., & Ruemmele, W. P. (2021). Investigating the growth of algae under low atmospheric pressures for potential food and oxygen production on mars. investigating the growth of algae under low atmospheric pressures for potential food and oxygen production on mars. Frontiers in Microbiology, 12, 12. doi:10.3389/fmicb.2021.733244
- Dahmen-Ben Moussa, I., Athmouni, K., Chtourou, H., Ayadi, H., Sayadi, S., & Dhouib, A. (2018). Phycoremediation potential, physiological, and biochemical response of Amphora subtropica and Dunaliella sp. to nickel pollution. Journal of Applied Phycology, 30, 931–941. doi:10.1007/s10811-017-1315-z
- Duan, X., Xie, C., Hill, D. R. A., Barrow, C. J., Dunshea, F. R., Martin, G. J. O., & Suleria, H. A. R. (2023). Bioaccessibility, bioavailability and bioactivities of carotenoids in microalgae: A review. Food Reviews International, 1–30. doi:10.1080/87559129.2023.2165095
- Dufossé, L., Galaup, P., Yaron, A., Arad, S. M., Blanc, P., Chidambara Murthy, K. N., & Ravishankar, G. A. (2005). Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends in Food Science & Technology, 16, 389–406. doi:10.1016/j.tifs.2005.02.006
- Dunal, F. (1838). Extrait d’un mémoire sur les algues qui colorent en rouge certains eaux des marais salants méditerranéens. Ann Sc Nat Bot Sér, 18, 172.
- Du, J., Wang, S., You, H., & Zhao, X. (2013). Understanding the toxicity of carbon nanotubes in the environment is crucial to the control of nanomaterials in producing and processing and the assessment of health risk for human: A review. Environmental Toxicology and Pharmacology, 36, 451–462. doi:10.1016/j.etap.2013.05.007
- el Agawany, N., Kaamoush, M., El-Zeiny, A., & Ahmed, M. (2021). Effect of heavy metals on protein content of marine unicellular green alga Dunaliella tertiolecta. Environmental Monitoring and Assessment, 193. doi:10.1007/s10661-021-09353-y
- EL Arroussi, H., Benhima, R., Elbaouchi, A., Sijilmassi, B., EL Mernissi, N. … Smouni, A. (2018). Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). Journal of Applied Phycology, 30, 2929–2941. doi:10.1007/s10811-017-1382-1
- Feltl, L., Pacakova, V., Stulik, K., & Volka, K. (2006). Reliability of carotenoid analyses: A review. Current Analytical Chemistry, 1, 93–102. doi:10.2174/1573411052948424
- Feng, S., Li, X., Xu, Z., & Qi, J. (2014). Dunaliella salina as a novel host for the production of recombinant proteins. Applied Microbiology and Biotechnology, 98, 4293–4300. doi:10.1007/s00253-014-5636-4
- Feng, S., Xue, L., Liu, H., & Lu, P. (2009). Improvement of efficiency of genetic transformation for Dunaliella salina by glass beads method. Molecular Biology Reports, 36, 1433–1439. doi:10.1007/s11033-008-9333-1
- Fernandes, P., & Cabral, J. M. S. (2007). Phytosterols: Applications and recovery methods. Bioresource Technology, 98, 2335–2350. doi:10.1016/j.biortech.2006.10.006
- Francavilla, M., Kamaterou, P., Intini, S., Monteleone, M., & Zabaniotou, A. (2015). Cascading microalgae biorefinery: Fast pyrolysis of Dunaliella tertiolecta lipid extracted-residue. Algal Research, 11, 184–193. doi:10.1016/j.algal.2015.06.017
- Francavilla, M., Trotta, P., & Luque, R. (2010). Phytosterols from Dunaliella tertiolecta and Dunaliella salina: A potentially novel industrial application. Bioresource Technology, 101, 4144–4150. doi:10.1016/j.biortech.2009.12.139
- Fu, W., Guðmundsson, Ó., Paglia, G., Herjólfsson, G., Andrésson, Ó. S., Palsson, B. Ø., & Brynjólfsson, S. (2013). Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Applied Microbiology and Biotechnology, 97, 2395–2403. doi:10.1007/s00253-012-4502-5
- Gamlieli-Bonshtein, I., Korin, E., & Cohen, S. (2002). Selective separation ofcis-trans geometrical isomers of b-carotene via CO2 supercritical fluid extraction. Biotechnology and Bioengineering, 80, 169–174. doi:10.1002/bit.10357
- Gao, M., Li, F., Su, R., Wang, K., Li, X., & Lu, W. (2014). Antifouling potential of the marine microalga Dunaliella salina. World Journal of Microbiology and Biotechnology, 30, 2899–2905. doi:10.1007/s11274-014-1717-x
- García, F., Freile-Pelegrín, Y., & Robledo, D. (2007). Physiological characterization of Dunaliella sp. (Chlorophyta, Volvocales) from Yucatan, Mexico. Bioresource Technology, 98, 1359–1365. doi:10.1016/j.biortech.2006.05.051
- Garg, S., Li, Y., Wang, L., & Schenk, P. M. (2012). Flotation of marine microalgae: Effect of algal hydrophobicity. Bioresource Technology, 121, 471–474. doi:10.1016/j.biortech.2012.06.111
- Ghoshal, D., Mach, D., Agarwal, M., Goyal, A., & Goyal, A. (2002). Osmoregulatory isoform of dihydroxyacetone phosphate reductase from Dunaliella tertiolecta: Purification and characterization. Protein Expression and Purification, 24, 404–411. doi:10.1006/prep.2001.1588
- Gim, G. H., Kim, J. K., Kim, H. S., Kathiravan, M. N., Yang, H., Jeong, S.-H., & Kim, S. W. (2014). Comparison of biomass production and total lipid content of freshwater green microalgae cultivated under various culture conditions. Bioprocess and Biosystems Engineering, 37, 99–106. doi:10.1007/s00449-013-0920-8
- Gonabadi, E., Samadlouie, H. R., & Shafafi Zenoozian, M. (2022). Optimization of culture conditions for enhanced Dunaliella salina productions in mixotrophic culture. Preparative Biochemistry and Biotechnology, 52, 154–162. doi:10.1080/10826068.2021.1922917
- Gonçalves, A. M., Pedro, A. Q., Maia, C., Sousa, F., Queiroz, J. A., & Passarinha, L. A. (2013). Pichia pastoris: A recombinant microfactory for antibodies and human membrane proteins. Journal of Microbiology and Biotechnology, 23, 587–601. doi:10.4014/jmb.1210.10063
- Goncalves, E. C., Wilkie, A. C., Kirst, M., & Rathinasabapathi, B. (2016). Metabolic regulation of triacylglycerol accumulation in the green algae: Identification of potential targets for engineering to improve oil yield. Plant Biotechnology Journal, 14, 1649–1660. doi:10.1111/pbi.12523
- Gonzalez, M. A., Coleman, A. W., Gomez, P. I., & Montoya, R. (2001). Phylogenetic relationship among various strains of Dunaliella (Chlorophyceae) based on nuclear its rDNA sequences. Journal of Phycology, 37, 604–611. doi:10.1046/j.1529-8817.2001.037004604.x
- Guan, Z. J., Guo, B., Huo, Y. L., Guan, Z. P., Dai, J. K., & Wei, Y. H. (2013). Recent advances and safety issues of transgenic plant-derived vaccines. Applied Microbiology and Biotechnology, 97, 2817–2840. doi:10.1007/s00253-012-4566-2
- Guillard, R. R. L. (1975). Culture of phytoplankton for feeding marine invertebrates. In W. L. Smith & M. H. Chanley (Eds.), Culture of Marine Invertebrate Animals (pp. 29–60). Springer US. doi:10.1007/978-1-4615-8714-9_3
- Gupta, S. K., Trivedi, D., Srivastava, S., Joshi, S., Halder, N., & Verma, S. D. (2003). Lycopene attenuates oxidative stress induced experimental cataract development: An in vitro and in vivo study. Nutrition, 19, 794–799. doi:10.1016/S0899-9007(03)00140-0
- Hadi, M. R., Shariati, M., & Afsharzadeh, S. (2008). Microalgal biotechnology: Carotenoid and glycerol production by the green algae Dunaliella isolated from the Gave-Khooni salt marsh, Iran. Biotechnology and Bioprocess Engineering, 13, 540–544. doi:10.1007/s12257-007-0185-7
- Harvey, P. J., & Ben-Amotz, A. (2020). Towards a sustainable Dunaliella salina microalgal biorefinery for 9-cis β-carotene production. Algal Research, 50, 102002. doi:10.1016/j.algal.2020.102002
- Havas, F., Krispin, S., Cohen, M., Loing, E., Farge, M., Suere, T., & Attia-Vigneau, J. (2022). A Dunaliella salina extract counteracts skin aging under intense solar irradiation thanks to its antiglycation and anti-inflammatory properties. Marine Drugs, 20, 104. doi:10.3390/md20020104
- Hejazi, M. A., de Lamarliere, C., Rocha, J. M. S., Vermuë, M., Tramper, J., & Wijffels, R. H. (2002). Selective extraction of carotenoids from the microalga Dunaliella salina with retention of viability. Biotechnology and Bioengineering, 79, 29–36. doi:10.1002/bit.10270
- Hejazi, M. A., Holwerda, E., & Wijffels, R. H. (2004). Milking microalga Dunaliella salina for β-carotene production in two-phase bioreactors. Biotechnology and Bioengineering, 85, 475–481. doi:10.1002/bit.10914
- He, Q., Lin, Y., Tan, H., Zhou, Y., Wen, Y. … Zhang, Q. (2020). Transcriptomic profiles of Dunaliella salina in response to hypersaline stress. BMC Genomics, 21, 1–17. doi:10.1186/s12864-020-6507-2
- He, Q., Qiao, D., Bai, L., Zhang, Q., Yang, W., Li, Q., & Cao, Y. (2007). Cloning and characterization of a plastidic glycerol 3-phosphate dehydrogenase cDNA from Dunaliella salina. Journal of Plant Physiology, 164, 214–220. doi:10.1016/j.jplph.2006.04.004
- He, Q., Qiao, D., Zhang, Q., Li, Y., Xu, H. … Cao, Y. (2004). Cloning and expression study of a putative high-affinity nitrate transporter gene from Dunaliella salina. Journal of Applied Phycology, 16, 395–400. doi:10.1023/B:JAPH.0000047950.76549.ce
- Herrero, M., Jaime, L., Martín-Álvarez, P. J., Cifuentes, A., & Ibáñez, E. (2006). Optimization of the extraction of antioxidants from Dunaliella salina microalga by pressurized liquids. Journal of Agricultural and Food Chemistry, 54, 5597–5603. doi:10.1021/jf060546q
- Hirschberg, J. (2001). Carotenoid biosynthesis in flowering plants. Current Opinion in Plant Biology, 4, 210–218. doi:10.1016/S1369-5266(00)00163-1
- Hirschberg, J., Cohen, M., Harker, M., Lotan, T., Mann, V., & Pecker, I. (1997). Molecular genetics of the carotenoid biosynthesis pathway in plants and algae. Pure and Applied Chemistry, 69, 2151–2158. doi:10.1351/pac199769102151
- Hosseini Tafreshi, A., & Shariati, M. (2009). Dunaliella biotechnology: Methods and applications. Journal of Applied Microbiology, 107, 14–35. doi:10.1111/j.1365-2672.2009.04153.x
- Hu, C.-C., Lin, J.-T., Lu, F.-J., Chou, F.-P., & Yang, D.-J. (2008). Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chemistry, 109, 439–446. doi:10.1016/j.foodchem.2007.12.043
- Huntley, M. E., & Redalje, D. G. (2007). CO2 mitigation and renewable oil from photosynthetic microbes: A new appraisal. Mitigation and Adaptation Strategies for Global Change, 12, 573–608. doi:10.1007/s11027-006-7304-1
- Iglesias, M. J., Soengas, R., Probert, I., Guilloud, E., Gourvil, P. … Ortiz, F. L. (2019). NMR characterization and evaluation of antibacterial and antiobiofilm activity of organic extracts from stationary phase batch cultures of five marine microalgae (Dunaliella sp ., D. salina, Chaetoceros calcitrans, C. gracilis and Tisochrysis lutea). Phytochemistry, 164, 192–205. doi:10.1016/j.phytochem.2019.05.001
- Ismaiel, M. M. S., El-Ayouty, Y. M., Said, A. A., & Fathey, H. A. (2018). Transformation of Dunaliella parva with PSY gene: Carotenoids show enhanced antioxidant activity under polyethylene glycol and calcium treatments. Biocatalysis and Agricultural Biotechnology, 16, 378–384. doi:10.1016/j.bcab.2018.09.011
- Jayappriyan, K. R., Rajkumar, R., & Rengasamy, R. (2011). Unusual occurrence of non carotenogenic strains of Dunaliella bardawil and Dunaliella parva in India. Journal of Basic Microbiology, 51, 473–483. doi:10.1002/jobm.201000384
- Jayappriyan, K. R., Rajkumar, R., Venkatakrishnan, V., Nagaraj, S., & Rengasamy, R. (2013). In vitro anticancer activity of natural β-carotene from Dunaliella salina EU5891199 in PC-3 cells. Biomedicine & Preventive Nutrition, 3, 99–105. doi:10.1016/j.bionut.2012.08.003
- Jin, E. S., & Melis, A. (2003). Microalgal biotechnology: Carotenoid production by the green algae Dunaliella salina. Biotechnology and Bioprocess Engineering, 8, 331–337. doi:10.1007/BF02949276
- Jin, E., & Polle, J. (2009). Carotenoid Biosynthesis in Dunaliella (Chlorophyta). In A. Ben-Amotz, J. E. W. Polle, & D. V. S. Rao (Eds.), The Alga Dunaliella (pp. 147–171). Science Publishers.
- Johnson, M. K., Johnson, E. J., MacElroy, R. D., Speer, H. L., & Bruff, B. S. (1968). Effects of salts on the halophilic alga Dunaliella viridis. Journal of Bacteriology, 95, 1461–1468. doi:10.1128/JB.95.4.1461-1468.1968
- Jones, H. D., & Sparks, C. A. (2009). Stable transformation of plants. In J. P. Gustafson, P. Langridge, & D. J. Somers (Eds.), Methods in Molecular Biology (Vol. 513, pp. 111–130). Humana Press. doi:10.1007/978-1-59745-427-8_7
- Katz, A., Paz, Y., & Pick, U. (2019). Salinity tolerance and iron deprivation resistance mechanisms revealed by proteomic analyzes in Dunaliella salina. In A. Ben-Amotz (Ed.), The Alga Dunaliella (1st ed. pp. 341–358). CRC Press. doi:10.1201/9780429061639-14
- Katz, A., & Pick, U. (2001). Plasma membrane electron transport coupled to Na+ extrusion in the halotolerant alga Dunaliella. Biochimica Et Biophysica Acta (BBA) - Bioenergetics, 1504, 423–431. doi:10.1016/S0005-2728(01)00157-8
- Khadim, S. R., Singh, P., Singh, A. K., Tiwari, A., Mohanta, A., & Asthana, R. K. (2018). Mass cultivation of Dunaliella salina in a flat plate photobioreactor and its effective harvesting. Bioresource Technology, 270, 20–29. doi:10.1016/j.biortech.2018.08.071
- Khramov, D. E., Matalin, D. A., Karpichev, I. V., Balnokin, Y. V., & Popova, L. G. (2019). Expression of P-Type atpases of marine green microalga Dunaliella maritima under hyperosmotic salt shock. Doklady Biochemistry and Biophysics, 488, 327–331. doi:10.1134/S1607672919050119
- Klausner, A. (1986). Algaculture: Food for Thought. Bio/technology, 4, 947–953. doi:10.1038/nbt1186-947
- Knaut, J., & Richtler, H. J. (1985). Trends in industrial uses of palm and lauric oils. Journal of the American Oil Chemists’ Society, 62, 317–327. doi:10.1007/BF02541398
- Kocberber Kilic, N., Erdem, K., & Donmez, G. (2018). Bioactive compounds produced by Dunaliella species, antimicrobial effects and optimization of the efficiency. Turkish Journal of Fisheries and Aquatic Sciences, 19, 923–933. doi:10.4194/1303-2712-v19_11_04
- Koh, L. P., & Ghazoul, J. (2008). Biofuels, biodiversity, and people: Understanding the conflicts and finding opportunities. Biological Conservation, 141, 2450–2460. doi:10.1016/j.biocon.2008.08.005
- Kroumov, A. D., Módenes, A. N., Trigueros, D. E. G., Espinoza-Quiñones, F. R., Borba, C. E., Scheufele, F. B., & Hinterholz, C. L. (2016). A systems approach for CO2 fixation from flue gas by microalgae—Theory review. Process Biochemistry, 51, 1817–1832. doi:10.1016/j.procbio.2016.05.019
- Kumudha, A., & Sarada, R. (2016). Characterization of vitamin B12 in Dunaliella salina. Journal of Food Science and Technology, 53, 888–894. doi:10.1007/s13197-015-2005-y
- Laakel, M., Lebrihi, A., Khaoua, S., Schneider, F., Lefebvre, G., & Germain, P. (1994). Relationship between valine, fatty acids, and spiramycin biosynthesis in Streptomyces ambofaciens. Canadian Journal of Microbiology, 40, 672–676. doi:10.1139/m94-106
- Lamers, P. P., Janssen, M., De Vos, R. C. H., Bino, R. J., & Wijffels, R. H. (2008). Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends in Biotechnology, 26, 631–638. doi:10.1016/j.tibtech.2008.07.002
- Lamers, P. P., van de Laak, C. C. W., Kaasenbrood, P. S., Lorier, J., Janssen, M. … Wijffels, R. H. (2010). Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnology and Bioengineering, 106, 638–648. doi:10.1002/bit.22725
- Lange, B. M., Rujan, T., Martin, W., & Croteau, R. (2000). Isoprenoid biosynthesis: The evolution of two ancient and distinct pathways across genomes. Proceedings of the National Academy of Sciences, 97, 13172–13177. doi:10.1073/pnas.240454797
- León-Bañares, R., González-Ballester, D., Galván, A., & Fernández, E. (2004). Transgenic microalgae as green cell-factories. Trends in Biotechnology, 22, 45–52. doi:10.1016/j.tibtech.2003.11.003
- Lewis, M. A., Weber, D. E., & Stanley, R. S. (1998). Comparative animal and plant toxicities of 10 treated effluents discharged to near-coastal areas of the Gulf of Mexico. Water Environment Research, 70, 1108–1117. doi:10.2175/106143098X123471
- Lichtenthaler, H. K., Schwender, J., & Müller, C. (1998). The 1-deoxy-d-xylulose-5-phosphate pathway for biosynthesis of carotenoids and other plastidic isoprenoids. In G. Garab (Ed.), Photosynthesis: Mechanisms and Effects (pp. 3215–3220). Springer Netherlands. doi:10.1007/978-94-011-3953-3_751
- Li, Q., Gao, X., Sun, Y., Zhang, Q., Song, R., & Xu, Z. (2006). Isolation and characterization of a sodium-dependent phosphate transporter gene in Dunaliella viridis. Biochemical and Biophysical Research Communications, 340, 95–104. doi:10.1016/j.bbrc.2005.11.144
- Lin, H., & Lee, Y. K. (2017). Genetic engineering of medium-chain-length fatty acid synthesis in Dunaliella tertiolecta for improved biodiesel production. Journal of Applied Phycology, 29, 2811–2819. doi:10.1007/s10811-017-1210-7
- Lin, H., Shen, H., & Lee, Y. K. (2018). Cellular and molecular responses of Dunaliella tertiolecta by expression of a plant medium chain length fatty acid specific acyl-ACP thioesterase. Frontiers in Microbiology, 9, 1–17. doi:10.3389/fmicb.2018.00619
- Lippold, F., Vom Dorp, K., Abraham, M., Hölzl, G., Wewer, V. … Dörmann, P. (2012). Fatty acid phytyl ester synthesis in chloroplasts of arabidopsis. The Plant Cell, 24, 2001–2014. doi:10.1105/tpc.112.095588
- Liu, L., Yang, H., Shin, H. D., Chen, R. R., Li, J., Du, G., & Chen, J. (2013). How to achieve high-level expression of microbial enzymes: Strategies and perspectives. Bioengineered, 4, 212–223. doi:10.4161/bioe.24761
- Lyukevich, A. A., Mouradyan, E. A., & Los, D. A. (2003). Molecular cloning and stress-dependent expression of a gene encoding ω3-fatty acid desaturase in the microalga Dunaliella salina. Russian Journal of Plant Physiology, 50, 481–486. doi:10.1023/A:1024764522062
- Madeira, M. S., Cardoso, C., Lopes, P. A., Coelho, D., Afonso, C., Bandarra, N. M., & Prates, J. A. M. (2017). Microalgae as feed ingredients for livestock production and meat quality: A review. Livestock Science, 205, 111–121. doi:10.1016/j.livsci.2017.09.020
- Matalin, D. A., Khramov, D. E., Shuvalov, A. V., Volkov, V. S., Balnokin, Y. V., & Popova, L. G. (2021). Cloning and characterization of two putative p-type ATPases from the marine microalga Dunaliella maritima similar to plant H+-ATPases and their gene expression analysis under conditions of hyperosmotic salt shock. Plants, 10, 2667. doi:10.3390/plants10122667
- Mata, T. M., Martins, A. A., & Caetano, N. S. (2010). Microalgae for biodiesel production and other applications: A review. Renewable and Sustainable Energy Reviews, 14, 217–232. doi:10.1016/j.rser.2009.07.020
- Matsumoto, G. I., Shioya, M., & Nagashima, H. (1984). Occurrence of 2-hydroxy acids in microalgae. Phytochemistry, 23, 1421–1423. doi:10.1016/S0031-9422(00)80478-1
- Mayne, S. T., Janerich, D. T., Greenwald, P., Chorost, S., Tucci, C. … McKneally, M. F. (1994). Dietary beta carotene and lung cancer risk in U.S. nonsmokers. JNCI Journal of the National Cancer Institute, 86, 33–38. doi:10.1093/jnci/86.1.33
- Michaud, D. S., Feskanich, D., Rimm, E. B., Colditz, G. A., Speizer, F. E., Willett, W. C., & Giovannucci, E. (2000). Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. The American Journal of Clinical Nutrition, 72, 990–997. doi:10.1093/ajcn/72.4.990
- Monte, J., Ribeiro, C., Parreira, C., Costa, L., Brive, L. … Crespo, J. G. (2020). Biorefinery of Dunaliella salina: Sustainable recovery of carotenoids, polar lipids and glycerol. Bioresource Technology, 297. doi:10.1016/j.biortech.2019.122509
- Monte, J., Sá, M., Galinha, C. F., Costa, L., Hoekstra, H., Brazinha, C., & Crespo, J. G. (2018). Harvesting of Dunaliella salina by membrane filtration at pilot scale. Separation and Purification Technology, 190, 252–260. doi:10.1016/j.seppur.2017.08.019
- Moulton, T. P., & Burford, M. A. (1990). The mass culture of Dunaliella viridis (Volvocales, Chlorophyta) for oxygenated carotenoids: Laboratory and pilot plant studies. Hydrobiologia, 204, 401–408. doi:10.1007/BF00040263
- Naik, P. S., Chanemougasoundharam, A., Khurana, S. M. P., & Kalloo, G. (2003). Genetic manipulation of carotenoid pathway in higher plants. In Current Science (Vol. 85, pp. 1423–1430). Current Science Association. doi:10.2307/24108823.
- Newman, J. D., & Chappell, J. (1999). Isoprenoid biosynthesis in plants: Carbon partitioning within the cytoplasmic pathway. Critical Reviews in Biochemistry and Molecular Biology, 34, 95–106. doi:10.1080/10409239991209228
- Ng, D. H. P., Low, C. S., Chow, Y. Y. S., & Lee, Y. K. (2014). Intracellular glycerol accumulation in light limited Dunaliella tertiolecta culture is determined by partitioning of glycerol across the cell membrane. FEMS Microbiology Letters, 357, n/a–n/a. doi:10.1111/1574-6968.12514
- Nishshanka, G. K. S. H., Anthonio, R. A. D. P., Nimarshana, P. H. V., Ariyadasa, T. U., & Chang, J.-S. (2022). Marine microalgae as sustainable feedstock for multi-product biorefineries. Biochemical Engineering Journal, 187, 108593. doi:10.1016/j.bej.2022.108593
- Nonomura, A. M. (1987). Process for producing a naturally-derived carotene⁄oil composition by direct extraction from algae. US Patent 4680314.
- Ohlrogge, J. B. (1994). Design of new plant products: Engineering of fatty acid metabolism. Plant Physiology, 104, 821–826. doi:10.1104/pp.104.3.821
- Ohlrogge, J., & Browse, J. (1995). Lipid biosynthesis. The Plant Cell, 7, 957–970. doi:10.1105/tpc.7.7.957
- Oren, A. (2005). A hundred years of Dunaliella research: 1905-2005. Saline Systems, 1, 2. doi:10.1186/1746-1448-1-2
- Oren, A. (2009). Saltern evaporation ponds as model systems for the study of primary production processes under hypersaline conditions. Aquatic Microbial Ecology, 56, 193–204. doi:10.3354/ame01297
- Oren, A. (2014). The ecology of Dunaliella in high-salt environments. Journal of Biological Research-Thessaloniki, 21, 23. doi:10.1186/s40709-014-0023-y
- Park, S., Lee, Y., & Jin, E. (2013). Comparison of the responses of two Dunaliella strains, Dunaliella salina CCAP 19/18 and Dunaliella bardawil to light intensity with special emphasis on carotenogenesis. ALGAE, 28, 203–211. doi:10.4490/algae.2013.28.2.203
- Pasquet, V., Morisset, P., Ihammouine, S., Chepied, A., Aumailley, L. … Picot, L. (2011). Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Marine Drugs, 9, 819–831. doi:10.3390/md9050819
- Patrick, L. (2000). Beta-carotene: The controversy continues. Alternative Medicine Review, 5, 530–545.
- Pick, U. (1999). Dunaliella acidophila — a most extreme acidophilic alga. Enigmatic Microorganisms and Life in Extreme Environments, 465–478. doi:10.1007/978-94-011-4838-2_36
- Pick, U., & Avidan, O. (2017). Triacylglycerol is produced from starch and polar lipids in the green alga Dunaliella tertiolecta. Journal of Experimental Botany, 68, 4939–4950. doi:10.1093/jxb/erx280
- Pirwitz, K., Rihko-Struckmann, L., & Sundmacher, K. (2015). Comparison of flocculation methods for harvesting Dunaliella. Bioresource Technology, 196, 145–152. doi:10.1016/j.biortech.2015.07.032
- Polle, J., Tran, D., & Ben-Amotz, A. (2009). History, distribution, and habitats of algae of the genus Dunaliella Teodoresco (Chlorophyceae). In A. Ben-Amotz, J. Polle, D. Subba Rao, & N. Enfield (Eds.), The Alga Dunaliella. Biodiversity, physiology, genomics and biotechnology (pp. 1–13). Boca Raton, USA: Science Publishers.
- Pragya, N., Pandey, K. K., & Sahoo, P. K. (2013). A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renewable and Sustainable Energy Reviews, 24, 159–171. doi:10.1016/j.rser.2013.03.034
- Primrose, S. (1991). Molecular biotechnology (2nd ed.). Oxford: Blackwell Science.
- Puente-Sánchez, F., Olsson, S., & Aguilera, A. (2016). Comparative transcriptomic analysis of the response of Dunaliella acidophila (Chlorophyta) to short-term cadmium and chronic natural metal-rich water exposures. Microbial Ecology, 72, 595–607. doi:10.1007/s00248-016-0824-7
- Pulz, O. (2001). Photobioreactors: Production systems for phototrophic microorganisms. Applied Microbiology and Biotechnology, 57, 287–293. doi:10.1007/s002530100702
- Pulz, O., & Gross, W. (2004). Valuable products from biotechnology of microalgae. Applied Microbiology and Biotechnology, 65, 635–648. doi:10.1007/s00253-004-1647-x
- Raja, R., Hemaiswarya, S., & Rengasamy, R. (2007). Exploitation of Dunaliella for β-carotene production. Applied Microbiology and Biotechnology, 74, 517–523. doi:10.1007/s00253-006-0777-8
- Rittschof, D., Lai, C.-H., Kok, L.-M., & Teo, S. L.-M. (2003). Pharmaceuticals as antifoulants: Concept and principles. Biofouling, 19, 207–212. doi:10.1080/0892701021000083769
- Rohmer, M., Knani, M., Simonin, P., Sutter, B., & Sahm, H. (1993). Isoprenoid biosynthesis in bacteria: A novel pathway for the early steps leading to isopentenyl diphosphate. Biochemical Journal, 295, 517–524. doi:10.1042/bj2950517
- Ruane, M. (1974). Extraction of Caroteniferous Materials from Algae. Australian Patent 7239574.
- Saçan, M. T., & Balcıoğlu, I. A. (2006). A case study on algal response to raw and treated effluents from an aluminum plating plant and a pharmaceutical plant. Ecotoxicology & Environmental Safety, 64, 234–243. doi:10.1016/j.ecoenv.2005.03.017
- Sandmann, G. (2001). Carotenoid biosynthesis and biotechnological application. Archives of Biochemistry and Biophysics, 385, 4–12. doi:10.1006/abbi.2000.2170
- Santín-Montanyá, I., Sandín-España, P., García Baudín, J. M., & Coll-Morales, J. (2007). Optimal growth of Dunaliella primolecta in axenic conditions to assay herbicides. Chemosphere, 66, 1315–1322. doi:10.1016/j.chemosphere.2006.07.019
- Sathya, A. B., Thirunavukkarasu, A., Nithya, R., Nandan, A., Sakthishobana, K. … Deepanraj, B. (2023). Microalgal biofuel production: Potential challenges and prospective research. Fuel, 332, 126199. doi:10.1016/j.fuel.2022.126199
- Schmidt-Dannert, C. (2000). Engineering novel carotenoids in microorganisms. Current Opinion in Biotechnology, 11, 255–261. doi:10.1016/S0958-1669(00)00093-8
- Schubert, B. A., Timofeeff, M. N., Lowenstein, T. K., & Polle, J. E. W. (2010). Dunaliella cells in fluid inclusions in halite: Significance for long-term survival of prokaryotes. Geomicrobiology Journal, 27, 61–75. doi:10.1080/01490450903232207
- Schwender, J., Seemann, M., Lichtenthaler, H. K., & Rohmer, M. (1996). Biosynthesis of isoprenoids (carotenoids, sterols, prenyl side-chains of chlorophylls and plastoquinone) via a novel pyruvate/glyceraldehyde 3-phosphate non-mevalonate pathway in the green alga Scenedesmus obliquus. Biochemical Journal, 316, 73–80. doi:10.1042/bj3160073
- Seemann, M., Tse Sum Bui, B., Wolff, M., Miginiac-Maslow, M., & Rohmer, M. (2006). Isoprenoid biosynthesis in plant chloroplasts via the MEP pathway: Direct thylakoid/ferredoxin-dependent photoreduction of GcpE/IspG. FEBS Letters, 580, 1547–1552. doi:10.1016/j.febslet.2006.01.082
- Sekler, I., & Pick, U. (1993). Purification and properties of a plasma membrane H+-ATPase from the extremely acidophilic alga Dunaliella acidophila. Plant Physiology, 101, 1055–1061. doi:10.1104/pp.101.3.1055
- Shaish, A., Harari, A., Hananshvili, L., Cohen, H., Bitzur, R. … Harats, D. (2006). 9-cis β-carotene-rich powder of the alga Dunaliella bardawil increases plasma HDL-cholesterol in fibrate-treated patients. Atherosclerosis, 189, 215–221. doi:10.1016/j.atherosclerosis.2005.12.004
- Shewmaker, C. K., Sheehy, J. A., Daley, M., Colburn, S., & Ke, D. Y. (1999). Seed-specific overexpression of phytoene synthase: Increase in carotenoids and other metabolic effects. Plant Journal, 20, 401–412. doi:10.1046/j.1365-313x.1999.00611.x
- Siefermann-Harms, D., Joyard, J., & Douce, R. (1978). Light-induced changes of the carotenoid levels in chloroplast envelopes. Plant Physiology, 61, 530–533. doi:10.1104/pp.61.4.530
- Silva, M. R. O. B. D., Moura, Y. A. S., Converti, A., Porto, A. L. F., Viana Marques, D. D. A., & Bezerra, R. P. (2021). Assessment of the potential of Dunaliella microalgae for different biotechnological applications: A systematic review. Algal Research, 58, 102396. doi:10.1016/j.algal.2021.102396
- Singh, P., Baranwal, M., & Reddy, S. M. (2016). Antioxidant and cytotoxic activity of carotenes produced by Dunaliella salina under stress. Pharmaceutical Biology, 54, 2269–2275. doi:10.3109/13880209.2016.1153660
- Slocombe, S. P., Zhang, Q., Ross, M., Anderson, A., Thomas, N. J. … Day, J. G. (2015). Unlocking nature’s treasure-chest: Screening for oleaginous algae. Scientific Reports, 5, 9844. doi:10.1038/srep09844
- Song, H. G., Byeon, S. Y., Chung, G. Y., Jung, S.-M., Choi, J. I., & Shin, H. S. (2018). A systematic correlation analysis of carotenoids, chlorophyll, non-pigmented cell mass, and cell number for the blueprint of Dunaliella salina culture in a photobioreactor. Bioprocess and Biosystems Engineering, 41, 1295–1303. doi:10.1007/s00449-018-1957-5
- Spolaore, P., Joannis-Cassan, C., Duran, E., & Isambert, A. (2006). Commercial applications of microalgae. Journal of Bioscience and Bioengineering, 101, 87–96. doi:10.1263/jbb.101.87
- Stolz, P., & Obermayer, B. (2005). Manufacturing microalgae for skin care. Cosmetics and Toiletries, 120, 99–106.
- Sui, Y., Muys, M., Vermeir, P., D’Adamo, S., & Vlaeminck, S. E. (2019). Light regime and growth phase affect the microalgal production of protein quantity and quality with Dunaliella salina. Bioresource Technology, 275, 145–152. doi:10.1016/j.biortech.2018.12.046
- Sui, Y., & Vlaeminck, S. E. (2020). Dunaliella microalgae for nutritional protein: An undervalued asset. Trends in Biotechnology, 38, 10–12. doi:10.1016/j.tibtech.2019.07.011
- Sun, Y., Yang, Z., Gao, X., Li, Q., Zhang, Q., & Xu, Z. (2005). Expression of foreign genes in Dunaliella by electroporation. Molecular Biotechnology, 30, 185–192. doi:10.1385/MB:30:3:185
- Supamattaya, K., Kiriratnikom, S., Boonyaratpalin, M., & Borowitzka, L. (2005). Effect of a Dunaliella extract on growth performance, health condition, immune response and disease resistance in black tiger shrimp (Penaeus monodon). Aquaculture, 248, 207–216. doi:10.1016/j.aquaculture.2005.04.014
- Takagi, M., Karseno, & Yoshida, T. (2006). Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. Journal of Bioscience and Bioengineering, 101, 223–226. doi:10.1263/jbb.101.223
- Takimura, O., Fuse, H., Murakami, K., Kamimura, K., & Yamaoka, Y. (1996). Uptake and reduction of arsenate by Dunaliella sp. Applied Organometallic Chemistry, 10, 753–756. doi:10.1002/(SICI)1099-0739(199611)10:9<753:AID-AOC573>3.0.CO;2-V
- Talbot, P., & de la Noüe, J. (1993). Tertiary treatment of wastewater with Phormidium bohneri (Schmidle) under various light and temperature conditions. Water Research, 27, 153–159. doi:10.1016/0043-1354(93)90206-W
- Tan, C., Qin, S., Zhang, Q., Jiang, P., & Zhao, F. (2005). Establishment of a micro-particle bombardment transformation system for Dunaliella salina. Journal of Microbiology, 43, 361–365.
- Taylor, R., & Fletcher, R. L. (1998). Cryopreservation of eukaryotic algae - a review of methodologies. Journal of Applied Phycology, 10, 481–501. doi:10.1023/A:1008094622412
- Teodoresco, E. (1905). Organisation et développement du Dunaliella, nouveau genre de Volvocacée-Polyblepharidée. Beih Z Bot Centralbl, 18, 215–232.
- Thakkar, M., Mitra, S., & Wei, L. (2016). Effect on growth, photosynthesis, and oxidative stress of single walled carbon nanotubes exposure to marine alga Dunaliella tertiolecta. Journal of Nanomaterials, 2016, 1–9. doi:10.1155/2016/8380491
- Thomas, K. V., & Brooks, S. (2010). The environmental fate and effects of antifouling paint biocides. Biofouling, 26, 73–88. doi:10.1080/08927010903216564
- Tillman, J. A., Seybold, S. J., Jurenka, R. A., & Blomquist, G. J. (1999). Insect pheromones - an overview of biosynthesis and endocrine regulation. Insect Biochemistry and Molecular Biology, 29, 481–514. doi:10.1016/S0965-1748(99)00016-8
- Törnwall, M. E., Virtamo, J., Korhonen, P. A., Virtanen, M. J., Taylor, P. R., Albanes, D., & Huttunen, J. K. (2004). Effect of tocopherol and b-carotene supplementation on coronary heart disease during the 6-year post-trial follow-up in the ATBC study. European Heart Journal, 25, 1171–1178. doi:10.1016/j.ehj.2004.05.007
- Tredici, M. R., & Zitelli, G. C. (1997). Cultivation of Spirulina (Arthrospira) platensis in flat plate reactors. In A. Vonshak (Ed.), Spirulina platensis (Arthrospira): Physiology, cell-biology and biotechnology (pp. 117–130). Boca Raton, USA: Taylor and Francis.
- Tsuji, N., Hirayanagi, N., Okada, M., Miyasaka, H., Hirata, K., Zenk, M. H., & Miyamoto, K. (2002). Enhancement of tolerance to heavy metals and oxidative stress in Dunaliella tertiolecta by Zn-induced phytochelatin synthesis. Biochemical and Biophysical Research Communications, 293, 653–659. doi:10.1016/S0006-291X(02)00265-6
- Uduman, N., Qi, Y., Danquah, M. K., Forde, G. M., & Hoadley, A. (2010). Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. Journal of Renewable and Sustainable Energy, 2, 012701. doi:10.1063/1.3294480
- Uriarte, I., Farías, A., Hawkins, A. J. S., & Bayne, B. L. (1993). Cell characteristics and biochemical composition of Dunaliella primolecta Butcher conditioned at different concentrations of dissolved nitrogen. Journal of Applied Phycology, 5, 447–453. doi:10.1007/BF02182737
- Vandamme, D., Foubert, I., Fraeye, I., Meesschaert, B., & Muylaert, K. (2012). Flocculation of Chlorella vulgaris induced by high pH: Role of magnesium and calcium and practical implications. Bioresource Technology, 105, 114–119. doi:10.1016/j.biortech.2011.11.105
- Vandamme, D., Foubert, I., & Muylaert, K. (2013). Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends in Biotechnology, 31, 233–239. doi:10.1016/j.tibtech.2012.12.005
- Vanitha, A., Narayan, M. S., Murthy, K. N. C., & Ravishankar, G. A. (2007). Comparative study of lipid composition of two halotolerant alga, Dunaliella bardawil and Dunaliella salina. International Journal of Food Sciences and Nutrition, 58, 373–382. doi:10.1080/09637480701252252
- van Poppel, G. (1993). Carotenoids and cancer: An update with emphasis on human intervention studies. European Journal of Cancer, 29, 1335–1344. doi:10.1016/0959-8049(93)90087-V
- Vigani, M., Parisi, C., Rodríguez-Cerezo, E., Barbosa, M. J., Sijtsma, L., Ploeg, M., & Enzing, C. (2015). Food and feed products from micro-algae: Market opportunities and challenges for the EU. Trends in Food Science & Technology, 42, 81–92. doi:10.1016/j.tifs.2014.12.004
- Villar, R., Laguna, M., Calleja, J., & Cadavid, I. (1992). Effects of Phaeodactylum tricornutum and Dunaliella tertiolecta extracts on the central nervous system. Planta Medica, 58, 405–409. doi:10.1055/s-2006-961501
- Volkman, J. K., Barrett, S. M., Blackburn, S. I., Mansour, M. P., Sikes, E. L., & Gelin, F. (1998). Microalgal biomarkers: A review of recent research developments. Organic Geochemistry, 29, 1163–1179. doi:10.1016/S0146-6380(98)00062-X
- Walne, P. R. (1970). Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea. Crassostrea, Mercenaria and Mytilus In Fishery Investigations, 26, 1–62, HMSO, London.
- Wan, C., Alam, M. A., Zhao, X.-Q., Zhang, X.-Y., Guo, S.-L. … Bai, F.-W. (2015). Current progress and future prospect of microalgal biomass harvest using various flocculation technologies. Bioresource Technology, 184, 251–257. doi:10.1016/j.biortech.2014.11.081
- Wright, S., & Burton, H. (1981). The biology of Antarctic saline lakes. Hydrobiologia, 82, 319–338. doi:10.1007/BF00048723
- Xu, L., Gao, F., Feng, J., Lv, J., Liu, Q. … Xie, S. (2022). Relationship between β-carotene accumulation and geranylgeranyl pyrophosphate synthase in different species of Dunaliella. Plants, 11, 27. doi:10.3390/plants11010027
- Yamaoka, Y., Takimura, O., Fuse, H., & Murakami, K. (1999). Effect of glutathione on arsenic accumulation by Dunaliella salina. Applied Organometallic Chemistry, 13, 89–94.
- Yamashita, Y., Takeuchi, T., Endo, Y., Goto, A., Sakaki, S. … Yamashita, H. (2020). Dietary supplementation with dunaliella tertiolecta prevents whitening of brown fat and controls diet-induced obesity at thermoneutrality in mice. Nutrients, 12, 1686. doi:10.3390/nu12061686
- Yao, S., Lu, J., Sárossy, Z., Baggesen, C., Brandt, A., & An, Y. (2016). Neutral lipid production in Dunaliella salina during osmotic stress and adaptation. Journal of Applied Phycology, 28, 2167–2175. doi:10.1007/s10811-016-0794-7
- Ye, Z.-W., Jiang, J.-G., & Wu, G.-H. (2008). Biosynthesis and regulation of carotenoids in Dunaliella: Progresses and prospects. Biotechnology Advances, 26, 352–360. doi:10.1016/j.biotechadv.2008.03.004
- Yilancioglu, K., Cokol, M., Pastirmaci, I., Erman, B., Cetiner, S., & Campbell, D. A. (2014). Oxidative stress is a mediator for increased lipid accumulation in a newly isolated Dunaliella salina strain. PLoS ONE, 9, e91957. doi:10.1371/journal.pone.0091957
- Yi, Y., Qiao, D., Bai, L., Xu, H., Li, Y., Wang, X., & Cao, Y. (2007). Cloning, expression, and functional characterization of the Dunaliella salina 5-enolpyruvylshikimate-3-phosphate synthase gene in Escherichia coli. Journal of Microbiology (Seoul, Korea), 45, 153–157.
- Yi, Y., Yi, C., Qian, L., Min, L., Long, C. … Dairong, Q. (2006). Cloning and sequence analysis of the gene encoding (6-4)photolyase from Dunaliella salina. Biotechnology Letters, 28, 309–314. doi:10.1007/s10529-005-5716-8
- Zhu, Y.-H., Jiang, J.-G., & Chen, Q. (2008). Characterization of cDNA of lycopene β-cyclase responsible for a high level of β-carotene accumulation in Dunaliella salina. Biochemistry and Cell Biology, 86, 285–292. doi:10.1139/O08-012


![Figure 1. Number of publications related to the term [Dunaliella] between the years 2000–2019, in PubMed database (NCBI), and respective linear regression.](/cms/asset/d605b5fe-df6d-4476-b4c1-b94b7e86daee/tapy_a_2222318_f0001_b.gif)