Abstract
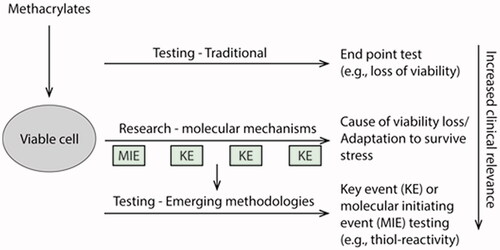
Biological evaluation of resin-based dental composites has traditionally been based on in vitro endpoint tests with different methods to determine loss of cell viability and cell morphology changes after exposure to the material or monomer constituents. The data reveals a potential for biological effects, but clinical relevance of such data is limited. Positive allergy tests and allergic clinical reactions to dental monomers are observed in dental personnel and patients. The aim of this review is to address newer research on molecular events caused by exposure to resin-based composites to have a better understanding of the potential for clinical adverse effects. A more accurate understanding of the biological aspects of dental composite materials has been found after studying parameters like glutathione depletion, oxidative stress, genotoxicity, and immunomodulatory key effects in various cell culture models. Using omics-based approaches allow for a broader and non-specified search of changes caused by methacrylate exposure. Defense mechanisms and adaption are observed in cells exposed to monomer concentrations relevant to clinical exposure. The above-mentioned methods are the foundations for modified testing strategies. The clinical relevance of most available in vitro endpoint tests is of limited relevance for the patient. Research focusing on molecular mechanisms has given new insight into methacrylate toxicity in exposed cells. Using this knowledge from mechanistic studies to develop standardized in vitro biocompatibility tests will likely improve their clinical relevance.
Introduction
Since its introduction in the 1960s [Citation1], dental composites have evolved from a relatively basic silica-filled resin-based material to a wide range of complex, specialized products. When used as intended, modern composites function well clinically and are aesthetically sovereign compared to dental amalgam, the previous first choice for filling therapy. Although the composition of composites has changed over the years, their main components remain a polymer matrix and reinforcing filler particles or short fibers. A coupling agent chemically binds the matrix and filler, further strengthening the composite. In addition to the three main components, composites usually contain small amounts of initiator and stabilizer components.
Modern composites have improved in several qualities compared to earlier types. To mention some, they have better wear resistance, lower polymerization shrinkage, and smoother surfaces. Current composites are also available for a comprehensive range of indications. The latter is achieved mainly by altering the monomer mixture that forms the organic phase and the filler, mainly by changing particle size and shape [Citation2]. Enamel and dentin adhesives are an integrated part of a composite restoration to improve the bond between the tooth and the composite material. Adhesive systems typically contain resin monomers, often with hydrophilic groups and sometime inorganic filler [Citation3]. Some commonly used methacrylates are shown in .
Figure 1. Structural formulas of mono- and di-methacrylate monomers. Commonly used constituents of dental composites are UDMA (urethane dimethacrylate), Bis-GMA (bisphenol A-glycidyl methacrylate) and TEGDMA (triethylene glycol dimethacrylate). HEMA (2-Hydroxylethyl methacrylate) is found in dental adhesives as part of the resin based dental filling, and EMA (ethyl methacrylate) as an impurity in dental adhesives [Citation75] and positive patch test to EMA has been used to confirm MMA (methyl methacrylate) denture base allergy [Citation5].
![Figure 1. Structural formulas of mono- and di-methacrylate monomers. Commonly used constituents of dental composites are UDMA (urethane dimethacrylate), Bis-GMA (bisphenol A-glycidyl methacrylate) and TEGDMA (triethylene glycol dimethacrylate). HEMA (2-Hydroxylethyl methacrylate) is found in dental adhesives as part of the resin based dental filling, and EMA (ethyl methacrylate) as an impurity in dental adhesives [Citation75] and positive patch test to EMA has been used to confirm MMA (methyl methacrylate) denture base allergy [Citation5].](/cms/asset/e4e2da0d-704c-4067-b821-d4a72e4e5b1d/iabo_a_2223223_f0001_b.jpg)
The ability to perform as desired without eliciting undesirable effects is commonly referred to as the biocompatibility of a biomaterial. Since the composition of available composites varies depending on the intended application, biocompatibility may also differ. Primarily, dental composites perform well in their task of restoring tooth function. However, both in vitro studies and clinical observations reveal a potential in dental composites to cause side effects. Awareness of such properties and understanding the underlying causes are essential when aiming for the safe use of composites.
Clinical findings
It is well known that monomers in resin-based composites have an allergic potential [Citation4] Dental personnel handling uncured materials on a daily basis is more prone to develop allergic reactions compared to the patient having the cured restoration. A number of publications have described positive allergy tests and allergic clinical reactions to dental monomers. Test files at the Finnish Institute of Occupational Health from 1994 to 2006 were reviewed for allergic reactions to acrylic monomers in dental personnel and 32 sensitized patients were found. 2-Hydroxyethyl methacrylate (HEMA) was the most important allergen in dentists and dental nurses, and methyl methacrylate (MMA) and ethylene glycol dimethacrylate (EGDMA) in dental technicians [Citation5]. Reactions to bisphenol A-glycidyl methacrylate (bis-GMA), diethylene glycol diacrylate (DEGDA), triethylene glycol diacrylate (TREGDA), ethyl methacrylate (EMA) and ethyl acrylate (EA) were relevant in some patients [Citation5]. Raposo and colleagues reviewed files of patients with allergic contact dermatitis caused by (meth)acrylates related to nail cosmetic products. Patch testing with 2-HEMA was positive in more than 90% of cases of the (meth) acrylate allergy patients and patch testing to 2-hydroxypropyl methacrylate (2-HPMA) was positive in 64.1% [Citation6]. Patients with allergic contact dermatitis from acrylic monomers often show reactions to several acrylic monomers when patch tested even though exposure has probably not occurred to all the patch test-positive acrylic monomers [Citation4,Citation7]. It was suggested that acrylic monomers cross-react in that allergic sensitization induced by one acrylic compound extends to one or more other acrylic compounds. Therefore, sensitized individuals are often multi-allergic and, accordingly, should not be exposed to any of the acrylic compounds [Citation8]. Reaction may occur after low level exposure also from materials where residual monomer content did not exceed international standards for the material. In this case report, the patient used a removable retainer made from auto-polymerized methylmethacrylate resin [Citation9].
Only a limited number of studies have addressed patient exposure to monomers from resin-based materials. Most studies have addressed methacrylate exposure from removable prostheses. The maximum concentration of monomer released into saliva peaked 1 day after the insertion of complete dentures. The methyl methacrylate (MMA) content was 0.4 ± 0.1 µM 1 h after insertion, 3 ± 1 µM, and 0.5 ± 0.1 µM on the first- and third-days post insertion, respectively. Although the released MMA was not at toxic levels, it could potentially sensitize the patient or elicit an allergic reaction [Citation10]. In another study, healthy human dentate subjects wore recently made auto-polymerized or heat-polymerized poly methyl methacrylate palatal appliances for 5 min. MMA is released into saliva from auto-polymerized appliances, with a maximum concentration of 0.45 mM in whole saliva and 1.8 mM in the salivary film on the fitting surface. Monomer was not found in saliva from subjects wearing properly heat-polymerized MMA appliances, whereas the maximum MMA concentration in saliva was 63 mM when the polymerization time was shorter than recommended. MMA was not detected in blood or urine [Citation11].
Unstimulated saliva samples taken from 10 patients before and after placing a two or three-surface composite restoration and the samples were analyzed for 5 specific monomers [Citation12]. Ten minutes after treatment, the saliva concentrations of Bis-GMA ranged from 0.05 to 20 µM, of HEMA 0.1 − 1.5 µM, and of urethane dimethacrylate (UDMA) 0 − 2.6 µM. Four samples contained triethyleneglycol dimethacrylate (TEGDMA) but the concentration was below the quantification limit. Bis-EMA was not detected in any of the samples. It was not possible to identify any of the monomers in saliva samples collected before treatment, or 24 h and 7 d after treatment.
Discussion of clinical findings
Monomers used in composite and resin-based denture materials are known sensitizers and allergy reactions are reported after exposure to such monomers [Citation5]. There is a higher risk for such reactions in dental personnel as they handle the uncured material compared to the patients in who the material are in a cured state. The few studies estimating leakage from resin-based materials have shown leakage only shortly after placement [Citation12] indicating that adverse reactions may be transient. One exception is cured methyl-methacrylate dentures. Monomer was found to leach out after 10 days post-curing in vitro [Citation13] and this could occur in vivo. In general, there is a lack of data regarding the occurrence of adverse effects of resin-based composite in patients and research in this field is encouraged.
In vitro findings
In vitro studies are the primary source of current knowledge of methacrylate monomer effects on living cells. Selected parameters are measured in various cell culture models after exposure to some commonly used methacrylates. Although composites vary in resin composition and new monomers are introduced, most studies have focused on methacrylates that are found to leak into aqueous solutions [Citation14,Citation15]. The effects of water-soluble (at least to some extent) methacrylates such as HEMA, TEGDMA, and MMA (used in removal prostheses) have been extensively investigated. However, many of the observed effects may suggest that different methacrylates similarly affect living cells, although their potency to cause damage may differ.
Cell viability
A well-documented dose-dependent viability loss in methacrylate-exposed cells points towards a general cytotoxic potential of the monomers. Methods measuring mitochondrial activity, cell membrane integrity, and cell growth pattern are commonly used to evaluate methacrylate monomers’ cytotoxic potential. The MTT and XTT assays in ISO 10993-5 [Citation16] measure mitochondrial succinate dehydrogenase (SDH) activity assumed to be constant in living cells and absent in dead cells. These widely used methods, therefore, may detect either cell death or growth inhibition but cannot discriminate between these effects. It is also important to note that the dose resulting in a cytotoxic response seems to depend strongly on the choice of the cell culture model [Citation17,Citation18].
Cell cycle arrest [Citation19,Citation20] and apoptotic and necrotic cell death [Citation21,Citation22] have all been reported in methacrylate-exposed cells. As mentioned above, all these responses may explain a reduction in the number of viable cells. As with the SDH activity, the effect on cell death patterns and growth inhibition varies among cell lines [Citation18]. These results show that these chemicals can potentially harm cells, but the interpretation of the results for risk evaluation of clinical use is challenging.
Glutathione depletion and oxidative stress
Reduced levels of glutathione (GSH) and increased levels of reactive oxygen species (ROS) are found in a range of cell cultures exposed to various methacrylates [Citation18,Citation23]. In addition to cellular functions like phase 2 metabolism [Citation24] and post-translational modification of proteins [Citation25], GSH is part of an essential cellular antioxidant system (GSH – GSSG). The finding that several methacrylates spontaneous form adducts with GSH [Citation26–28] supports the hypothesis that increased ROS level primarily links to GSH-depletion () [Citation27]. However, research aiming to explore a possible association between these events and reduced cell viability is inconsistent. Studies that compare the effects of GSH-depletion after methacrylate exposure with inhibition of GSH synthesis don’t support this hypothesis. In contrast, the antioxidant N-acetylcystein (NAC) seems to prevent methactylate induced cell death [Citation20,Citation29–31].
Figure 2. The antioxidant effect of glutathione is briefly outlined in Figure (A). The antioxidant capacity is mediated by oxidizing the redox-active thiol group (2 GSH -> GSSG) as glutathione reduces target molecules. GSSG (glutathione disulfide) is recycled to GSH in a reaction catalyzed by glutathione-disulfide reductase (GSR). Protection against oxidative stress by this system depends on the maintenance of the GSH concentration. Figure (B) shows the suggested mechanism that results in increased ROS in methacrylate-exposed cells. Methacrylates cause GSH depletion by direct binding to GSH. The reduced antioxidant capacity (red arrows) may initiate a redox imbalance. Oxidative damage on cellular macromolecules may result from this imbalance.

Immunomodulatory effects
There is limited literature regarding possible immunological effects caused by methacrylate monomer exposure in vitro, and the published results vary due to differences in study design and choice of cell culture model. In a study utilizing peripheral blood mononuclear cells (PBMCs), Alizadehgharib and coworkers [Citation32] measured increased release of several pro‐inflammatory cytokines (IL‐1β, IL‐8, IL‐18) after exposure to HEMA (0.5-1 mM) and TEGDMA (0.5-1 mM). In TEGDMA-exposed cells, the release of IL‐6 and TNF‐α was also increased.
In contrast to these results, no measurable changes in IL-1β and TNFα release were measured after exposure of the mouse macrophage-like cell line Raw 264.7 to HEMA (100-200 µM [Citation33]) and TEGDMA (50-200 µM [Citation33] and 3 mM [Citation34]). It was also found that both monomers counteracted the IL-1β and TNFα release provoked by lipopolysaccharide from Escherichia coli. Although all these studies suggest immunomodulatory effects, the diversity of the results makes it difficult to draw conclusions.
Genotoxicity of methacrylates
In methacrylate-exposed cells, some studies have indicated DNA damage as the cause of observed cell responses like cell cycle arrest and increased gH2AX foci formation [Citation19,Citation35–37]. Increased micronuclei formation and chromosomal abnormalities [Citation38,Citation39] and increased DNA damage response (DDR) signaling are also reported [Citation19,Citation40–42]. Both direct interaction between electrophilic methacrylates and nucleophilic centers in DNA, and oxidative damage caused by increased oxidative load could initiate such responses [Citation23,Citation40]. However, data shedding light on the initiating events are still limited.
Omics methodologies
Most in vitro studies that have addressed possible adverse effects of methacrylate exposure have used cell-based assays that measure specific endpoints, as described above. However, principles, methodology, and toxicity testing techniques have changed over time, and the recent introduction of ‘omics’ techniques (genomics, transcriptomics, proteomics, and metabolomics) allows new toxicity targets to be detected. Since clinical exposure to monomers occurs at low levels, such studies could be very useful. Although no phenotypic changes would occur when using clinical exposure levels in conventional in vitro studies, the effects of low methacrylate concentrations may be detected because the techniques identify changes before phenotypic changes are measurable.
Using omics-based approaches allow for a broader and non-specified search of changes caused by methacrylate exposure. By utilizing a microarray analysis to measure the effect of 12 h exposure of 10 mM MMA on L929 cells, Ishikawa and coworkers [Citation43] found significantly altered expression of 44 genes. The genes were associated with Nrf2-activated transcription and mainly indicated increased defense against oxidative stress.
Schweikl and coworkers [Citation44] performed a similar study using 3 mM TEGDMA and human skin fibroblast. They also detected many genes with altered expression. By categorizing the genes into specific networks, the altered gene expression could primarily be associated with cell cycle regulation, cell growth, and cell death. The authors further concluded that the underlying mechanisms of the changes seemed to be caused by oxidative stress.
Both these studies used methacrylate concentrations that reduced cell viability. Using doses below the threshold that causes cell death allows for cell adaptation to stress caused by the exposure. Using this approach, two studies measured altered gene expression after TEGDMA-exposure to dental pulp cells [Citation45], and after HEMA-exposure to bronchial epithelial cells [Citation31]. In TEGDMA-exposed pulp cells, most of the altered gene regulations were associated with inflammation, response to oxidative stress, regulation of apoptosis, and cell proliferation. Adaptation to increased oxidative stress was also concluded from the study on HEMA-exposed bronchial cells. In addition, increased transcription of SQSTM1/p62 and Heat shock protein (HSP70) led the authors to suggest increased Endoplasmic Reticulum (ER) stress and autophagic capacity in the exposed cells [Citation31].
Two other studies using the ‘adaption approach’ suggest similar effects by analyzing alterations on the cellular proteome after methacrylate exposure. In addition to an increased level of proteins related to antioxidant function, increased HSP70 and p62 protein levels were seen in human monocytes after exposure to TEGDMA [Citation46] and HEMA [Citation47]. In the HEMA-exposed cells, the findings also pointed towards an interaction with the NFkB signaling pathway and reduced capacity for pyroptotic cell death. In the TEGDMA-exposed monocytes, Nilsen and coworkers detect an altered level of proteins that could be related to DNA damage. Despite these differences, both studies point towards an increased Nrf2-regulated transcription as the cause of altered protein levels.
Discussion of in vitro findings
Several research projects have aimed to map the underlying mechanism that leads to the observed viability loss in cells after exposure to methacrylate monomers. Two possibly related events common for most investigated cell types are glutathione depletion and increased levels of reactive oxygen species (ROS) [Citation18,Citation23]. The GSH depletion seems to be caused by direct adduct formation with methacrylates [Citation27,Citation28]. Being an important cellular antioxidant, glutathione sequestering by methacrylates can cause a redox imbalance in cells, thereby causing increased ROS levels. ROS may further lead to altered cell signaling, altered growth, oxidative damage of macromolecules, and so on. Hence, many of the reported methacrylate effects may originate from this GSH depletion ().
Most methacrylate toxicity studies are performed in vitro and have dealt with lethal concentrations of the monomers that are relatively high compared to measured concentrations in the clinic (usually cell death occurs in the mM range while measurements in the clinic are in the µM range). In such studies, severe cell damage and cell death signaling can potentially overshadow other critical cellular events. Hence, some confounding factors could be avoided by focusing on adaption to cellular stress at methacrylate doses below the threshold that causes cell death as illustrated in . By combining this strategy with omics methodologies [Citation31,Citation46,Citation47], Nrf2-directed cytoprotection seems to be important. Shared findings in these studies indicate increased defense against oxidative stress and increased capacity to remove damaged cellular components like misfolded proteins by autophagy.
Figure 3. Methacrylate toxicity studies performed in vitro commonly use higher monomer concentrations than those measured in the clinic. At these concentrations, irreversible damage and cell death can occur. Severe cell damage and cell death signaling can potentially overshadow other cellular events of importance for biocompatibility. Focusing on doses below the threshold of cell death may reveal such events.

In addition to facilitating the safety assessment of single compounds without using test animals, molecular understanding of interactions of xenobiotics with living cells may also provide hints on how exposure to mixtures will affect humans. Ultimately, the effect of a combined exposure scenario may be foreseen, and for methacrylate-exposed dental patients/personnel, such knowledge is essential to avoid increased side-effect risk by combined exposure.
The reported inhibition of cytokine production in LPS-challenged macrophages by methacrylate monomers is one combined exposure scenario likely to occur in the clinic. Inhibitory effect of the autophagosome on TLR signaling has been suggested as one possible explanation for this effect [Citation48]. Another possible interaction on the molecular level is the finding of increased Pirin in methacrylate-exposed monocytes [Citation46,Citation47]. Pirin transcription can be regulated by Nrf2 and is an Iron-binding nuclear protein involved in the regulation of NFkB transcription [Citation49]. NfKB regulates multiple aspects of innate and adaptive immune functions, including the transcription of pro-inflammatory cytokines and chemokines. Although the modulatory effect on in vitro cytokine production is evident, further clarification on the molecular mechanisms is needed to understand the clinical importance of these findings.
There are studies that suggest DNA damage in methacrylate-exposed cells. The underlying mechanism, however, has not been investigated in detail and more data is necessary for an appropriate evaluation. So far, there is no clinical data pointing toward such effects.
General considerations on the in vitro approach
The in vitro situation is quite different from the clinical situation. Most studies regarding methacrylate toxicity utilize mammalian cell lines of clonal origin. It is unlikely that these homogeneous models are able to mimic the complex interactions in human tissues. This must be considered when trying to link such studies to possible outcomes of patient exposure.
The exposure situation also varies. In cell culture, the cell media contains the chemicals in question facilitating cell surface binding and absorption. In vivo, with few exceptions, chemicals need penetrating cell layers, absorption into blood and distribution to the target organs implying crossing a number of cell membranes. The concentrations used in cell culture studies can be compared to the exposure in vivo, but usually not to the target organ concentration. The documented patient exposure to monomers was usually of much lower concentration than those used for in vitro experiments [Citation12,Citation32]. Many in vitro studies could therefore be classified as ‘accelerated’ tests, where the concentration of extract or methacrylate is relatively high compared to in vivo measurements and the exposure time is relatively short compared to the clinical setting. Effects that take place only at concentrations higher than clinical exposure levels may have limited value for evaluating the biocompatibility of materials. On the other hand, effects caused by long-term exposure to lower concentrations may be difficult to detect in such ‘accelerated’ tests. This weakness may be valid for several analyses of specific endpoints.
There are several advantages associated with an in vitro approach as well. A significant benefit is an ability to control experimental variables in detail without the modulating influences likely to occur in a complex organism. The opportunity to reproduce experiments in homogeneous cell populations makes it a valuable tool for identifying early cellular reactions after a toxic exposure. The cell response to toxic exposure, however, may vary widely among cell types [Citation18]. It is, therefore, essential to study and compare the response of different cell systems to toxic exposure. The range of cell types investigated for methacrylate responses is extensive. Among these studies, several findings seem to be shared both between different cell types and between different methacrylates.
Another similarity among most cell lines is their infinite life span. This property is often related to genetic modifications of the cells. Alterations in genes related to processes like cell growth control, DNA-damage response and antioxidant responses are found in several commonly used cell lines. Awareness of such defects is essential when interpreting information on the underlying mechanisms of toxicity experiments. Lack of knowledge could potentially lead to faulty conclusions. Defects in genes like p53 (common in many cancer-derived cell lines; for instance, V79 [Citation50] and THP-1 [Citation51]) and the genes of the Nrf2/ARE pathway (for instance, A549 [Citation52]) can potentially affect DNA-damage responses and antioxidant responses, respectively.
Testing strategies
Biological testing is part of the safety evaluation of materials and devices used in dentistry. The ISO standard ISO 10993-1 [Citation53] addresses the type of tests needed based on clinical use. Cell culture studies form the initial evaluation often with reference to ISO 10993-5 [Citation16] methods that evaluate cytotoxicity by various parameters for cell death and cell growth. Such parameters do not shed light on the mechanisms behind the cytotoxic effects but are still in common use also in the evaluation of resin-based composites.
Most clinical reactions to resin-based composites involve an allergic immune response [Citation6,Citation54]. Testing of resin-based composites according to reactions like sensitization as described in ISO 10993-10 [Citation55] and irritation (ISO 10993-23) [Citation56] is clinically relevant. There are currently three animal assays available for the determination of the skin-sensitizing potential of chemicals. These include 2 guinea pig assays and one murine assay [Citation57]. The murine local lymph node assay was internationally accepted as an OECD test guideline in 2010 [Citation58] for testing single chemicals as a stand-alone alternative to the guinea pig assay. This is now the preferred in vivo assay for sensitization testing of chemicals. Due to animal welfare requirements and the wish for reducing animal studies, non-animal methods for skin sensitization have been presented in the ISO 10993-10. A number of assays have been mentioned [Citation59,Citation60]. However, in vitro methods for skin sensitization testing are not yet routinely used, but in view of new regulations in Europe which ban the use of animal tests for cosmetics (EU ban), it seems likely that novel strategies will become available for the identification of skin sensitizers.
ISO 10993-23 presents one animal test and one in vitro test for irritation. The in vitro test called the reconstructed human epidermis model which is a three-dimensional epidermal model comprising the main basal, suprabasal, spinous and granular layers and a functional stratum corneum [Citation61]. Another irritation test is the HET-CAM method which is commonly used for testing cosmetics [Citation62,Citation63] and has also been used in research on dental resin-based materials [Citation64–66]. This method, however, has not found its way into the ISO 10993 series of standards.
Discussion of testing strategies
Test protocols are based on research findings, and according to ISO rules, their validity is evaluated every five years. Revised standards for sensitization and irritation include new in vitro alternatives [Citation55,Citation56].
The ability to activate Nrf2 is an observed property in a range of sensitizers [Citation67]. This knowledge has been used in the development of in vitro and chemical assays to test substances and materials for sensitizing potential [Citation68]. The initial step in Nrf2 activation is modifications of cysteine thiols of the Keap1 protein, either by oxidation or by direct binding of electrophiles. The latter is the presumed mechanism of Nrf2 activation by sensitizers [Citation69]. Assays that measure either Nrf2 activity in cultures cells or reactivity towards synthetic peptides containing cysteine are described in OECD protocols TG442c (keratinosense) [Citation70] and TG442d (peptide-binding assay) [Citation71], respectively. The replacement of animal tests by animal-free laboratory protocols is in line with the ‘3 R principle’ of animal testing (Refine, Reduce, and Replace) [Citation72]. Following this principle, methods for the safety assessment of drugs and medical devices have increased. The recent revision of the ISO standard for evaluating the sensitizing potential of medical devices now includes a description of animal-free alternatives [Citation55]. The understanding of the molecular and cellular mechanisms leading to adverse effects (adverse outcome pathways; AOPs) is essential in the development of such protocols. Both the Keratinosense assay (442c) and the peptide binding assay (442d) are reported to return good accuracy [Citation73,Citation74]. The previously reported spontaneous binding of several methacrylate monomers to GSH (a cysteine-containing tripeptide) may suggest an even easier and cheaper assay to measure sensitizing potential. By assuming that the thiol reactivity is the property of allergens that lead to sensitization (TG442C), the cell-based in vitro assays could potentially return false positives by compounds that oxidize Keap-1 thiols. Hence, cell-free systems, i.e. peptide binding assays, could be a better choice to avoid false positives, at least for screening purposes.
Conclusion
The current strategies to verify the biocompatibility of dental composites measure in vitro endpoints of limited relevance for the patient. Research focusing on molecular mechanisms has given new insight into methacrylate toxicity in exposed cells. Using this knowledge from mechanistic studies to develop standardized in vitro biocompatibility tests will likely improve their clinical relevance.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bowen RL. Properties of a silica-reinforced polymer for dental restorations. J Am Dent Assoc. 1963;66:57–64. doi: 10.14219/jada.archive.1963.0010.
- Bayne SC. Beginnings of the dental composite revolution. J Am Dent Assoc. 2013;144(8):880–884. doi: 10.14219/jada.archive.2013.0205.
- Van Landuyt KL, Snauwaert J, De Munck J, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28(26):3757–3785. doi: 10.1016/j.biomaterials.2007.04.044.
- Kanerva L, Estlander T, Jolanki R. Allergic contact dermatitis from dental composite resins due to aromatic epoxy acrylates and aliphatic acrylates. Contact Dermatitis. 1989;20(3):201–211. doi: 10.1111/j.1600-0536.1989.tb04657.x.
- Aalto-Korte K, Alanko K, Kuuliala O, et al. Methacrylate and acrylate allergy in dental personnel. Contact Dermatitis. 2007;57(5):324–330. doi: 10.1111/j.1600-0536.2007.01237.x.
- Raposo I, Lobo I, Amaro C, et al. Allergic contact dermatitis caused by (meth)acrylates in nail cosmetic products in users and nail technicians - a 5-year study. Contact Dermatitis. 2017;77(6):356–359. doi: 10.1111/cod.12817.
- Jordan WP. Jr., Cross-sensitization patterns in acrylate allergies. Contact Dermatitis. 1975;1(1):13–15. doi: 10.1111/j.1600-0536.1975.tb05304.x.
- Kanerva L. Cross-reactions of multifunctional methacrylates and acrylates. Acta Odontol Scand. 2001;59(5):320–329. doi: 10.1080/000163501750541200.
- Goncalves TS, Morganti MA, Campos LC, et al. Allergy to auto-polymerized acrylic resin in an orthodontic patient. Am J Orthod Dentofacial Orthop. 2006;129(3):431–435. doi: 10.1016/j.ajodo.2005.10.017.
- Singh RD, Gautam R, Siddhartha R, et al. High performance liquid chromatographic determination of residual monomer released from heat-cured acrylic resin. An in vivo study. J Prosthodont. 2013;22(5):358–361. doi: 10.1111/jopr.12004.
- Baker S, Brooks SC, Walker DM. The release of residual monomeric methyl methacrylate from acrylic appliances in the human mouth: an assay for monomer in saliva. J Dent Res. 1988;67(10):1295–1299. doi: 10.1177/00220345880670101001.
- Michelsen VB, Kopperud HB, Lygre GB, et al. Detection and quantification of monomers in unstimulated whole saliva after treatment with resin-based composite fillings in vivo. Eur J Oral Sci. 2012;120(1):89–95. doi: 10.1111/j.1600-0722.2011.00897.x.
- Kopperud HM, Kleven IS, Wellendorf H. Identification and quantification of leachable substances from polymer-based orthodontic base-plate materials. Eur J Orthod. 2011;33(1):26–31. doi: 10.1093/ejo/cjq020.
- Tuna EB, Aktoren O, Oshida Y, et al. Elution of residual monomers from dental composite materials. Eur J Paediatr Dent. 2010;11(3):110–114.
- Putzeys E, Nys S, Cokic SM, et al. Long-term elution of monomers from resin-based dental composites. Dent Mater. 2019;35(3):477–485. doi: 10.1016/j.dental.2019.01.005.
- ISO, ISO 10993-5 Biological evaluation of medical devices, part 5: tests for in vitro cytotoxicity., International Standard Organization, Geneva, Switzerland, 2009.
- Juranova J. Illuminating the cellular and molecular mechanism of the potential toxicity of methacrylate monomers used in biomaterials. Drug Chem Toxicol. 2020;43(3):266–278.
- Morisbak E, Uvslokk S, Samuelsen JT. In vitro effects of dental monomer exposure - Dependence on the cell culture model. Toxicol In Vitro. 2020;67:104906. doi: 10.1016/j.tiv.2020.104906.
- Samuelsen JT, Holme JA, Becher R, et al. HEMA reduces cell proliferation and induces apoptosis in vitro. Dent Mater. 2008;24(1):134–140. doi: 10.1016/j.dental.2007.08.006.
- Morisbak E, Ansteinsson V, Samuelsen JT. Cell toxicity of 2-hydroxyethyl methacrylate (HEMA): the role of oxidative stress. Eur J Oral Sci. 2015;123(4):282–287. doi: 10.1111/eos.12189.
- Samuelsen JT, Dahl JE, Karlsson S, et al. Apoptosis induced by the monomers HEMA and TEGDMA involves formation of ROS and differential activation of the MAP-kinases p38, JNK and ERK. Dent Mater. 2007;23(1):34–39. doi: 10.1016/j.dental.2005.11.037.
- Spagnuolo G, D'Anto V, Valletta R, et al. Effect of 2-hydroxyethyl methacrylate on human pulp cell survival pathways ERK and AKT. J Endod. 2008;34(6):684–688. doi: 10.1016/j.joen.2008.02.040.
- Krifka S, Spagnuolo G, Schmalz G, et al. A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials. 2013;34(19):4555–4563. doi: 10.1016/j.biomaterials.2013.03.019.
- Klaassen CD. Casarett & Doull’s toxicology: the basic science of poisons., 7th ed., McGraw-Hill Medical, New York, 2008.
- Demasi M, Netto LE, Silva GM, et al. Redox regulation of the proteasome via S-glutathionylation. Redox Biol. 2014;2:44–51. doi: 10.1016/j.redox.2013.12.003.
- Engelmann J, Leyhausen G, Leibfritz D, et al. Effect of TEGDMA on the intracellular glutathione concentration of human gingival fibroblasts. J Biomed Mater Res. 2002;63(6):746–751. doi: 10.1002/jbm.10465.
- Samuelsen JT, Kopperud HM, Holme JA, et al. Role of thiol-complex formation in 2-hydroxyethyl- methacrylate-induced toxicity in vitro. J Biomed Mater Res A. 2011;96(2):395–401. doi: 10.1002/jbm.a.32993.
- Ansteinsson V, Kopperud HB, Morisbak E, et al. Cell toxicity of methacrylate monomers-the role of glutathione adduct formation. J Biomed Mater Res A. 2013;101(12):3504–3510. doi: 10.1002/jbm.a.34652.
- Spagnuolo G, D'Anto V, Cosentino C, et al. Effect of N-acetyl-L-cysteine on ROS production and cell death caused by HEMA in human primary gingival fibroblasts. Biomaterials. 2006;27(9):1803–1809. doi: 10.1016/j.biomaterials.2005.10.022.
- Paranjpe A, Cacalano NA, Hume WR, et al. N-acetyl cysteine mediates protection from 2-hydroxyethyl methacrylate induced apoptosis via nuclear factor kappa B-dependent and independent pathways: potential involvement of JNK. Toxicol Sci. 2009;108(2):356–366. doi: 10.1093/toxsci/kfp010.
- Becher R, Valen H, Olderbo BP, et al. The dental monomer 2-hydroxyethyl methacrylate (HEMA) causes transcriptionally regulated adaptation partially initiated by electrophilic stress. Dent Mater. 2019;35(1):125–134. doi: 10.1016/j.dental.2018.11.008.
- Alizadehgharib S, Ostberg AK, Dahlgren U. Effects of the methacrylate/acrylate monomers HEMA, TEGDMA, DEGDA, and EMA on the immune system. Clin Exp Dent Res. 2017;3(6):227–234. doi: 10.1002/cre2.93.
- Bolling AK, Samuelsen JT, Morisbak E, et al. Dental monomers inhibit LPS-induced cytokine release from the macrophage cell line RAW264.7. Toxicol Lett. 2013;216(2-3):130–138. doi: 10.1016/j.toxlet.2012.11.010.
- Krifka S, Petzel C, Hiller KA, et al. Resin monomer-induced differential activation of MAP kinases and apoptosis in mouse macrophages and human pulp cells. Biomaterials. 2010;31(11):2964–2975. doi: 10.1016/j.biomaterials.2010.01.005.
- Schweikl H, Altmannberger I, Hanser N, et al. The effect of triethylene glycol dimethacrylate on the cell cycle of mammalian cells. Biomaterials. 2005;26(19):4111–4118. doi: 10.1016/j.biomaterials.2004.10.026.
- Eckhardt A, Muller P, Hiller KA, et al. Influence of TEGDMA on the mammalian cell cycle in comparison with chemotherapeutic agents. Dent Mater. 2010;26(3):232–241. doi: 10.1016/j.dental.2009.10.005.
- Urcan E, Scherthan H, Styllou M, et al. Induction of DNA double-strand breaks in primary gingival fibroblasts by exposure to dental resin composites. Biomaterials. 2010;31(8):2010–2014. doi: 10.1016/j.biomaterials.2009.11.065.
- Schweikl H, Schmalz G, Rackebrandt K. The mutagenic activity of unpolymerized resin monomers in Salmonella typhimurium and V79 cells. Mutat Res. 1998;415(1–2):119–130. doi: 10.1016/s1383-5718(98)00067-9.
- Schweikl H, Schmalz G, Spruss T. The induction of micronuclei in vitro by unpolymerized resin monomers. J Dent Res. 2001;80(7):1615–1620. doi: 10.1177/00220345010800070401.
- Ansteinsson V, Solhaug A, Samuelsen JT, et al. DNA-damage, cell-cycle arrest and apoptosis induced in BEAS-2B cells by 2-hydroxyethyl methacrylate (HEMA). Mutat Res. 2011;723(2):158–164. doi: 10.1016/j.mrgentox.2011.04.011.
- Krifka S, Petzel C, Bolay C, et al. Activation of stress-regulated transcription factors by triethylene glycol dimethacrylate monomer. Biomaterials. 2011;32(7):1787–1795. doi: 10.1016/j.biomaterials.2010.11.031.
- Eckhardt A, Gerstmayr N, Hiller KA, et al. TEGDMA-induced oxidative DNA damage and activation of ATM and MAP kinases. Biomaterials. 2009;30(11):2006–2014. doi: 10.1016/j.biomaterials.2008.12.045.
- Ishikawa A, Jinno S, Suzuki T, et al. Global gene expression analyses of mouse fibroblast L929 cells exposed to IC50 MMA by DNA microarray and confirmation of four detoxification genes’ expression by real-time PCR. Dent Mater J. 2006;25(2):205–213. doi: 10.4012/dmj.25.205.
- Schweikl H, Hiller KA, Eckhardt A, et al. Differential gene expression involved in oxidative stress response caused by triethylene glycol dimethacrylate. Biomaterials. 2008;29(10):1377–1387. doi: 10.1016/j.biomaterials.2007.11.049.
- Cho SG, Lee JW, Heo JS, et al. Gene expression change in human dental pulp cells exposed to a low-level toxic concentration of triethylene glycol dimethacrylate: an RNA-seq analysis. Basic Clin Pharmacol Toxicol. 2014;115(3):282–290. doi: 10.1111/bcpt.12197.
- Nilsen BW, Simon-Santamaria J, Ortengren U, et al. Dose- and time-dependent effects of triethylene glycol dimethacrylate on the proteome of human THP-1 monocytes. Eur J Oral Sci. 2018;126(5):345–358. doi: 10.1111/eos.12559.
- Samuelsen JT, Michelsen VB, Bruun JA, et al. The dental monomer HEMA causes proteome changes in human THP-1 monocytes. J Biomed Mater Res A. 2019;107(4):851–859. doi: 10.1002/jbm.a.36601.
- Tilija Pun N, Park PH. Role of p62 in the suppression of inflammatory cytokine production by adiponectin in macrophages: involvement of autophagy and p21/Nrf2 axis. Sci Rep. 2017;7(1):393. doi: 10.1038/s41598-017-00456-6.
- Liu F, Rehmani I, Esaki S, et al. Pirin is an iron-dependent redox regulator of NF-kappaB. Proc Natl Acad Sci U S A. 2013;110(24):9722–9727. doi: 10.1073/pnas.1221743110.
- Chaung W, Mi LJ, Boorstein RJ. The p53 status of Chinese hamster V79 cells frequently used for studies on DNA damage and DNA repair. Nucleic Acids Res. 1997;25(5):992–994. doi: 10.1093/nar/25.5.992.
- Sugimoto K, Toyoshima H, Sakai R, et al. Frequent mutations in the p53 gene in human myeloid leukemia cell lines. Blood. 1992;79(9):2378–2383. doi: 10.1182/blood.V79.9.2378.bloodjournal7992378.
- Singh A, Misra V, Thimmulappa RK, et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3(10):e420. doi: 10.1371/journal.pmed.0030420.
- ISO, ISO 10993-1 Biological evaluation of medical devices -, part 1: evaluation and testing within a risk management process. Geneva, Switzerland: International Standard Organization, 2018.
- Chen AY, Zirwas MJ. Denture stomatitis. Skinmed. 2007;6(2):92–94. doi: 10.1111/j.1540-9740.2007.05867.x.
- ISO, ISO 10993-10 Biological evaluation of medical devices -, part 10: tests for skin sensitization. Geneva, Switzerland: International Standard Organization, 2021.
- ISO, ISO 10993-23 Biological evaluation of medical devices -, part 23: tests for irritation. Geneva, Switzerland: International Standard Organization, Geneva, Switzerland, 2021.
- Frankild S, Volund A, Wahlberg JE, et al. Comparison of the sensitivities of the buehler test and the Guinea pig maximization test for predictive testing of contact allergy. Acta Derm Venereol. 2000;80(4):256–262.
- OECD, Test No. 429: skin Sensitisation, 2010.
- Ankley GT, Bennett RS, Erickson RJ, et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29(3):730–741. doi: 10.1002/etc.34.
- Urbisch D, Honarvar N, Kolle SN, et al. Peptide reactivity associated with skin sensitization: the QSAR toolbox and TIMES compared to the DPRA. Toxicol In Vitro. 2016;34:194–203. doi: 10.1016/j.tiv.2016.04.005.
- Kim H, Choi J, Lee H, et al. Skin corrosion and irritation test of nanoparticles using reconstructed three-dimensional human skin model, EpiDerm(TM. Toxicol Res. 2016;32(4):311–316. doi: 10.5487/TR.2016.32.4.311.
- Spielmann H, Kalweit S, Liebsch M, et al. Validation study of alternatives to the draize eye irritation test in Germany: cytotoxicity testing and HET-CAM test with 136 industrial chemicals. Toxicol In Vitro. 1993;7(4):505–510. doi: 10.1016/0887-2333(93)90055-a.
- Rivero MN, Lenze M, Izaguirre M, et al. Comparison between HET-CAM protocols and a product use clinical study for eye irritation evaluation of personal care products including cosmetics according to their surfactant composition. Food Chem Toxicol. 2021;153:112229. doi: 10.1016/j.fct.2021.112229.
- Dahl JE. Irritation of dental adhesive agents evaluated by the HET-CAM test. Toxicol In Vitro. 1999;13(2):259–264. doi: 10.1016/s0887-2333(98)00086-1.
- Lonnroth EC, Dahl JE, Shahnavaz H. Evaluating the potential occupational hazard of handling dental polymer products using the HET-CAM technique. Int J Occup Saf Ergon. 1999;5(1):43–57. doi: 10.1080/10803548.1999.11076410.
- Dahl JE. Potential of dental adhesives to induce mucosal irritation evaluated by the HET-CAM method. Acta Odontol Scand. 2007;65(5):275–283. doi: 10.1080/00016350701589286.
- Natsch A, Emter R. Skin sensitizers induce antioxidant response element dependent genes: application to the in vitro testing of the sensitization potential of chemicals. Toxicol Sci. 2008;102(1):110–119. doi: 10.1093/toxsci/kfm259.
- Gerberick GF, Vassallo JD, Bailey RE, et al. Development of a peptide reactivity assay for screening contact allergens. Toxicol Sci. 2004;81(2):332–343. doi: 10.1093/toxsci/kfh213.
- Natsch A. The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers–functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicol Sci. 2010;113(2):284–292. doi: 10.1093/toxsci/kfp228.
- OECD, Test No. 442C: in Chemico Skin Sensitisation, 2015.
- OECD, Test No. 442D: in Vitro Skin Sensitisation, 2015.
- Russell WMS, Burch RL. The principles of humane experimental technique. Special edition ed. South Mimms: Universities Federation for Animal Welfare, 1992.
- Natsch A, Emter R, Ellis G. Filling the concept with data: integrating data from different in vitro and in silico assays on skin sensitizers to explore the battery approach for animal-free skin sensitization testing. Toxicol Sci. 2009;107(1):106–121. doi: 10.1093/toxsci/kfn204.
- van der Veen JW, Rorije E, Emter R, et al. Evaluating the performance of integrated approaches for hazard identification of skin sensitizing chemicals. Regul Toxicol Pharmacol. 2014;69(3):371–379. doi: 10.1016/j.yrtph.2014.04.018.
- Henriks-Eckerman ML, Suuronen K, Jolanki R, et al. Methacrylates in dental restorative materials. Contact Dermatitis. 2004;50(4):233–237. doi: 10.1111/j.0105-1873.2004.00336.x.