Abstract
Objective
In this article, we analyzed the important categories capable of interfering with the determinants of scientific advancement in the type of study, considering seven leading journals over a 20-year.
Methodology
A bibliometric review was performed at the website of well-established implant dentistry journals in five-time points defined to represent a 20-year period of observation. The measures associated with the type of study design were: the country of origin of the article, country income, continent of the corresponding author, inter-institutional collaboration, interdisciplinary collaboration, type of funding, and topic of research. Logistic regression was used in the multiple models to identify the exploratory factors associated with the type of study.
Results
From a total of 1,944 articles, 50.6% comprised clinical studies. High-income countries and continents stood out for developing more clinical research than others. Since research funders request more collaborative research, overall clinical studies depended upon more inter-institutional collaboration than the others. Most clinical studies were partly supported by research institutes or universities and by industry. About the research topic, the majority of the clinical and animal studies disclosed surgical procedures.
Conclusions
High-income countries and continents are more likely to develop clinical studies in the surgical procedures field. The highest collaborations in terms of the number of institutions and funding sources are more prevalent in clinical research designs. Indeed, most in vivo studies in dental implant fields are performed to evaluate new materials or even new surgical procedures.
1. Introduction
The number of patients rehabilitated with dental implants has grown over the years as the medical device have been used routinely worldwide.Citation1–4 Concomitantly, the global dental implants market has expanded significantly in the last years, with an annual growth expectation rating of 9.8% between 2023 and 2030 (https://www.grandviewresearch.com/industry-analysis/dental-implants-market). The popularity of dental implant therapy translates to the direct impact of research discoveries on clinical applications, given the high success rates and survival in the first year after dental implant placement.Citation5 Notedly, for research outcomes to be commercialized and finally reach society, clinical efficacy, regulatory approval, and commercial viability must be provided. In other words, the impressive technological advancement within the dental implant field is supported by research of quality, and the clinical application of scientific knowledge depends on the most appropriate type of study.Citation6
The evidence pyramid depicts the types of different research studies according to the relevancy and quality of the evidence. Importantly, the type of study chosen by a researcher will depend on the type of clinical question. In general, in vitro, ex vivo, and in silico models are carried out at the beginning of the idea construction to validate the methodology and reflect the natural process of knowledge development.Citation6–9 However, the persistence at this stage might reflect a lack of financial support and/or unsolved preclinical issues. After that, researchers may move forward to the preclinical trials within animal subjects to visualize potential interactions, safety, toxicity, and efficacy of a product within a living organism.Citation6–8 At the peak of the pyramid are clinical research, which act to conclude and validate the meaningful scientific results to be translated into the real setting.
Indeed, financial support in the tools of science ensures efficient functional structures to perform research of quality and increase the ability of a country in competing with others. Therefore, the real reason behind choosing a type of study might be beyond the research question. High-, upper-middle and low-income countries are defined according to the gross national income (GNI) per capita, calculated using the World Bank Atlas method, which means that high-income economies are those with a GNI per capita of $13,846 or more (https://data.worldbank.org/country/XD). High‐income countries make substantial investments in research and innovation and evidently publish more articles under the heading of "clinical trials".Citation10 This can be explained by the fact that, in general, clinical studies are more costly, extensive, and complex to perform than animal or in vitro studies.Citation6 On the other hand, upper-middle and low-income countries comprise insufficient economic capacity, which reflects the greater proportion of in vitro or animal studies.Citation10
The origin of funding may also vary depending on the continent and might influence the university innovation performance. According to Guam et al. 2005, university-industry collaboration is responsible for driving industrial and technological innovation and the welfare of a country.Citation11 High-income countries from Europe and North America, for example, leaded beneficiaries of industry funds from private companies,Citation10 probably because countries such as the USA and Switzerland disclose the most important and well-established dental implant companies. By contrast, South American countries, such as Brazil, or Asian countries, such as China or Korea, are more susceptible to government funds.Citation12 In an attempt to overcome this point at issue, strategic partnerships between high- and upper-middle/lower-income countries and even between universities and industry might open up new avenues through scientific collaboration.
Inter-institutional collaborations and multidisciplinary team sets are appointed as important aspects that raise the types of study and chances of a study being funded by any type of source.Citation13–15 Indeed, international collaboration has been intensified since it is thought to be an excellent tool to improve the scientific output, mainly between high-income and upper-middle-/lower-income countries.Citation16,Citation17 Scientific advancement also requires a team with a multidisciplinary skillset able to address different areas of development and facilitate innovative ideas, methods development, and data interpretation improvement.Citation18 The impact of overall collaborations on financial investment might be estimated by increasing industry partnership motivations. In turn, industry partnerships might signalize the recognition of the research on clinical practice. Recently, Pereira et al. 2022, showed, through a bibliometric study, that interinstitute collaboration is a critical factor in underpinning the industry’s sponsorship interest.Citation13
At long last, it is necessary to recognize the impact of study design and the factors involved in each step of research development, as an essential element, in boosting the science progression. Unraveling the factors capable of interfering with the type of study, and therefore, the high level of evidence, may help to fix the disparity between the production burden and the production of health knowledge through clinical studies. Scientific advancement requires financial investment, which means that national economic growth can interfere with scientific capacity. This statement predicts a direct association between high-income countries and research advancement from laboratorial studies to clinical trials. In an attempt to gain a better understanding about the factors that guide the type of study, in this article, we analyzed the impact of country of origin of the article, country income, continent of the corresponding author, international collaboration, inter-institutional collaboration, interdisciplinary collaboration, type of funding and topic of research as important categories capable of interfering with the determinants of scientific advancement considering seven leading journals over a 20-year observational period. We also discussed key actions that could improve the research team’s ability to progress through these categories.
2. Material and methods
A bibliometric review was performed by hand-search at the website of seven well-established implant dentistry journals: Clinical Implant Dentistry and Related Research (CIDRR), Clinical Oral Implants Research (COIR), Implant Dentistry (ID), Journal of Clinical Periodontology (JCP), The International Journal of Oral & Maxillofacial Implants (JOMI), the Journal of Oral Implantology (JOI), and The Journal of Prosthetic Dentistry(JPD), in five time-points defined to represent a 20-year period of observation: 1999, 2004, 2009, 2014 and 2019. The journals were selected based on the journals’ scope and the journals’ tradition in the implant dentistry field, and the different implant dentistry organizations that they represent. As inclusion criteria, only original peer-reviewed research articles related to implant dentistry were included in the study. The exclusion criteria were as follows: (a) non-related articles to implant dentistry; (b) non-original articles such as reviews, abstracts, case reports, techniques, letters to editors, opinions, book reviews, symposium reports, and association reports.
The data was carried out by two independent investigators (M.M.A.P. and C.D.). To ensure consistency in the collected data, calibration was performed before the data collection and analysis. The title and abstract checklist of each journal for the first volume of 2019 were tested for calibration. The interrater assessment was calculated using Cohen’s kappa (κ) coefficient. Generally, a kappa of less than 0 indicates no agreement, 0.01–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect agreement.Citation19 Here, the data confirmed the excellent level of agreement with the Kappa value of κ = 0.97. Divergences between investigators were solved through discussion with two experts (V.A.R.B. and E.D.A.).
After the calibration process, the full text of all included articles was downloaded from the journals’ websites. Each article was reviewed for the following information: general information such as publication year, name of the journal, title of publication, and number of authors, as well as type of study, country of origin, country income, continent of origin, international collaboration, inter-institutional collaboration, interdisciplinary collaboration, type of funding and topic of the research of each article, were collected. All parameters collected are described in detail in .
Table 1. Detailed description of all collected parameters.
Graph- Pad Prism version 5.0c was used to create the overall frequency distribution of each factor evaluated per type of study. SPSS 21.0 (IBM Corporation) was used to analyze the data. Descriptive data regarding all variables evaluated were summarized. Bivariate analysis was performed to assess the associations and distribution between type of study (dependent variables) and exploratory factors, including publication year, number of authors, country of origin, country income, continent of origin, international collaboration, inter-institutional collaboration, interdisciplinary collaboration, type of funding and topic of the research of each article. The exploratory variables with p ≤ 0.20 were included in the multiple models. Finally, logistic regression was used in the multiple models to identify the exploratory factors associated with the type of study, considering a significance level of 5%. Odds ratio (OR) values and 95% confidence intervals (CI) were estimated.
3. Results
3.1. Distribution of articles according to the type of study
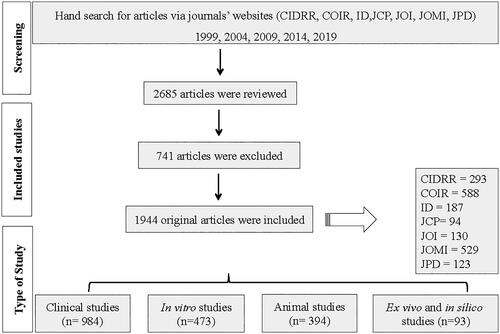
From a total of 2,685 articles published in seven journals between 1999 and 2019, 741 manuscripts were excluded, and 1,944 met the inclusion criteria. Among them, 984 articles (50.6%) comprised clinical studies, 473 (24.3%) in vitro studies, 394 (20.3%) animal studies, and finally, 93 articles (4.8%) reported ex vivo and in-silico studies ().
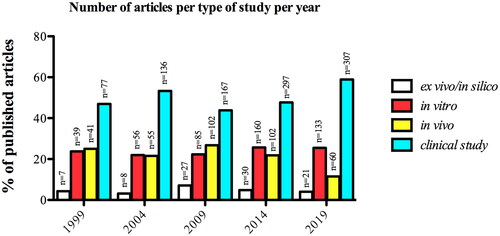
Throughout the years, the distribution of the types of studies kept a similar trend, with the majority of the articles reporting clinical studies, followed by in vitro and animal studies, and the minority of the articles reporting ex vivo and in-silico studies. Particularly, in 2019, it is possible to observe an expressive decrease of 42.2% in animal studies and an increase of 26.6% in clinical studies compared with 2014 (). Looking over the distribution of the total number of articles according to the type of study, we noticed that the clinical studies kept a crescent increase in the number of articles over the investigated period, while the other types of studies had an increase from 1999 to 2014 and a decrease in 2019 ().
3.2. Type of study versus geographic origins and income countries
With regards to the geographic origins, the included articles came from 56 different countries. The United States of America (USA) represented the most frequent country of published articles (n = 297), followed by Brazil (n = 178), Italy (n = 175), Germany (n = 167), Sweden (n = 129), China (n = 115), Switzerland (n = 99), Korea (n = 92), Japan (n = 82) and Spain (n = 77). Concerning the continent origins, Europe published the largest number of articles within clinical (55.59%) and animal studies (41.88%) subjects, followed by Asia (clinical studies = 20.43%; animal studies = 27.41%) and North America (clinical studies = 15.65%; animal studies = 14.21%). Europe and Asia also detained similar distribution of in vitro studies articles, whereas Asia published the majority of the ex vivo and in-silico studies ().
Figure 3. Distribution of total number of published articles according to the type of study per continent; “n” refers to total number of published articles according to the type of study.
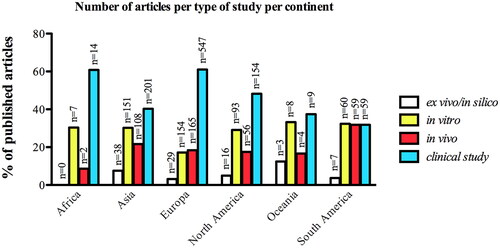
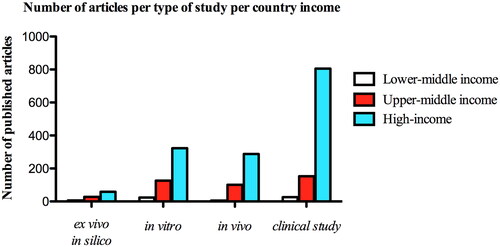
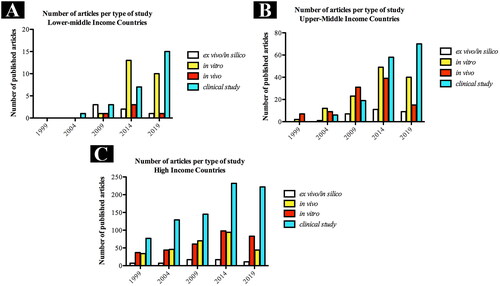
With regards to the developed economic of overall countries, we observed that high-income countries hold the largest number of published articles under clinical topics (). Unraveling the distribution of the type of study according to the general income of the countries over the years, we observed that there was an increase in the number of clinical articles published from 1999 to 2019, regardless of the countries’ income ().
Figure 5. Distribution of total number of published articles according to the income country and type of study over the years. (A) Lower-middle income countries, (B) Upper-middle income countries and (C) High income countries, according to the World Bank country and lending groups (september 2021).
Further bivariate analysis showed that high-income countries were more likely to publish articles within clinical study subject than others (OR = 1.61; =95% CI, 0.96–2.71; p = 0.069) (Supplementary Table 1S). In addition, Europe stood out for developing more clinical research, with 3.35 more chances of carrying it out compared to other types of studies (OR = 3.35; 95% CI, 2.39–4.70; p < 0.001), followed by North American continent (OR = 1.99; 95% CI, 1.36–2.91; p < 0.001). As shown in the multiple models, clinical studies disclosed a 2.97 times greater chance of having conducted by countries from Europe compared to animal, in vitro, ex vivo, and in silico (OR = 2.97; 95% CI, 2.06–4.28; p < 0.001) ().
Table 2. Multiple regression model for indicators of type of study by number of authors, country income, number of institution (interinstitutional collaboration), topic of research, and funding factors.
3.3. Type of study versus scientific collaboration
Concerning the overall scientific collaboration, clinical studies stand out for involving a greater number of institutions and disciplines in published articles in comparison to the others (). Moreover, clinical studies showed a well-established collaboration, with most of the published articles comprising 5 or more authors ().
Figure 6. Distribution of total number of published articles according to the number of disciplines from each author’s affiliation per type of study (A) and number of institutions involved from each author’s affiliation per type of study.
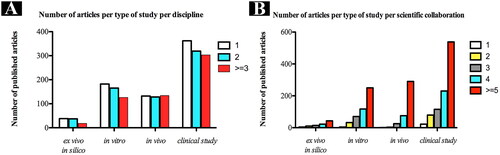
In addition, regardless of the type of study, a positive correlation might be observed between scientific collaboration (number of authors, number of institutions, and disciplines) and number of published articles. However, bivariate analysis suggests no association between clinical study outcome and number of authors (OR = 0.93; 95% CI, 0.85–1.01; p = 0.109), number of disciplines, and number of institutions (OR = 1.06; 95% CI, 0.98–1.15; p = 0.091). For the multiple models, as it is shown in , our findings revealed that with the increase in the numbers of institutions, clinical studies were 1.14 times more likely to be conducted than other types of research (OR = 1.14; 95% CI, 1.04–1.25; p = 0.003).
3.4. Type of study versus type of funding
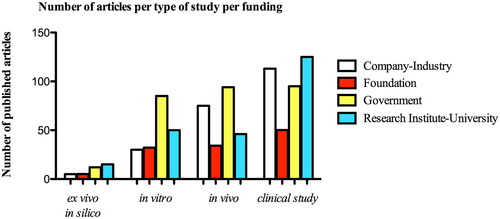
The impact of type of study on research financial funding to knowledge generate has been investigated at multiple levels: governments, research institutes or universities, and industry levels. In total, 1,017 (52.3%) articles were screened with some type of support. The overall support might include funding, design, implementation, data analysis, or reporting. Out of 1,017 articles included, 866 (44.5%) of the total studies were supported financially by any source of funding. Of the included articles, 383 were related to clinical studies, followed by 249 animal studies, 197 in vitro studies, and only 37 published articles were under ex vivo and in silico topics. Overall, the majority of clinical studies were supported in part by research institutes or universities (125 studies; 29.3%) and by industry (113 studies; 26.5%). Regarding in vitro studies, 85 studies were supported by the governments (31.95%), and only 30 (25.9%) received any type of source from industry ().
In the multiple logistic regression analysis (), articles that publish clinical research were 1.5 times more likely to be funded by a research institute or university (OR = 1.48; 95% CI, 0.99–2.21; p = 0.05) compared with other types of studies ().
3.5. Type of study versus topic of the research
About the topic of the research, the majority of the clinical and animal studies disclosed surgical procedures, whereas in vitro, ex vivo, and in-silico studies were related to prosthodontic topics ().
Figure 8. Distribution of 5 main research topics in dental implant research field according to the type of study; “n” refers to total number of published articles according to the type of study.
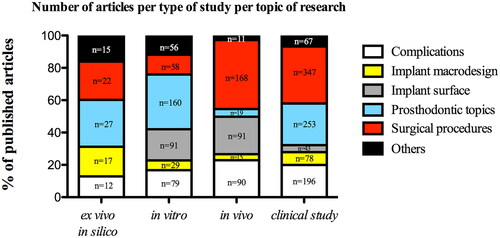
Bivariate analysis showed that surgical procedures were more likely to be conducted by clinical studies than those from other topics (OR = 1.29; 95% CI, 0.99–1.67; p = 0.053). However, when we calculated the probability of clinical studies being conducted depending on research topics sets through the multiple logistic regression analysis (), we found a slight difference among prosthodontics topics, implant design, and surgical procedures, with 1.18 (OR = 1.18; 95% CI, 0.88–1.57; p = 0.263), 1.16 (OR = 1.16; 95% CI, 0.76–1.77; p = 0.469) and 1.15 (OR = 1.15; 95% CI, 0.87–1.52; p = 0.301) more chances to be conducted by clinical studies compared with other research topics.
4. Discussion
Scientific progress is related to the development of technologies to improve human health through the process in which basic science discoveries are integrated into clinical applications. Therefore, the type of research studies might be taken as indicators or measures of scientific progress. According to the new evidence pyramid, clinical trials are always assigned the top on levels of evidence because they provide the basis for developing and marketing new biological products, medical devices, and drugs, for example.Citation20 In this study, we demonstrated that high-income countries and continents are more likely to develop clinical studies in the dental implant field. Importantly, we also revealed that the highest collaborations in terms of number of institutions and funding sources are more prevalent in clinical research designs.
Overall investments in late-stage clinical studies could improve population access to new health products, therapeutics, and diagnostics. High-income countries understand the worth of research and invest strongly in that sector.Citation21 Accordingly, our outcomes revealed that high-income countries hold a higher number of published articles in the dental implant field. Payne and Siow (2003) demonstrated the impact of financial support in science, asserting that a $1 million increase in funding yields 10 to 16 additional scientific articles.Citation22 Further analysis confirmed a strong correlation between the type of study and the income of countries and continents. According to this evidence, the clinical studies stand out with an impressive numerical difference in relation to the others. Unlike, upper-middle- and lower-income countries are still far behind in prioritizing research and development for economic growth and national development. In a recent study, the authors justified the few randomized clinical trials performed by upper-middle- and lower-income countries due to the lack of research funding, lack of research infrastructure, and inadequate budgets.Citation23 Although our results have demonstrated that high-income countries are more likely to develop clinical studies, in terms of the total number of publications, upper-middle-income, such as Brazil and China, also presented high scientific production. The high frequency in number of publications might be justified by the network of international collaboration. Indeed, high-income countries hold greater resources and offer quality infrastructure, which attracts and enables a greater number of collaborations with upper-middle and low-income countries, consequently affecting scientific production.Citation14,Citation19,Citation24
The crucial goal of interinstitutional scientific teams and multidisciplinary skillsets is to integrate knowledge from diverse groups to develop novel insights and innovations. Moreover, research funders are requesting more interdisciplinary and collaborative research as an attempt to bridge the gap between research and clinical application.Citation25 Although the multivariate logistic regression analysis did not show a substantial probability of increasing clinical studies publication in comparison to other researches studies, depending on multiple sets of collaborations: number of authors, disciplines, and institutions, our descriptive results demonstrated an important impact of collaboration to advance in a clinical set with the majority of the articles being composed for 5 or more authors from different institutions. In addition, the results in the present article also revealed a multidisciplinary team concentrated, with authors from different disciplines, on clinical study design compared to the others. The findings are consistent with a recent article from our research group that reported an increasing trend in collaboration between countries over the years in the dental implant field.Citation14 Previous study has also demonstrated a growth in scientific production linked to increased collaboration, mainly between high-income and upper-middle/lower-income countries.Citation19 Further reasons for overall collaboration include rising investments, easier access to financial resources, intellectual influences, increased scientific productivity, and exchange programs.Citation26,Citation27
Looking over clinical studies, the dissemination gap also has a time component to conduct a project through the development processes. A review suggested that it might take around 17 years for 14% of original (i.e. discovery) research to launch a product into practice.Citation28 Certainly, the clinical research stage can vary depending on the product being developed and the regulatory rules of each country. However, besides the investment required to move forward with research development, it is mandatory to conclude each step of preclinical studies. Recent evidence has pointed out that only 21 articles published clinical outcomes considering the topic of antibacterial dental implant surfaces independent of the countries’ income.Citation6 This number is extremely low in view of the 1,778 articles included through a systematic search.Citation6 The conclusion of the stagnation in clinical application could also be attributed to the complexity of the factors involved in antimicrobial coating development.Citation6 Although the challenges encompassed specific topics within the dental implants field should be considered as interfering variables in the number of preclinical and clinical studies, we can suggest that countries which manage to invest heavily in scientific resources will certainly be able to evolve faster in the type of study design than the others. In a recent study, the authors analyzed the projects submitted to the ITI International Team for Implantology for funding and observed that the USA was the country and the University of Bern, in Switzerland, was the institution with the largest number of financed projects and published papers.Citation29
Another important result that drew our attention is the major financial support from research institutes or universities and overall industries directed to animal and clinical studies compared to the others. When we disassembled both studies, we observed that the investment in clinical research was even greater. A similar trend was observed by our research team, which found that randomized clinical trials performed by high-income countries were more likely to be funded by industry compared with upper- and lower-middle-income countries.Citation14 This result is consistent with all said in this discussion since high-income countries might provide better structural and financial conditions. In addition, most reputable and well-known dental implant companies were developed in USA and Switzerland, which certainly, favor more industry support directed to both countries (Website (2020). Accessed January 2022. https://meticulousblog.org/ top-10-companies-in-dental-implants-market/). According to Markham, the trajectory of an idea conception is followed by such steps: idea and research, commercial importance, promisors’ results, strategy team, influence, formal process, and product launch. Inevitably, resources must be present in all steps of the process to the scientific development of the product are completed.Citation30 Consistently, the majority of the clinical and animal studies disclosed surgical procedures, while in vitro, ex vivo, and in-silico studies are about prosthodontic topics. Indeed, most in vivo studies in dental implant fields are performed to evaluate new materials or even though new surgical procedures.
The present study disclosed shortcomings regarding the period represented by 20-year observational data sets since that only 5-time points were selected to facilitate data extraction. We also selected only 7 dental implant journals based on the journals’ scope in relation to the subject. We are also aware of other biases that this study may present, even regarding the type of funding received and the amount of collaboration. Regarding this last, we considered only the collaboration between countries when the first author’s country was different from the other authors.
In fact, scientific research generated at universities and research organizations plays an important role for science to reach society.Citation31,Citation32 As already mentioned above, clinical study represents the last stage during the development of a product, which highlights the impact of clinical studies not only extends to the individual patient through rehabilitation but also to society as a whole by enhancing the value of health care provided.
5. Conclusion
Based on the analysis of articles published in seven dental implant journals, we demonstrated that high-income countries and continents are more likely to develop clinical studies in the surgical procedures field. Importantly, we also revealed that the highest collaborations in terms of number of institutions and funding sources are more prevalent in clinical research designs. Indeed, most in vivo studies in dental implant fields are performed to evaluate new materials or even new surgical procedures.
Authors’ contributions
V.A.R.B and E.D.A. conceived the ideas; C.D. and M.M.A.P. screened the initial entries and selected the articles; C.D. and M.M.A.P. collected the data; J.G.S.S. performed the statistical analysis; C.D., M.M.A.P., J.G.S.S., E.D.A. and V.A.R.B. analyzed the data; M.M.A.P. led the writing; V.A.R.B., E.D.A., and J.G.S.S. made the critical review of article content and writing.
Supplemental Material
Download MS Word (16 KB)Acknowledgments
This study was funded by the State of Sao Paulo Research Foundation (FAPESP, Brazil) (grant numbers 2021/10762-1 to M.M.A.P.; 2021/09434-0 to E.D.A; 2018/20719-3 to E.D.A; 2020/05231-4 to V.A.R.B.), the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq, Brazil) (#307471/2021-7 to V.A.R.B.), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior e Brazil (CAPES, Brazil) (Finance code 001).
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Pjetursson BE, Asgeirsson AG, Zwahlen M, et al. Improvements in implant dentistry over the last decade: comparison of survival and complication rates in older and newer publications. Int J Oral Maxillofac Implants. 2014;29(Supplement):1–11. doi: 10.11607/jomi.2014suppl.g5.2.
- Zitzmann NU, Krastl G, Hecker H, et al. Endodontics or implants? A review of decisive criteria and guidelines for single tooth restorations and full arch reconstructions. Int Endodontic J. 2009;42(9):757–774. doi: 10.1111/j.1365-2591.2009.01561.x.
- Zitzmann NU, Krastl G, Hecker H, et al. Strategic considerations in treatment planning: deciding when to treat, extract, or replace a questionable tooth. J Prosthet Dent. 2010;104(2):80–91. doi: 10.1016/S0022-3913(10)60096-0.
- Zitzmann NU, Scherrer SS, Weiger R, et al. Preferences of dental care providers in maintaining compromised teeth in relation to their professional status: implants instead of periodontally involved maxillary molars? Clin Oral Implants Res. 2011;22(2):143–150. doi: 10.1111/j.1600-0501.2010.02062.x.
- French D, Ofec R, Levin L. Long term clinical performance of 10 871 dental implants with up to 22 years of follow-up: a cohort study in 4247 patients. Clin Implant Dent Rel Res. 2021;23(3):289–297. doi: 10.1111/cid.12994.
- de Avila ED, Nagay BE, Pereira MMA, et al. Race for applicable antimicrobial dental implant surfaces to fight Biofilm-Related disease: advancing in laboratorial studies vs stagnation in clinical application. ACS Biomater Sci Eng. 2022;8(8):3187–3198. doi: 10.1021/acsbiomaterials.2c00160.
- Andrade EL, Bento AF, Cavalli J, et al. Non-clinical studies required for new drug development – part I: early in silico and in vitro studies,new target discovery and validation,proof of principles and robustness of animal studies. Brazilian J Med Biol Res. 2016;49(11):1–9. doi: 10.1590/1414-431x20165644.
- Danchin A. In vivo, in vitro and in silico: an open space for the development of microbe-based applications of synthetic biology. Microb Biotechnol. 2022;15(1):42–64. doi: 10.1111/1751-7915.13937.
- Gowing G, Svendsen S, Svendsen CN. Ex vivo gene therapy for the treatment of neurological disorders. Prog Brain Res. 2017;230:99–132. doi: 10.1016/bs.pbr.2016.11.003.
- Barão VA, Shyamsunder N, Yuan JC, et al. Trends in funding, internationalization, and types of study for original articles published in five implant-related journals between 2005 and 2009. Int J Oral Maxillofac Implants. 2012;27:69–76.
- Guan J, Zhao Q. The impact of university—industry collaboration networks on innovation in nanobiopharmaceuticals. Technol Forecast Soc Change. 2013;80(7):1271–1286. doi: 10.1016/j.techfore.2012.11.013.
- Bastani P, Mohammadpour M, Mehraliain G, et al. What makes inequality in the area of dental and oral health in developing countries? A scoping review. Cost Eff Resour Alloc. 2021;19(1):54. 26 doi: 10.1186/s12962-021-00309-0.
- Pereira MMA, Dini C, Souza JGS, et al. Industry support for dental implant research: a metatrend study of industry partnership in the development of new technologies. J Prosthet Dent. 2022;3913(22):00355–00359. doi: 10.1016/j.prosdent.2022.05.026.
- Dini C, Pereira MMA, Souza JGS, et al. Association between industry support and the reporting of study outcomes in randomized clinical trials of dental implant research from the past 20 years. Clin Implant Dent Rel Res. 2022;24(1):94–104. doi: 10.1111/cid.13065.
- Barão VAR, Shyamsunder N, Yuan JCC, et al. Authorship, collaboration, and funding trends in implantology literature: analysis of five journals from 2005 to 2009. Implant Dent. 2011;20(1):68–75. doi: 10.1097/ID.0b013e3181fce302.
- Abramo G, D’Angelo CA, Solazzi M. The relationship between scientists’ research performance and the degree of internationalization of their research. Scientometrics. 2011;86(3):629–643. doi: 10.1007/s11192-010-0284-7.
- Way SF, Morgan AC, Larremore DB, et al. Productivity, prominence, and the effects of academic environment. Proc Natl Acad Sci USA. 2019;116(22):10729–10733. doi: 10.1073/pnas.1817431116.
- Disis M, Slattery JT. The road we must take: multidisciplinary team science. Sci Transl Med. 2010;2(22):22–29. doi: 10.1126/scitranslmed.3000421.
- Tarazona B, Vidal-Infer A, Alonso-Arroyo A. Bibliometric analysis of the scientific production in implantology (2009–2013). Clinical Oral Implants Res. 2017;28(7):864–870. doi: 10.1111/clr.12891.
- Murad NH, Asi N, Alsawas M, et al. New evidence pyramid. Evid Based Med. 2016;21(4):125–127. doi: 10.1136/ebmed-2016-110401.
- Yegros-Yegros A, van de Klippe W, Abad-Garcia MA, et al. Exploring why global health needs are unmet by research efforts: the potential influences of geography, industry and publication incentives. Health Res Policy Sys. 2020;18(1):47. doi: 10.1186/s12961-020-00560-6.
- Payne A, Siow A. Does federal research funding increase university research output? Advances in Economic Analysis & Policy. 2003;3(1):1018–1018. doi: 10.2202/1538-0637.1018.
- Rubagumya F, Hopman WM, Gyawali B, et al. Participation of lower and upper Middle–income countries in oncology clinical trials led by high-income countries. JAMA Netw Open. 2022;5(8):e2227252. doi: 10.1001/jamanetworkopen.2022.27252.
- Dini C, Pereira MMA, Souza JGS, et al. Mapping the trends and impact of research collaboration between countries in oral implantology publications: a bibliometric analysis from 1999 to 2019. J Prosthet Dent. 2022;3913(22):00653-9) doi: 10.1016/j.prosdent.2022.10.009.
- Nyström ME, Karltun J, Keller C, et al. Collaborative and partnership research for improvement of health and social services: researcher’s experiences from 20 projects. Health Res Policy Sys. 2018;16(1):46. doi: 10.1186/s12961-018-0322-0.
- Dusdal J, Powell JJW. Benefits, motivations, and challenges of international collaborative research: a sociology of science case study. Science and Public Policy. 2021;48(2):235–245. doi: 10.1093/scipol/scab010.
- Luukkonen T, Persson O, Sivertsen G. Understanding patterns of international scientific collaboration. Science, Technology, & Human Values. 1992;17(1):101–126. doi: 10.1177/016224399201700106.
- Balas EA. From appropriate care to evidence-based medicine. Pediatr Ann. 1998;27(9):581–584. doi: 10.3928/0090-4481-19980901-11.
- Lazarin R, Ebenezer S, Benthaus K, et al. The impact of the ITI international team for implantology on implant dentistry: a retrospective and descriptive analysis of 30 years of research support. Int J Oral Maxillofac Implants. 2020;35(1):e1–e13. doi: 10.11607/jomi.7799.
- Markham S. Moving technologies from lab to market. Res. Technol. Manag. 2002;45(6):31–42. doi: 10.1080/08956308.2002.11671531.
- Fleming L, Greene H, Li G, et al. Government-funded research increasingly fuels innovation. Science. 2019;364(6446):1139–1141. doi: 10.1126/science.aaw2373.
- Poege F, Harhoff D, Gaessler F, et al. (2019) Science quality and the value of inventions. Sci Adv. 2019;5(12):eaay7323. doi: 10.1126/sciadv.aay7323.