ABSTRACT
Microplastics, identified as an emerging environmental pollutant in marine ecosystems, necessitate immediate and comprehensive attention from organizations across various levels. The microsize of these plastic particles poses a significant threat to the environment as they effortlessly disperse throughout the biosphere via ocean waves. This study focuses on investigating the physicochemical properties of microplastic waste in a specific Southeast Asian regional area and evaluating its potential conversion into gaseous products through the pyrolysis process. Remarkably, the pyrolysis of microplastics resulted in an average syngas yield of 34.79%, demonstrating the viability of microplastic valorization. Furthermore, the pyro-solid-char produced exhibits a highly porous structure with minimal amounts of metal oxides, suggesting its potential utilization as fertilizer or catalyst.
Introduction
The myriad of microplastics (1 µm < plastic particles < 5 mm) in the global biosphere has caused serious concern, especially in the marine ecosystem. These tiny particles are found in large amounts from the breakdown of our daily products (i.e., cosmetics, chemical agents, fabric clothing). Researchers have proven that microplastics are everywhere in the deep oceans, including the sea region near Arctic and Antarctic ice, imposing threats to shellfish, dolphins, and turtles (Lim Citation2021). Significant impact on marine environments as they can be ingested by marine animals, causing harm to their health and potentially entering the food chain (Koelmans et al. Citation2022). Additionally, even worse, microplastic debris could absorb toxic pollutants (e.g., pesticides and industrial chemicals), increasing the adverse health effects to marine life (Gontard et al. Citation2022). For instance, when being digested, microplastics can block the digestive tracts of animals, leading to starvation or a buildup of toxins in the animal’s body. They can also physically damage internal organs and tissues. In addition, microplastics can act as a vector for the accumulation of harmful chemicals in marine animals, physically damaging the internal organs and tissues (Nunes et al. Citation2023).
Over the years, extensive research has provided compelling evidence that pyrolysis of bulk plastic waste is a viable and effective process, yielding high selectivity toward high-quality long-chain hydrocarbons and syngas (Miandad et al. Citation2019). For instance, Hu et al. (Citation2020) investigated the thermal decomposition of four individual pure plastic (e.g. high-density polyethylene (HDPE), low-density polyethylene (LDPE), polypropylene (PP), and polystyrene (PS)) using TGA-FTIR, revealing that CH4 is the major product from the degradation of HDPE, LDPE, and PP, while the aromatics and C – H containing compounds are the main products from the degradation of PS. Mumbach et al. (Citation2019) conducted a study using Gas Chromatography-Mass Spectrometry (GC-MS) to investigate the thermal degradation of plastic solid waste and analyze the composition of resulting liquid products. Their research revealed that the pyrolytic oil primarily comprised hydrocarbons, with a significant proportion being aliphatic hydrocarbons (i.e., 26.31% paraffins and 46.02% olefins), while aromatics constituted the remaining. The high presence of aliphatic hydrocarbons in the liquid product is favorable for potential applications as an energy source due to high calorific value and lower viscosity (Tian et al. Citation2011). Recently, Inayat and coauthors (Inayat et al. Citation2022) have reported the bio-oil production from co-pyrolysis of date seeds and plastic waste. The optimal blending ratio of 70% plastic to 30% date seed resulted in the highest bio-oil yield of 62.57%, with the remaining 37.43% comprising a combination of syngas and biochar.
While there is extensive literature supporting the promise of converting plastic waste into value-added chemicals, the research on elucidating the fundamental thermal degradation of microplastics, which often comprise a blend of various pure plastic compositions in a micro-scale are still very limited (Tang et al. Citation2022). This is because microplastics can have a wide range of shapes, sizes, and compositions, making them difficult to study and understand. Some research has focused on characterizing the physical properties such as their size, shape, composition, and thermal properties; also, chemical properties of microplastics such as their toxicity and the potential for them to absorb pollutants. However, it is important to note that microplastics are diverse and the results obtained from one sample may not be the same for another sample. To date, there are only limited studies reporting the thermal valorization of microplastics. For example, Ni et al. (Citation2020) have investigated the effect of pyrolysis temperature on the microplastic sample degradation. They found that the pyrolysis temperature should reach 450°C and above to ensure 99.7% of microplastic in the sludge to be fully decomposed. In 2020, Picó and Barceló (Citation2020) have examined the organic matter in microplastics using pyrolysis gas chromatography-mass spectrometry (Py-GC-MS). A series of organic compounds such as p-Methoxy-tert-butylbenzene, Phenol, Benzene, and p-Vinylphenol were discovered with the precise indicator ions (m/z), which is crucial information for fundamental-semi quantification analysis.
In this context, gaining further insights into the fate and risks posed by microplastics, particularly in the marine ecosystem, is of paramount information to mitigate microplastic pollution. The primary objective of this study is to provide a systematic summary of the classification, physical properties, morphology, and degradation profile of microplastics collected from the marine environment. Firstly, the key characteristics of microplastic samples (i.e. carbon content, hydrogen content, oxygen content, nitrogen content, moisture content, particle size, and maximum degradation temperature of the microplastics) were examined. Secondly, the potential of transforming microplastic into syngas was studied. Then, the pyro-solid-char microplastic ash was analyzed. Lastly, the future directions and challenges obtained from the work were analyzed through a Strengths, Weaknesses, Opportunities, and Threats (SWOT) study.
Materials and methods
Data collection
The samples are collected form the Port Klang location 2.999° N, 101.523° E in the year of 2022 (shown in supplementary Figure S1), where Port Klang is the main and largest gateway port in Malaysia which is situated in close proximity to many industries. The sampling was conducted at three-month intervals within the same year (total of four samples). The main reason of selecting Port Klang as the data sampling location is because high amount of municipal industrial waste (e.g. product packaging, bottles, and appliances) have been found in both water and sediments near to the port, suggesting that Port Klang is the major source of microplastic.
Sampling and analysis technique
Sample preparation
Standard microplastic preparation protocols proposed by Al-Azzawi et al. have been employed in this study (Al-Azzawi et al. Citation2020). To prevent contamination, the samples were sterilized and washed with 10 wt.% NaOH/Ethanol to remove impurities before placed into an aluminum tray. Then, the samples were dried in an oven at 60°C for 12 h until a constant dry weight (wt.%) was obtained. Afterward, a 100 g of each sample was stored in a closed-cap bottle to prevent in contact with moisture (see supplementary Figure S2) (Unsworth et al. Citation2021). On the other hand, the samples were identified, classified, and separated into either fibers or non-fiber using high-resolution microscope (Leica DM4000 B Fluorescence Motorized Microscope). Then, we sorted the plastic types based on the standardized classification from the Marine & Environmental Research Institutes’: Guide to microplastic identification (Boshoff, Robinson, and von der Heyden Citation2023). The remaining non-fibers residues such as pellets or beads, fragments or any unknown were filtered and discarded accordingly.
Sample characterization
Moisture content was quantified by introducing approximately 1 g of microplastic samples into the moisture content analyzer (Halogen Moisture Analyzer, HC103). The analysis was repeated thrice to ensure a high accuracy. The ultimate analysis of the samples was determined using LECO CHNS-932 elemental analyzer to estimate the elemental compositions such as C, H, N, S, and O. Approximately 1 mg of sample is grounded and homogenized into fine powder before being subjected into the analyzer. Meanwhile, the surface morphology of the bulk microplastic samples (before and after pyrolysis) was analyzed using scanning electron microscopy (SEM) with a BSD detector at an accelerating voltage of 5 kV. The SEM analysis was performed using the Phenom XL Desktop SEM system from Thermo Scientific, USA. A high-resolution image of the microplastic was taken using Field emission gun scanning electron microscopy (Model: FEI Nova NanoSEM 450 FEGSEM, 30 kV) coupled with energy-dispersive X-ray spectroscopy.
Thermal degradation- gas product distribution analysis
The thermal degradation analysis was carried out using TGA 8000™ Thermogravimetric analyzer. Approximately 0.5 mg of microplastic samples was placed on a ceramic crucible in the analyzer under an inert atmosphere of argon. A flowrate of 100 mL min−1 of argon was fed into the system for 20 min at the temperature of 100°C to remove the entrapment of other gases (avoid unwanted oxidation of the sample in the pyrolysis zone). Subsequently, all samples were heated from 100°C to 800°C at the heating rate of 10°C min−1. The gas products released from each sample were collected using a gas bag (at after phase II temperature, Tmax ±10°C) and analyzed using a micro-gas chromatography (Agilent 490 Micro GC).
Results and discussion
Physicochemical properties
indicates that the average moisture and volatile matters of microplastic waste were 0.25 wt.% and 86.5 wt.%, respectively. The results of this analysis revealed that the microplastic has a very low moisture content but with high volatile matter, indicating that they were highly suitable feedstock for energy production. Additionally, the ultimate analysis of the microplastic showed that the average amount of carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and sulfur (S) content are 85.25%, 6.99%, 7.04%, 0.36%, and 0.36%, respectively. The low S and N content of microplastic implies that the emission of nitrogen oxides (NOx) and sulfur dioxide (SO2) during the pyrolysis process was minimal (Loy et al. Citation2018). The high carbon content in microplastic can also lead to an increase in the yield of gaseous products (such as CO and CO2) due to weak Van de Waals C–C bond in between the molecules, microplastic a promising feedstock for pyro-gas production (less solid carbon char) (Gan et al. Citation2018; Yap et al. Citation2022). It is also important to note that these findings are only preliminary and further characterization is necessary to confirm their validity and generalizability to other feedstocks and conditions.
Table 1. Physiochemical properties of marine microplastic wastes.
Thermal degradation of microplastics
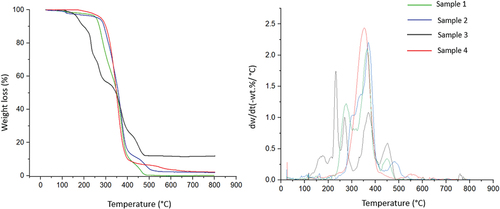
The degradation profiles of samples, including information on mass transfer and kinetic parameters under non-isothermal conditions were obtained using TGA; this data is crucial for understanding the fundamental thermal degradation or reaction engineering mechanisms of a material, facilitating the design or fabrication of equipment or reactors (Gan et al. Citation2018). shows the degradation profile of the four samples (a) Thermogravimetric profile (TG) and (b) derivative thermogravimetric (DTG) curves, respectively. Basically, three stages of degradation were observed from the degradation profile, namely: Stage I (water evaporation and dissipation of light volatile compounds stage) occurred at temperatures ranging from room temperature (~25°C) to approximately 120°C; Stage II (main decomposition stage to yield volatile matter) occurred at a temperature of 120–400°C, and Stage III (high-temperature decomposition stage) occurred at above 400°C. The temperature range specifically in Stage II is also known as the main decomposition temperature since most of the volatile matters will be transform into gaseous products at this temperature range, Tmax (Minh Loy et al. Citation2018)
Whereas the peak intensity shown in the DTG curve represents the reactivity of the samples during the pyrolysis reaction; a low peak intensity indicates that a complete thermal degradation process can be achieved faster at a lower activation energy in the system. Thus, the difference in peak intensity observed in this study can be deducted due to the different decomposition mechanisms of organic and inorganic constituents, the length and distribution of chains within the constituents, and the different compositions of microplastic (Yu et al. Citation2019). From the degradation profile analysis, it shows that sample three was having the highest maximum degradation temperature among all samples, further confirming that polylactic acid (PLA) is exhibiting a higher ultimate strength as compared to other plastic compositions (see supplementary Figure S3 and S4). Also, we can conclude that a minimum temperature of 400°C was required for degrading the microplastic waste (calculated based on the average results of the microplastic samples).
Gaseous products distribution from microplastic valorization
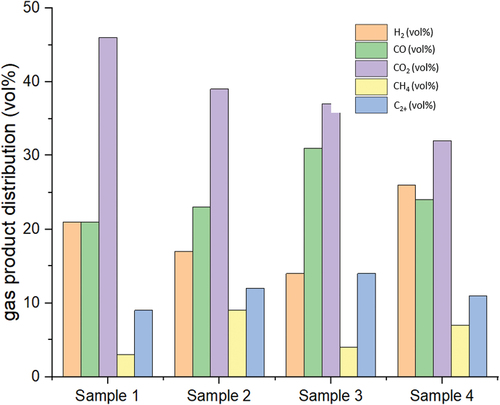
presents the gaseous product analysis obtained from each sample collected during the second phase of valorization. This phase is crucial as it represents the period where volatile components undergo decomposition and degradation, resulting in the formation of gaseous products. The composition of syngas, which consists of hydrogen (H2) and carbon monoxide (CO), follows a specific trend among the samples. Sample 4 exhibits the highest syngas composition, followed by Sample 3, Sample 1, and Sample 2. It is noteworthy to mention that samples containing polyethylene (PE) and polyurethane (PU) show a higher selectivity toward syngas production, which is consistent with previous studies (Hasanzadeh et al. Citation2022; Mojaver, Azdast, and Hasanzadeh Citation2021). Also, the small fraction of C2+ products were corresponded to the light aliphatic hydrocarbons due to the breaking of C–C bond (Supriyanto, Richards, and Richards Citation2021). Furthermore, the average syngas production and hydrogen-to-carbon (H/C) ratio of the microplastic samples were determined to be 34.79% and 0.25, respectively (see ). These values indicate the potential of microplastic waste as a suitable candidate for blending with other feedstocks, particularly lignocellulosic biomass. The poor properties of lignocellulosic biomass, such as a high oxygen-to-carbon (O/C) ratio and low H/C ratio, could be compensated by the high (H/C) characteristics of microplastic waste through blending, resulting in improved product quality during co-pyrolysis (Gale, Nguyen, and Gilliard-AbdulAziz Citation2023).
Table 2. Yield, selectivity, and conversion of the microplastic to different products.
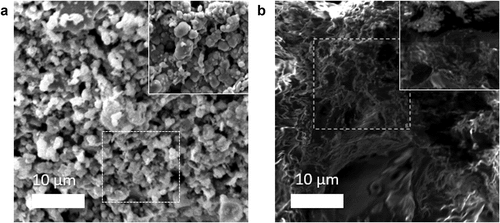
In addition to the gaseous product analysis, scanning electron microscopy (SEM) micrographs were obtained for both pre- and post-degradation samples of microplastic (specifically, Sample 3 was chosen due to its highest syngas composition). These micrographs were taken to observe any structural changes that occurred during the degradation process. The SEM images revealed a notable transformation in the sample’s structure. Prior to pyrolysis, the microplastic sample exhibits a “bubble-like” morphology. However, after the degradation process, the structure of the sample transforms into a “porous sponge-like” char. This change in structure suggests that the surface area of the microplastic samples has been significantly altered (see ). The post-degradation microplastic sample has more holes in it because of some elements (Fe, Al, and Si), which means it could be used in other areas (see additional Figure S5). For instance, it may serve as a valuable soil additive or be utilized as a support material for catalysts (Wijesekara et al. Citation2021).
Conclusion
The presence of microplastics in the environment has become a growing concern as emerging particulate pollutants. These tiny plastic particles can be found globally on both land and sea, raising concerns about their impact on both the environment and human health. To address these concerns, reliable information on the levels of microplastics, both physical and chemical, in the marine environment is highly crucial. In this study, we have presented a quantitative analysis of the physical properties of microplastics found in the regional marine environment of Malaysia, thereby establishing a baseline for future investigation. Moreover, the microplastics are valorized into pyro-gas to investigate the potential of aping for the “hazardous-to-wealth” concept. Notably, a considerable good yield of hydrogen and syngas was achieved (15.78%; 34.79% average value) in a pyrolyzed condition without the addition of a catalyst, indicating that the valorization of microplastics through a thermal process is highly feasible. However, taking note that the research also showed that the levels of microplastic pollution may differ in specific locations, the way forward for improving the study has been derived based on the SWOT analysis, as shown:
The introduction of synchrotron infrared or coupling TGA-FTIR-MS spectroscopy can be the way forward, allowing precise understanding of the characteristics of microplastic particles in a spatial resolution mode. For example, the TGA-FTIR-MS spectroscopy could identify the degradation profile of mix polymer types in bulk microplastics in real-time mode, as well as provide information on the possible liquid and gas products. As mentioned in the above section, the non-homogeneous, small, and diverse microplastics are very challenging to analyze. Additionally, there is a lack of standardized protocols for sampling, analyzing, and reporting microplastic data, which can lead to inconsistent data while comparing results across studies. For example, the choice of sample preparation (i.e., 0.1–10 μm) and characterization methodology can lead to significant differences in showcasing the microplastic characteristic.
The findings and insights obtained from this study can provide a benchmark for comparing and evaluating alternative pathways for plastic degradation, such as anaerobic digestion and enzymatic degradation. Moreover, the obtained microplastic degradation profile can be utilized in kinetic studies, which are crucial for designing efficient fixed-bed reactors. This work can act as an initial standpoint to drive further advancements in the field, such as co-pyrolysis, tri-combustion, and co-gasification. Last but not least, the degree to which particulate matter and heavy metals bind to the microplastic can act as threats in affecting the valorization temperature of the microplastic for volatile matters, making the whole degradation process more complex to understand.
Highlights
Physiochemical properties of microplastics have been studied.
Conversion of microplastic to syngas is feasible through thermochemical methods.
SWOT analysis has been carried out to elucidate the way forward of microplastic valorization.
More samplings are required to further support the results obtained in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s). However, we would like to thank our Malaysian collaborators to provide the data samplings. More information will be given upon request.
Additional information
Notes on contributors
Mohammed B. Al Rayaan
Mohammed B. Al Rayaan is a professional environmental engineer and hydrogeologist with more than a decade of experience in the field. Currently serving as a lead hydrogeologist at Saudi Aramco, Mohammed has been instrumental in spearheading numerous environmental projects. His expertise lies primarily in groundwater investigations, data collection and analysis, environmental evaluation, and project management. Throughout his tenure at Saudi Aramco, he has worked on significant projects, including leading the Aramco environmental team on several acquisition projects in different countries (environmental-related) and conducting comprehensive environmental assessments He also led projects including waste management, environmental impact assessment for several categories, and projects including marine protection, air quality, health hazards, etc.
Ali M. Qasem
Ali M. Qasem is an Environmental Consultant in the Marine Environment Protection Unit of Saudi Aramco Environmental Protection Department. He Joined Saudi Aramco in Dec. 1995 as Marine Scientist. He is also the Environmental Advisor of the Oil Spill Response Team in the company. He is also the leading expert in Saudi Aramco Mangrove Plantation program, Artificial Reef Program, Coastal and Offshore Monitoring and other Environmental Programs. In addition, He is also Saudi Aramco Environmental Protection Department SME in the offshore project environmental assessment, and the environmental focal point for all governmental discussion in these projects.
References
- Al-Azzawi, M. S. M., S. Kefer, J. Weißer, J. Reichel, C. Schwaller, K. Glas, O. Knoop, and J. E. Drewes. 2020. Validation of sample preparation methods for microplastic analysis in wastewater matrices—reproducibility and standardization. Water 12(9):2445. 10.3390/w12092445.
- Boshoff, B. J., T. B. Robinson, and S. von der Heyden. 2023. The role of seagrass meadows in the accumulation of microplastics: Insights from a South African estuary. Marine Pollution Bulletin 186: 114403. January 1. doi:10.1016/j.marpolbul.2022.114403.
- Gale, M., P. M. Nguyen, and K. L. Gilliard-AbdulAziz. 2023. Synergistic and antagonistic effects of the co-pyrolysis of plastics and corn stover to produce char and activated carbon. American Chemical Society Omega 8 (1):380–90. January 10. doi:10.1021/acsomega.2c04815.
- Gan, D. K. W., A. C. M. Loy, B. L. F. Chin, S. Yusup, P. Unrean, E. Rianawati, and M. N. Acda. 2018. Kinetics and thermodynamic analysis in one-pot pyrolysis of rice hull using renewable calcium oxide based catalysts. Bioresource Technology 265: 180–90. October 1. doi:10.1016/j.biortech.2018.06.003.
- Gontard, N., G. David, A. Guilbert, and J. Sohn. 2022. Recognizing the long-term impacts of plastic particles for preventing distortion in decision-making. Nature Sustainability 5 (6):472–78. June 1. doi:10.1038/s41893-022-00863-2.
- Hasanzadeh, R., P. Mojaver, S. Khalilarya, T. Azdast, A. Chitsaz, and M. Mojaver. 2022. Polyurethane foam waste upcycling into an efficient and low pollutant gasification syngas, (in eng). Polymers (Basel) 14 (22):4938. November 15. doi:10.3390/polym14224938.
- Hu, Q., Z. Tang, D. Yao, H. Yang, J. Shao, and H. Chen. 2020. Thermal behavior, kinetics and gas evolution characteristics for the co-pyrolysis of real-world plastic and tyre wastes. Journal of Cleaner Production 260: 121102. July 1. doi:10.1016/j.jclepro.2020.121102.
- Inayat, A., L. Rocha-Meneses, C. Ghenai, M. Abdallah, A. Shanableh, K. Al-Ali, A. Alghfeli, and R. Alsuwaidi. 2022. Co-pyrolysis for bio-oil production via fixed bed reactor using date seeds and plastic waste as biomass. Case Studies in Thermal Engineering 31: 101841. March 1. doi:10.1016/j.csite.2022.101841.
- Koelmans, A. A., P. E. Redondo-Hasselerharm, N. H. M. Nor, V. N. de Ruijter, S. M. Mintenig, and M. Kooi. 2022. Risk assessment of microplastic particles. Nature Reviews Materials 7 (2):138–52. February 1. doi:10.1038/s41578-021-00411-y.
- Lim, X. 2021. Microplastics are everywhere - but are they harmful?, (in eng). Nature 593 (7857):22–25. May. 10.1038/d41586-021-01143-3
- Loy, A. C. M., S. Yusup, M. K. Lam, B. L. F. Chin, M. Shahbaz, A. Yamamoto, and M. N. Acda. 2018. The effect of industrial waste coal bottom ash as catalyst in catalytic pyrolysis of rice husk for syngas production. Energy Conversion and Management 165: 541–54. June 1. doi:10.1016/j.enconman.2018.03.063.
- Miandad, R., M. Rehan, M. A. Barakat, A. S. Aburiazaiza, H. Khan, I. M. I. Ismail, J. Dhavamani, J. Gardy, A. Hassanpour, A.-S. Nizami, et al. 2019. Catalytic pyrolysis of plastic waste: Moving toward pyrolysis based biorefineries, (in English). Frontiers in Energy Research 7. March 19. doi:10.3389/fenrg.2019.00027.
- Minh Loy, A. C., S. Yusup, B. L. Fui Chin, D. K. Wai Gan, M. Shahbaz, M. N. Acda, P. Unrean, and E. Rianawati. 2018. Comparative study of in-situ catalytic pyrolysis of rice husk for syngas production: Kinetics modelling and product gas analysis. Journal of Cleaner Production 197: 1231–43. October 1. doi:10.1016/j.jclepro.2018.06.245.
- Mojaver, M., T. Azdast, and R. Hasanzadeh. 2021. Assessments of key features and Taguchi analysis on hydrogen rich syngas production via gasification of polyethylene, polypropylene, polycarbonate and polyethylene terephthalate wastes. International Journal of Hydrogen Energy 46 (58):29846–57. August 23. doi:10.1016/j.ijhydene.2021.06.161.
- Mumbach, G. D., J. L. F. Alves, J. C. G. Da Silva, R. F. De Sena, C. Marangoni, R. A. F. Machado, and A. Bolzan. 2019. Thermal investigation of plastic solid waste pyrolysis via the deconvolution technique using the asymmetric double sigmoidal function: Determination of the kinetic triplet, thermodynamic parameters, thermal lifetime and pyrolytic oil composition for clean energy recovery. Energy Conversion and Management 200:112031. November 15. doi:10.1016/j.enconman.2019.112031.
- Ni, B.-J., Z.-R. Zhu, W.-H. Li, X. Yan, W. Wei, Q. Xu, Z. Xia, X. Dai, and J. Sun. 2020. Microplastics mitigation in sewage sludge through pyrolysis: The role of pyrolysis temperature. Environmental Science & Technology Letters 7 (12):961–67. December 8. doi:10.1021/acs.estlett.0c00740.
- Nunes, B. Z., Y. Huang, V. V. Ribeiro, S. Wu, H. Holbech, L. B. Moreira, E. G. Xu, and I. B. Castro. 2023. Microplastic contamination in seawater across global marine protected areas boundaries. Environmental Pollution 316: 120692. January 1. doi:10.1016/j.envpol.2022.120692.
- Picó, Y., and D. Barceló. 2020. Pyrolysis gas chromatography-mass spectrometry in environmental analysis: Focus on organic matter and microplastics. TrAC Trends in Analytical Chemistry 130: 115964. September 1. doi:10.1016/j.trac.2020.115964.
- Supriyanto, P. Y., T. Richards, and T. Richards. 2021. Gaseous products from primary reactions of fast plastic pyrolysis. Journal of Analytical and Applied Pyrolysis 158: 105248. August 1. doi:10.1016/j.jaap.2021.105248.
- Tang, K. H. D., S. S. M. Lock, P.-S. Yap, K. W. Cheah, Y. H. Chan, C. L. Yiin, A. Z. E. Ku, A. C. M. Loy, B. L. F. Chin, and Y. H. Chai. 2022. Immobilized enzyme/microorganism complexes for degradation of microplastics: A review of recent advances, feasibility and future prospects. Science of the Total Environment 832: 154868. August 1. doi:10.1016/j.scitotenv.2022.154868.
- Tian, Y., W. Zuo, Z. Ren, and D. Chen. 2011. Estimation of a novel method to produce bio-oil from sewage sludge by microwave pyrolysis with the consideration of efficiency and safety. Bioresource Technology 102 (2):2053–61. January 1. doi:10.1016/j.biortech.2010.09.082.
- Unsworth, R. K. F., A. Higgs, B. Walter, L. C. Cullen-Unsworth, I. Inman, and B. L. Jones. 2021. Canopy accumulation: Are seagrass meadows a sink of microplastics? Oceans 2 (1):162–78. [Online]. https://www.mdpi.com/2673-1924/2/1/10.
- Wijesekara, D. A., P. Sargent, C. J. Ennis, and D. Hughes. 2021. Prospects of using chars derived from mixed post waste plastic pyrolysis in civil engineering applications. Journal of Cleaner Production 317: 128212. October 1. doi:10.1016/j.jclepro.2021.128212.
- Yap, T. L., A. C. M. Loy, B. L. F. Chin, J. Y. Lim, H. Alhamzi, Y. H. Chai, C. L. Yiin, K. W. Cheah, M. X. J. Wee, M. K. Lam, et al. 2022. Synergistic effects of catalytic co-pyrolysis chlorella vulgaris and polyethylene mixtures using artificial neuron network: Thermodynamic and empirical kinetic analyses. Journal of Environmental Chemical Engineering 10 (3): 107391.1 June. doi:10.1016/j.jece.2022.107391.
- Yu, J., P. Wang, F. Ni, J. Cizdziel, D. Wu, Q. Zhao, and Y. Zhou. 2019. Characterization of microplastics in environment by thermal gravimetric analysis coupled with Fourier transform infrared spectroscopy. Marine Pollution Bulletin 145: 153–60. August 1. doi:10.1016/j.marpolbul.2019.05.037.