Abstract
The demand for protein has increased over last few years. Plant-based proteins are appreciated to meet the increasing demand of proteins than animal protein with the growing population worldwide. Pulses are one of the good sources of protein. Chickpea is one of the oldest and most cultivated legume crops consumed as source of protein. Chickpeas can be classified into two types named as ‘kabuli’ and ‘desi’ on the basis of their physical parameters. They are recognized as a valuable dietary protein source due to their sufficient biological value, and protein bioavailability. Chickpeas contain a variety of bioactive compounds, including isoflavones and phenolic acid. Generally, chickpea and pulses serve as viable options for functional and nutritional proteins. Chickpea proteins are considered as the best alternative to animal proteins with high amino acid profile and cost effectiveness. They are significant in academic and food business domains. Chickpea protein isolates are commonly identified as having a minimum protein content of 90% on a dry weight basis. They offer excellent functional and therapeutic potential in mitigating various health ailments. Chickpea protein is also used in production of value-added products in various food industries including baking industries, meat industry, infant formula and nutraceutical industries. This review explores the production, nutritional composition, health perspectives and industrial applications of chickpea protein.
Introduction
Cicer arietinum L commonly known as Chickpea, is a leguminous crop that is cultivated annually. It is derived from herbaceous plants that produce pods and is typically grown in regions characterized by a temperate climate (Wallace et al., Citation2016). Multiple varieties of chickpeas belong to the Cicer genus and considered to be originated from Cicer reticulatum (wild species) (Sharma et al., Citation2013). The Middle East also referred to as the Fertile Crescent in ancient times, is recognized as the birthplace of chickpeas. The distribution of this particular leguminous plant corresponds with the historical patterns of commerce and migration originating from this geographical area and extending globally (Redden & Berger, Citation2007). Chickpeas can be classified into two primary classes, namely ‘kabuli’ and ‘desi’ on the basis of their physical parameters such as seed color, shape, and size. The ‘desi’ chickpeas, which are derived from the Urdu and Hindi term ‘native’, are characterized by dark brown, tiny, as well as furrowed seeds. These seeds are predominantly cultivated in semiarid regions, including the Indian subcontinent, Central America, Australia, and East Africa (Knights & Hobson, Citation2016). Winter crops are cultivated and necessitate approximately 16 inches of annual precipitation. These crops are typically grown after the cultivation of monsoon season crops (Knights & Hobson, Citation2016; Nasir & Sidhu, Citation2012). It is believed that the differentiation of the ‘kabuli’ variety took place later in the Mediterranean region compared to the ‘desi’ chickpea. Kabuli chickpeas are distinguished by their considerable seed dimensions, new exterior, and pale hue and are cultivated in regions with milder weather conditions (Mohammed Mubarak, Citation2009). During the sixteenth century, Portuguese and Spanish travelers introduced the ‘kabuli’ variety to Central and South America and became recognized by their Spanish name, ‘garbanzo’. Chickpeas of this variety are predominantly cultivated in the Mediterranean, North America, North Africa, and the Middle East (Knights & Hobson, Citation2016). Pulses are widely recognized for their capacity to enhance soil fertility through nitrogen fixation, thereby promoting agricultural systems that are both productive and sustainable. Legumes are commonly integrated into crop rotations as a sustainable method to decrease the need for nitrogen fertilizers and enhance the yields of subsequent crops. Research has demonstrated that chickpeas are especially efficacious in augmenting the yields of cereals (Korbu et al., Citation2020). Globally, there is a comparable trade volume for the ‘desi’ and ‘kabuli’ varieties of chickpeas. Nevertheless, there is a general inclination toward the ‘kabuli’ type, especially those with larger seeds, which are favored for their suitability for direct human consumption (Yadav et al., Citation2007). Based on the FAOSTAT statistics, the production of chickpea significantly increased between 1980 and 2018, resulting in a global production volume of approximately 17 million tons in 2018 (Food and Agriculture Organization of the United Nations \(FAO\), Citation2020). While, India was responsible for highest proportion of chickpea production, producing more than 11 million tons in 2018 (Food and Agriculture Organization of the United Nations \(FAO\), 2020). Chickpea nutritionally rich food source, characterized by elevated protein, fiber, and fat levels but a lower carbohydrate content relative to wheat. Additionally, chickpeas contain a variety of bioactive compounds, including isoflavones and phenolic acid (Rachwa-Rosiak et al., Citation2015). Chickpea is recognized as a valuable dietary protein source due to their sufficient biological value, and protein bioavailability. Chickpea comprises fewer sulfur-containing amino acids such as cystine and methionine (Jukanti et al., Citation2012). Additionally, the functional properties of chickpea proteins exhibit favorable characteristics, including foaming, solubility, gelling, and emulsifying properties for developing novel food products. The protein profile significantly influences these properties, processing parameters like temperature and pH, and the selection of extraction methodology (Boye et al., Citation2010; Day, Citation2013). Chickpea protein can be used to develop protein-enriched ingredients such as concentrates, isolates, and flour. These ingredients are commonly used to make protein snacks, infant formulas, merguez sausage, and noodles. The goal of the current article is to emphasize the composition, health perspectives as well as potential uses of chickpea protein within the food industry. Additionally, it will explore various approaches to improve the quality of protein and analyze the feasible applications of byproducts acquired throughout the protein extraction and processing stages. The information presented in this review may be helpful for researchers and industries interested in incorporating chickpea protein ingredients into developing and producing innovative food products.
Nutritional composition
The nutritional composition of chickpeas is a topic of interest in the field of food science. In addition to protein, chickpeas possess fat, carbohydrates, bioactive compounds, and minerals, all of which impact the efficacy of recuperation and crucial quality characteristics of chickpea protein constituents. The dietary composition of chickpea seeds and other pulses is subject to variability based on a range of factors, including environmental conditions, climate, soil nutrient content, and agronomic techniques (Shevkani et al., Citation2019). Differences in composition, particularly in protein content, are discernible between the ‘Desi’ and ‘Kabuli’ varieties of chickpeas.
Carbohydrates
Chickpeas contain starch as their primary carbohydrate component, comprising 47.4–66.9% of the total fraction. The remaining carbohydrate content is made up of soluble sugars, crude fiber, as well as dietary fiber (Singh, Citation1985). Starch accounts for 41.0–50.8% of the total carbohydrate content in chickpeas. Chickpeas possess a granular starch structure categorized as type C, characterized by a crystalline structure commonly observed in legumes. The amylose content of ‘Kabuli’ chickpeas is marginally higher than that ‘Desi’ chickpeas (Singh et al., Citation2004). However, both types of chickpeas contain a more significant amount of amylopectin than amylose. In contrast, cereals typically exhibit lower amylose and higher amylopectin content (Singh et al., Citation2004). The low glycemic index of chickpea starch can be attributed to its low amylose content and subsequent high rate of retrogradation (Kaur & Prasad, Citation2021). The residual starch content significantly impacts chickpea protein ingredients’ functional properties, which is partially eliminated during the protein component extraction process (Kaur & Prasad, Citation2021).
Protein
Chickpea seeds contain significant protein, usually ranging from 20 to 25%. Prolamin, albumin, glutelin, and globulin as the primary proteins in chickpeas, accounting for approximately 3–7%, 8–12%, 19–25%, and 53–60% of the total protein, respectively (Day, Citation2013). Differences in protein content between ‘desi’ and ‘kabuli’ seed types may be attributed to variations in a growth environment, varietal characteristics, agronomic practices, genotypic diversity, or storage conditions (Özer et al., Citation2010). A study has reported variations in amino acid patterns between ‘desi’ and ‘kabuli’ chickpeas. The amino acid profile of ‘desi’ and ‘kabuli’ chickpeas are presented in . Specifically, the methionine content was found to be 1.4 and 1.1 g/100 g of protein for ‘desi’ and ‘kabuli’ flours, respectively. Moreover, significant differences were observed in leucine, serine, and lysine content between the two seeds, with values of 4.2 and 2.5, 5.4 and 7.3, and 7.2 and 7.6 g/100 g of protein, respectively (Ghribi et al., Citation2015). The difference in the compositions of amino acids is influenced by genetic diversity, as well as environmental factors like soil quality, climate, and farming techniques. Numerous studies suggest that these factors can impact the synthesis and accumulation of amino acids in chickpeas, ultimately affecting the protein content (Frimpong et al., Citation2009).
Table 1. Amino acid profile of chickpea protein in Kabuli and Desi cultivars.
Fat
Chickpeas have a higher fat level than certain cereals and pulses but a lower fat content than oilseed legumes such as soybeans and peanuts (Grasso et al., Citation2022). The fat content of chickpeas varies between 3.10 and 5.67%, contingent upon the type of chickpea. Chickpea fat is comprised of approximately 15% saturated fatty acids, 19% monounsaturated fatty acids, and 66% polyunsaturated fatty acids. Linoleic and oleic acids are the predominant fatty acids, with palmitic acid present in lesser amounts. These fatty acids account for 61.6% and 51.2%, 22.3%, and 32.6%, and 9.1% and 9.4% of total fat, respectively, in ‘desi’ and ‘kabuli’ chickpeas, as reported by Jukanti et al. (2012) and Singh (Singh, Citation1985). In order to optimize the quality and quantity of chickpea protein ingredients, it is common practice to eliminate lipids through solvent extraction (Wang et al., Citation2022).
Dietary fiber
Chickpeas contain a significant amount of dietary fiber that cannot digested in the human small intestine. Specifically, chickpeas contain between 18–22 grams of dietary fiber per 100 grams, with 10–18 grams of insoluble fiber and 4–8 grams of soluble fiber (Tosh & Yada, Citation2010). Dietary fiber serves several crucial functions in maintaining gut health. Specifically, it promotes the growth of bacteria in the large intestine, thereby exhibiting prebiotic effects. Additionally, it diminishes colon transit time and, as a result, minimizes the duration of contact between toxic compounds and colon mucosa (Pathania & Kaur, Citation2022). The dietary fiber content of dehulled chickpeas is comparatively lower than that of other pulses, owing to the removal of the chickpea seed coat during the dehulling process. ‘Desi’ chickpeas exhibit elevated levels of insoluble dietary fiber compared to ‘kabuli’ chickpeas, owing to the thickness and fiber composition of the seed coat (Summo et al., Citation2019). The functional properties of chickpea protein ingredients are influenced by the residual fiber, much like the starch fraction (Xu et al., Citation2014).
Minerals and vitamins
Chickpeas contain essential minerals and vitamins in addition to other significant compounds. Compared to alternative legumes like common bean, black beans, kidney beans, and field pea, Chickpeas exhibit an excellent mineral composition, including zinc and phosphorous. Ethiopian Habru variety contains 375.24 mg/100 g phosphorus and 3.69 mg/100 g zinc. Furthermore, they contain water-soluble vitamins (B-complex and vitamins C) and fat-soluble vitamins (vitamins A, E, and K) (Yegrem, Citation2021). In addition, chickpeas are found to possess numerous phenolic compounds, such as isoflavones and formononetin, alongside carotenoids that are more abundant in black and brown chickpea cultivars (Hall et al., Citation2017; Serrano et al., Citation2017; Summo et al., Citation2019). Like other legumes, chickpeas also possess anti-nutritional constituents that can interfere with the digestive process. Notable examples of these constituents include trypsin, chymotrypsin inhibitors, and phytic acid, which can impede the body’s absorption of essential minerals such as calcium, zinc, and iron. The implementation of technological methods, like thermal processing and extrusion, has demonstrated a reduction in anti-nutritional compounds and modification of phenolic compound content in chickpea seeds or flour. Specifically, the application of pressure during the cooking of chickpeas has been shown to decrease by 20% of phytic acid (Xu & Chang, Citation2009).
Bioactive components
Chickpeas are a great source of flavonoids and polyphenols with excellent antioxidant properties. The darker the hue of the chickpeas, the higher the concentration of these substances, though the exact quantity varies depending on the specific color. The phenolic components vary based on the extraction process, reagents used, extraction duration, and analytical techniques. The beans contain 14.9 mg/kg of anthocyanin, while the polyphenolic component can vary between 0.72 and 1.81 mg/g. Although chickpeas are not rich in anthocyanins or polyphenols, they contain a significant amount of phenolic acids, including hydroxycinnamic, anise, caffeic, cinnamic, p-coumaric, chlorogenic, isoferulic, and piperonyl acids. The presence of these phenolic acids in the beans is responsible for their high antioxidant activity, which includes reducing oxidative stress and securely binding metal ions (Begum et al., Citation2023). Zhao et al. (Citation2021) analyzed six chickpea cultivars to determine their total phenolic, anthocyanin levels and flavonoid content. The study revealed that coats with darker pigmentation had higher levels of total phenolics, anthocyanin, and flavonoid content than coats with lower pigmentation. The concentration of flavonoids ranged from 0.021 to 0.1 mg Rutin/g across six distinct species. The carotenoid concentrations of black and brown chickpeas were 36.4 mg/kg and 35.2 mg/kg, respectively, compared to beige chickpeas. Researchers discovered that the desi chickpea has a more vibrant seed coat color, indicating a higher concentration of carotenoids than the Kabuli chickpea (Ashokkumar et al., Citation2015).
Constituents of chickpea protein
The investigation and utilization of chickpea protein constituents are significant in academic and business domains. Chickpeas and other pulses such as peas, lentils, and cowpeas are significant dietary constituents in regions where animal protein is prohibitively expensive or where they have a rich cultural heritage of consumption. In numerous regions across the globe, customary culinary preparations consist of a combination of chickpeas and various cereal such as wheat, rice, or others (Knights & Hobson, Citation2016). Incorporating chickpea proteins, as well as other pulse proteins, into formulated products can assist in fulfilling the daily recommended protein intake (Day, Citation2013). In addition, individuals who experience allergic reactions to milk, gluten, eggs, fish, or sesame, and do not exhibit cross-reactivity to soybean and peanut, chickpea, and pulses in general, serve as viable options for functional and nutritional proteins (Maryniak et al., Citation2022). Although pulses are not categorized as principal allergens, there have been reports of allergies to chickpeas in certain regions with significant consumption of chickpea-derived products (Wangorsch et al., Citation2020). The allergic reaction to chickpea protein is commonly linked to cross-reactivity with other legumes such as soybean or lentil and peanuts (Cox et al., Citation2021). In addition, pulses have been reported to contain only trace amounts of heavy metals, specifically arsenic, and chromium, which are in lower concentrations than in other commonly consumed staple foods like rice (Bessada et al., Citation2019).
Globulins
Globulins are a type of protein that can dissolve in salt (Okagu & Udenigwe, Citation2022). The primary globulins in legumes are the storage proteins known as Legumin (11S) and vicilin (7S), categorized based on their sedimentation coefficients (Boye et al., Citation2010; Day, Citation2013). Chickpeas contain a significant amount of Legumin, a hexameric protein, and the primary globulin found in them. The subunits of Legumin are connected through disulfide bonds, whereby the acidic chains are positioned on the molecule’s surface and the basic hydrophobic units are located internally, thereby restricting their interaction with water (Sim et al., Citation2021). Legumins are known to possess elevated levels of Sulfur-containing amino acids such as methionine and cysteine in comparison to vicilin. Conversely, vicilin lack cysteine residues, which precludes their ability to form disulfide bonds. Vicilin is a trimeric protein consisting of monomers that are interconnected via non-covalent hydrophobic bonds (Shevkani et al., Citation2019). Globulins exhibit a lower foaming capacity than albumins due to their structural characteristics, resulting in a diminished capacity to unfold and trap air. Conversely, the configuration of globulins holds significant ramifications for additional operative characteristics, such as emulsification potential and water absorption capacity (WAC) (Ghumman et al., Citation2016). In addition, it has been observed that globular proteins can undergo gelation due to physical interactions, specifically hydrophobic interactions and hydrogen bonding, which are induced by exposure to temperatures above a certain threshold that leads to protein unfolding (Papalamprou et al., Citation2009). The gelling properties and thermal behaviors of soy globulins, specifically 7S β-conglycinin and 11S glycinin, have been the subject of extensive research. These properties have been found to be similar to those of chickpea globulins in their molecular characteristics (Chang et al., Citation2012; Chen et al., Citation2016). Soy globulin is frequently regarded as a suitable benchmark gelling protein in various semi-solid food items, primarily owing to its extensively examined gelling characteristics (Bessada et al., Citation2019).
Prolamins
Prolamins are a type of alcohol-soluble protein that is commonly found in cereal proteins. Chickpeas constitute a minor proportion, accounting for approximately 3–7% of the total protein content. The entities mentioned above exhibit a notable abundance of glutamine and proline residues (Rachwa-Rosiak et al., Citation2015). It is believed that prolamins are accountable for the lower emulsifying and foaming characteristics in cereal flours in contrast to legume flours, which are abundant in globulins and albumins (Stone et al., Citation2019). Nevertheless, the characterization of chickpea prolamins still needs to be improved in literature (Chang et al., Citation2012).
Albumin
Chickpeas contain albumin, constituting approximately 8–11% of the total protein content, which is classified as a protein soluble in water. Legumes furnish a substantial quantity of essential amino acids and exhibit a more excellent ratio of amino acids containing sulphur than globulins (Bhatty, Citation1982). Albumins are known to be highly nutritious in chickpeas, they also contain several anti-nutritional elements, including trypsin and amylase inhibitors (Idate et al., Citation2021). Albumins can augment the foaming characteristics of pulses owing to their water solubility. Moreover, their facile interaction with the starch constituent has significant implications for the functional attributes of chickpea protein ingredients (Day, Citation2013; Ghumman et al., Citation2016).
Glutelin
Glutelin exhibit solubility in alkali detergents or diluted acid and in the presence of reducing agents (Okagu & Udenigwe, Citation2022). The legume seed of chickpea is distinguished by its unique glutelin content, which ranges from 19 to 25%. Glutelin is a subject of nutritional significance due to its comparatively elevated levels of methionine and cysteine compared to globulins (Singh & Jambunathan, Citation1982; Tripathi et al., Citation2021). The characterization of chickpea glutelin has been insufficient, similar to that of prolamins (Chang et al., Citation2012). As part of the seed’s protein matrix, these proteins are essential for chickpea seeds to retain their cohesive structure (Patil, Citation2023). In a study conducted by Serrano-Sandoval et al. (Citation2019), the antioxidant activity of globulin, albumin, and glutelin protein fractions isolated from the pepsin-pancreatin hydrolysate of selenium-enriched chickpea sprouts was investigated in CaCO-2 cells. The glutelin fraction showed the most antioxidant activity, which may be because it contains peptides with molecular weights lower than 10 kDa.
Protein isolates derived from chickpea
Protein isolates serve as the functional ingredients of food in various food products as well as increased the nutritional status of several food products described by various studies. Chickpea protein isolates have minimum protein content of 90% on dry weight basis (Singhal et al., Citation2016). The methods used to produce chickpea protein isolates are parallel to those methods used in manufacturing of chickpea protein concentrates. The effect of processing methods on physiochemical attributes of chickpea protein isolates were demonstrated by Papalamprou et al. (2009) Alkaline extraction techniques like ultrafiltration technology and isoelectric precipitation technology were used to prepared the protein isolates. Furthermore, it was noted that an isolate was acquired by ultrafiltration with conjugation of acidic extraction. The results of the electrophoretic analysis indicated that the primary protein fractions found in the chickpea protein isolates were legumin, globulin and vicilin obtained from the isoelectric precipitation method. The ultrafiltered isolate contained both globulin and albumin proteins, indicating that the ultrafiltration of protein extracts yields protein concentrates/isolates that exhibit a more diverse protein profile (Papalamprou et al., Citation2009). Dehulling chickpeas before their processing can improve protein recovery and enhance the functional properties of the resultant protein ingredients (Boukid, Citation2021). In a study conducted by Withana-Gamage et al. (Citation2011), it was discovered that manual dehulling of both kabuli and desi chickpea seed coats led to the production of chickpea protein isolates with high protein content approximately 72.8–85.3%. These protein isolates were found to be comparable to peas and soy protein isolates. Chang et al. (2012), conducted a study wherein they produced and analyzed chickpea protein isolates through alkaline extraction and cryoprecipitate. The latter is a method employed to generate a uniform globulin protein extract. The methodology employed in this study comprised alkaline extraction and centrifugation. The resulting extract was subjected to filtration and refrigeration for 18 hours to promote protein precipitation from the solution. The protein was subsequently retrieved through centrifugation and freeze-drying to obtain a cryoprecipitate. The utilization of electrophoresis led to the identification of two distinct proteins, namely Legumin and vicilin, in the cryoprecipitate. The cryoprecipitate method yielded a protein isolate with greater homogeneity than isoelectric precipitation. It was evidenced by the absence of other protein bands on the gel, except for globulins (Chang et al., Citation2012).
Health perspective of chickpea protein
Cancer
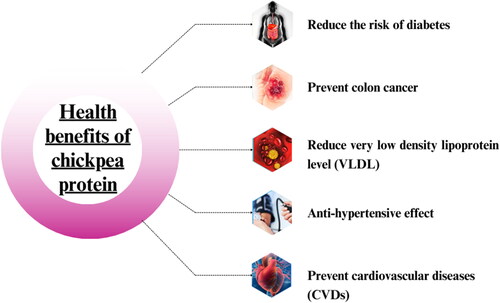
Cancer is a leading cause of death and one among the most common noncommunicable chronic diseases (Laxmi, Citation2022). According to the World Health Organization, colon cancer caused the deaths of 1 million people worldwide in 2015 (World Health Organization of the United Nations \(WHO\), Citation2022). The protein found in chickpeas has been shown to have the ability to lower the risk of developing colon cancer. Chickpea is the main source of bioactive compounds that showed anti-cancer potential through various mechanism. The antifungal proteins of chickpeas, known as C-25, were recently shown to be a powerful antifungal and anti-proliferative agent against a human oral carcinoma cell line. Its antiproliferative activity was assessed in targeting p38 MAP kinase at a concentration of 37.5 µg/mL (Kumar et al., Citation2014). Sanchez-Chino et al. (Citation2019) investigated the effect of chickpea protein hydrolysates on colon cancer caused by hypercaloric diet. Chickpea protein hydrolysates obtained through pepsin-pancreatin hydrolysis may reduce low density lipoproteins (LDL) and triglycerides (TC) levels after being consumed for 90 minutes at a dose of 30 mg/kg. while having no discernible effect on high density lipoproteins (HDL) levels and lowering the atherogenic index only at doses of 20 and 30 mg/kg. While, at doses of 20 and 30 mg/kg, it only lowers the atherogenic index and has no effect on HDL values. When chickpea proteins are broken down with pepsin and pancreatin, they release peptides which combat cancer and reduce cholesterol levels. These peptides could be used as a therapy to avoid colon cancer (Matemu et al., Citation2021).
Hypercholesterolemia
Hyperlipidemia is a lipid metabolic disorder resulting in elevated concentration of lipid serum. The risk factor of hyperlipidemia involved heredity, high cholesterol level, obesity and hypothyroidism. It was noted that dietary proteins, phytochemicals, raw extract, polyphenols and whole food were claimed to reduce the risk of hyperlipidemia (Stewart et al., Citation2020). Plant proteins, especially chickpea and soy proteins are better than animal proteins because they play pivotal role in lowering A the level of cholesterol and triacylglycerols in the blood. Most of the studies have been conducted on soybeans, and other legume seeds, including lentils (Dabai et al., Citation1996), or chickpeas (Wang & McIntosh, Citation1996; Zulet et al., Citation1999) have also been shown to lower cholesterol and triglycerides in people as well as animals. Limited literature is available on the impact of purified chickpea, soybean, and lentil proteins against VLDL and triglyceridemia metabolism. Yang et al. (Citation2007) investigated that dietary chickpea significantly normalized the hepatic lipase and epididymal adipose tissue lipoprotein lipase activity in rats when fed a high fat diet as compared to a normal fat diet. The health benefits of chickpea protein have been presented in .
Diabetes
Diabetes mellitus is a chronic as well as metabolic disease having harmful but preventable consequences. It is categorized in two types: Type 1 diabetes mellitus that is due to insulin deficiency (IDDM), Type 2 diabetes due to insulin resistance (NIDDM). These two types characterized by chronic hyperglycemic leading to an alteration in carbohydrate, lipid, and protein metabolism. According to an estimation, it is projected that the total number of diabetic people rise to 366 million in 2030 all over the world (Whiting et al., Citation2011). The utilization of chickpea flour in the manufacturing and composition of pasta items with a low glycemic index has been observed, potentially rendering them appropriate for individuals with diabetes. Commercially available chickpea pasta is produced by numerous food companies globally. According to Goñi and Valentín-Gamazo’s (Citation2003), chickpea flour in pasta has notably decreased the glucose release rate into the bloodstream. Hao et al. (Citation2009) identified proteinaceous α-amylase inhibitor having molecular weight 25.947 kDa from chickpeas. The amino acid sequence of the polypeptide from the isolated α-amylase inhibitor classified it as a protein called legumin, and experiments on its inhibitory activity demonstrated that it effectively blocked the activity of α-amylase from animals and plants but ineffective for inhibiting microbial α-amylase. So, the protein in chickpeas could help to lose weight and have better control of blood sugar after food intake.
Hypertension
Hypertension is considered as main cause of CVDs and stroke. The risk of hypertension is minimized by using angiotensin I-converting enzyme (ACE) inhibitors (Mark & Davis, Citation2000). Naturally, ACE inhibitor peptide showed antihypertensive properties but ACE is considered as the main cause of high blood pressure by converting inactive angiotensin I to the activated vasoconstrictor angiotensin II and prevents the vasodilator bradykinin (Fahmy et al., Citation2015). It was observed that chickpea imparted a potential role to reduce the risk of hypertension due to chickpea protein which contained bioactive peptides including ACE inhibitors. In a prior investigation, the protein hydrolysates from the chickpeas after enzymatic hydrolysis contained four ACE inhibitory peptides, each with a molecular weight of 900 Da and an IC50 of 0.1 mg/ml. This study demonstrated that two of them were the uncompetitive inhibitor of ACE and other two were competitive inhibitors (Pedroche et al., Citation2002).
Industrial application of chickpea protein ingredients
Baking industry
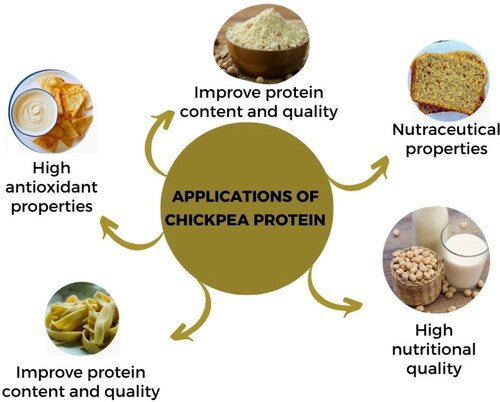
Chickpea proteins are primarily observed in food items such as cereal-based and bakery products, meat products and infant formulas. Nevertheless, there exists a possibility for their application in nutraceutical contexts (Boye et al., Citation2010; Shevkani et al., Citation2019). . shows the industrial applications of chickpea proteins. Chickpea protein constituents in cereal-based comestibles results in an amelioration of protein quantity and quality, as well as an augmentation of nutritional worth and certain sensory attributes of the food items. The addition of chickpea flour in place of wheat flour has been found to enhance the protein content and nutritional quality of food items derived from it, including baked goods such as bread and pasta. Additionally, this substitution has been observed to improve these products’ rheological, functional, and sensory characteristics in certain instances (Dandachy et al., Citation2019; Ouazib et al., Citation2016; Summo et al., Citation2019). The study conducted by Aider et al. (Citation2012) aimed to examine the impact of incorporating pulse protein concentrates, specifically lentils, peas, and chickpea, at varying levels on bread production through partial substitution of wheat flour. It exhibited that the bread’s mass volume, which was enriched with chickpea protein at 6% and 9%, was the greatest among the bread tested. However, it was still lower than the control bread made with wheat flour. Plant proteins had high water absorption capacity (WAC), which could have potentially reduced the formation of water vapor in the dough while it was being baked. According to recent reports, the addition of legume flours in bakery items such as pasta, biscuits, and bread has decreased these products’ in vitro glycemic response. It presents an opportunity to develop new food products that cater to individuals who require low glycemic index foods (Monnet et al., Citation2019). shown the food applications of chickpeas and chickpea proteins.
Table 2. Food applications of chickpea proteins.
Meat industry
Chickpea protein is mostly consumed legume than soy, faba and lentils. Chickpea protein has good texture, oil binding ability, water holding capacity and gelatinization. It had also comparable properties like stabilize emulsions, foaming and fat absorbing capacities. Chickpea protein imparts a positive effect on color of meat analogs. Several studies described that chickpea protein showed more reliable textural, color and acceptability attributes of meatless nuggets due to more carotenoid concertation (Kurek et al., Citation2022). According to Ghribi et al. (Citation2018) chickpea protein concentrate was explored to enhance the organoleptic characteristics of ‘Merguez’ sausage. The researchers incorporated chickpea protein concentrate at varying levels of 1.5%, 2.5%, and 5% protein content into the cooked sausages. The findings indicate that incorporating chickpea protein concentrates into meat products yields favorable sensory attributes, diminishes lipid oxidation levels, enhances color stability, and confers antioxidant properties. Kyriakopoulou et al. (Citation2021), have recently reported that chickpea protein ingredients possess favorable gelling properties, emulsifying abilities, and foam stability, rendering them promising substitutes for soy in the production of sausage-type meat analogs. In addition, there is a growing presence of novel yogurt substitutes that are derived from chickpeas, wherein chickpea protein concentrate is frequently incorporated into the product’s composition. According to the study of Bakr (Bakr, Citation1987), the sensory attributes of six meat sausages prepared with the substitution of chickpea protein and broad beans. It was observed that the products having chickpea and other legumes showed high score for color, taste and palatability as well as good quality of tenderness, water holding capacity and cooking losses. The results showed that value-added product with chickpea and faba beans had high desirability effects. It also indicated that protein quality of value-added sausages was high as compared to meat sausages. It was concluded that the sausages having 20% legumes had high status of amino acid, chemical composition and protein value as compared to all meat-based sausages (Bakr, Citation1987).
Infant formula
Infant formulas prepared by using chickpea protein could have low cost and still meet the Codex Aliment arias requirements for baby food in terms of S-containing amino acids (Valencia et al., Citation1988). According to Sotelo et al. (Citation1987), these formulas provide an option for lactose-intolerant infants and toddlers who cannot tolerate cow’s milk. Malunga et al. (Citation2014) conducted a study to examine the utilization of chickpea protein components derived from ‘desi’ and ‘kabuli’ varieties in the development of follow-on infant formulae. The formula developed has been determined to satisfy the nutritional standards established by the World Health Organization (WHO) regarding protein and carbohydrate levels, amino acid composition, and the majority of micronutrients while requiring only minimal supplementation of oils, minerals, and vitamins. Various preliminary techniques, including germination, dehulling, boiling, and enzymatic hydrolysis, were employed to mitigate anti-nutritional factors (Banti & Bajo, Citation2020).
Nutraceutical industry
Chickpea protein isolates can be used as bioactive peptides and capsules for micronutrient supplements in the nutraceutical industry. Bioactive peptides are used in nutrition to induce changes in the immunological, digestive, metabolic, and circulatory systems. The term ‘nutraceutical’ refers to any food or dietary component with health or medical benefits, including preventing or treating diseases. Chickpeas contain a variety of bioactive peptides that can improve health by regulating several biochemical processes (Tak et al., Citation2021). These bioactive peptides in chickpeas formed ACE inhibitory peptides when Alcalase breaks down the chickpea storage protein legumin (Pedroche et al., Citation2002). Hydrolyzed proteins from chickpea sprouts showed antiradical efficacy against hydroxyl and DPPH radicals when tested with a variety of enzymes, including alcalase, neutrase papain, and trypsin (Wali et al., Citation2021). Ariyarathna and Nedra Karunaratne (Citation2015), studied the utilization of chickpea protein isolates in the microencapsulation of folate. The utilization of proteins to encapsulate micronutrients is a burgeoning technological advancement that exhibits significant promise owing to the biocompatibility of proteins and their nutritional worth (Ariyarathna & Nedra Karunaratne, Citation2015). The study conducted by Karaca et al. (Citation2013) examined the microencapsulation of flaxseed oil by using the use of chickpea protein isolates. Polyunsaturated fatty acids make flaxseed oil very vulnerable to oxidative rancidity. During a 25-day storage period, microcapsules composed of chickpea protein isolates and maltodextrin inhibited oxidative rancidity. Research on the in-vitro digestibility of microcapsules composed of chickpea protein isolate and flaxseed oil demonstrated that chickpea protein isolate microcapsules had superior release characteristics to those made with lentil protein isolate. The existing literature on the encapsulation efficiency of chickpea protein isolates suggests their potential suitability for the encapsulation of bioactive compounds. However, further research is needed to explore their applications in nutraceutical industry.
Conclusion
There has been a notable rise in chickpea production on a global scale to cater to the demands of populations worldwide. Chickpeas are nutritionally valuable, offering a significant amount of protein, fat, carbohydrate, and fiber. Chickpea protein constituents have been extensively studied to create novel and modified food items, especially chickpea flour. Various applications have documented the potential of chickpea protein components for health-promoting effects, the development of novel food products, and the improvement of the nutrient composition of existing food products. The quantity of inquiries conducted on plausible applications of chickpea protein constituents has been on the rise, particularly in recent times, bridging the void concerning the potential utilization of said constituents in the food sector. Additional research on chickpea protein isolates and concentrates is necessary to cater to the demands of discerning consumers who seek superior protein, despite most published studies being conducted on chickpea flour. Moreover, utilizing food products derived from chickpea protein isolation results in more eco-friendly procedures.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Sidra Akram
Sidra Akram is a researcher in the department of Nutritional sciences. She has authored 4 publications in international journals. Her preliminary research focuses on extraction bioactive components. She has preliminary work on development of value added products enriched with iron to combat iron deficiency in women. She is currently working on improving community health through nutritional counseling.
Muhammad Faizan Afzal
Muhammad Faizan Afzal is a young Food Science and Nutrition researcher. He has authored 15 papers in various international journals. His preliminary research focuses on extraction and enrichment of bioactive components. He has preliminary work on development of value-added products. He is currently working on enrichment of edible oils using green technologies.
Kainat Anwer
Kainat Anwer is a young, motivated and goal-oriented Nutritionist and Public Health researcher. She has authored in 3 papers which published in different international Journals. Her basic research based on socioeconomic factors effecting the public health. Her primary work was on Intermittent fastening and its co relationship with CVD and GDM. The main focus was on Cardiometabolic diseases. Currently she is a student of Ph.D. in a Public university in Nutritional Sciences department.
Laiba Farman
Laiba Farman is M.Phil. scholar in the department of applied chemistry at University of Agriculture, Faisalabad. She has working on development of nanoparticles and encapsulation of bioactive components.
Muhammad Zubair
Muhammad Zubair is motivated and goal-oriented PhD scholar in department of Nutritional sciences. He has published 5 papers in different international Journals. His basic research based on preventing Diabetes by using bioactive compounds enrich value added products.
Safura Kousar
Safura Kousar is a dedicated PhD student with a passion for advancing knowledge in her field. With a keen interest in research, Safura has authored and co-authored a total of 13 papers, showcasing her commitment to scholarly inquiry and academic excellence. Her contributions to the academic community have been significant, with her papers making valuable contributions to the advancement of knowledge in her field. Safura is poised to make even greater contributions to her field in the years to come.
Touseef Iqbal
Dr. Touseef Iqbal has a PhD in Food and Nutrition subject from the Dept. of Food science and Human Nutrition at University of veterinary and animal sciences, Lahore. Dr. khan served as Head of the department (Nutrition) from 2019 to 2023 at Afro-Asian Institute Lahore. He is currently working as a faculty for BS Human Nutrition and Dietetics program at Dept. Of public health, institute of social and cultural studies, University of the Punjab. He has research interests in phytochemistry, extraction and characterization of bioactive compounds, Nutraceutical and Sports nutrition. He is expanding his thoughts nutrigenomics and personalized nutrition.
Waseem Khalid
Dr. Waseem Khalid working as Assistant Professor at the University Institute of Food Science & Technology, The University of Lahore, Pakistan. Waseem completed BS, MS, and Ph.D. in Food Technology from Government College University, Faisalabad, Pakistan, and obtained 1st position in both examinations. He also worked as Lecturer, at Government College University Faisalabad, Pakistan. His areas of interest for teaching and research include Raw Meat, Meat products, Irradiation, Functional Food, Plant-based Food, and Halal Food. He authored more than 145 articles and book chapters published in top-tier journals and conference proceedings. Currently, he is trying to develop sustainable food in Pakistan.
Mohammed Ahmed Elawad
Mohammed Ahmed Elawad has completed his Ph.D. from University of Khartoum, Khartoum, Sudan since 2012 in Public Health. He became assistant professor in University of Khartoum in 2012. He moved to work at Health Sciences College at Al-Leith, Umm Al-Qura University, Saudi Arabia and he is appointed as the head of the Department of Public Health. He has published many scientific researches in the field of public health and epidemiology.
Alshebli Ahmed
Alshebli Ahmed PhD from Khartoum University, Sudan, 2017. Presently he has been working as assistant professor in department in Public Health in Health Sciences College at Al-Leith, Umm Al-Qura University, Saudi Arabia Research interest: Public Health, Environmental Health and Occupational Health Is an active researcher in the Public Health and Environmental Health. He has published more than 24 scientific papers in international and ISI journals He has 26 years’ experience in both teaching and research activities. He has participated in several International, National conference, symposiums, workshops.
Muhammad Zubair Khalid
Muhammad Zubair Khalid is a young Food Science researcher. He has authored and co-authored 50 papers in various international journals. His preliminary research focuses on Extraction of Phytoconstituents from Agro Wastes. He has preliminary worked on clean label extraction technique. He is currently working on waste management and green extraction technologies.
Felix Kwashie Madilo
Felix Kwashie Madilo is a lecturer in the Food Science and Technology Department of Ho Technical University, Volta Region, Ghana. He is a Faculty of Applied Sciences and Technology board member and a member of Faculty Seminar, Workshop and Durbar committee. He also worked as a visiting researcher and a lecturer at the University of Zuĺuland, South Africa. His research interest includes but not limited to Food Microbiology, Food Fermentation, Food Safety and Hygiene, Food Quality and Management, and Food Processing and Preservation.
References
- Aider, M., Sirois-Gosselin, M., & Boye, J. I. (2012). Pea, lentil and chickpea protein application in bread making. Journal of Food Research, 1(4), 1. https://doi.org/10.5539/jfr.v1n4p160
- Ariyarathna, I. R., & Nedra Karunaratne, D. (2015). Use of chickpea protein for encapsulation of folate to enhance nutritional potency and stability. Food and Bioproducts Processing, 95, 76–15. https://doi.org/10.1016/j.fbp.2015.04.004
- Ashokkumar, K., Diapari, M., Jha, A. B., Tar’an, B., Arganosa, G., & Warkentin, T. D. (2015). Genetic diversity of nutritionally important carotenoids in 94 pea and 121 chickpea accessions. Journal of Food Composition and Analysis, 43, 49–60. https://doi.org/10.1016/j.jfca.2015.04.014
- Bakr, T. M. A. (1987). Nutritional evaluation of sausages containing chick peas and faba beans as meat protein extenders. Food Chemistry. 23(2), 143–150.
- Banti, M., & Bajo, W. (2020). Review on nutritional importance and anti-nutritional factors of legumes. International Journal of Nutrition and Food Sciences, 9(6), 138–149. https://doi.org/10.11648/j.ijnfs.20200906.11
- Begum, N., Khan, Q. U., Liu, L. G., Li, W., Liu, D., & Haq, I. U. (2023). Nutritional composition, health benefits and bio-active compounds of chickpea (Cicer arietinum L.). Frontiers in Nutrition, 10.
- Bessada, S. M. F., Barreira, J. C. M., & Oliveira, M. B. P. P. (2019). Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends in Food Science and Technology. 93(228), 53–68. https://doi.org/10.1016/j.tifs.2019.08.022
- Bhatty, R. S. (1982). Albumin proteins of eight edible grain legume species: Electrophoretic patterns and amino acid composition. Journal of Agricultural and Food Chemistry, 30(3), 620–622. https://doi.org/10.1021/jf00111a057
- Boukid, F. (2021). Chickpea (Cicer arietinum L.) protein as a prospective plant‐based ingredient: A review. International Journal of Food Science & Technology, 56(11), 5435–5444. https://doi.org/10.1111/ijfs.15046
- Boye, J. I., Aksay, S., Roufik, S., Ribéreau, S., Mondor, M., Farnworth, E., & Rajamohamed, S. H. (2010). Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Research International. 43(2), 537–546. https://doi.org/10.1016/j.foodres.2009.07.021
- Boye, J. I., Zare, F., & Pletch, A. (2010). Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Research International. 43(2), 414–431. https://doi.org/10.1016/j.foodres.2009.09.003
- Chang, Y. W., Alli, I., Molina, A. T., Konishi, Y., & Boye, J. I. (2012). Isolation and characterization of chickpea (Cicer arietinum L.) seed protein fractions. Food and Bioprocess Technology, 5(2), 618–625. https://doi.org/10.1007/s11947-009-0303-y
- Chen, N., Zhao, M., Chassenieux, C., & Nicolai, T. (2016). Thermal aggregation and gelation of soy globulin at neutral pH. Food Hydrocolloids, 61, 740–746. 06.028 https://doi.org/10.1016/j.foodhyd.2016
- Cox, A. L., Eigenmann, P. A., & Sicherer, S. H. (2021). Clinical relevance of cross-reactivity in food allergy. The Journal of Allergy and Clinical Immunology. In Practice, 9(1), 82–99. https://doi.org/10.1016/j.jaip.2020.09.030
- Dabai, F. D., Walker, A. F., Sambrook, I. E., Welch, V. A., Owen, R. W., & Abeyasekera, S. (1996). Comparative effects on blood lipids and faecal steroids of five legume species incorporated into a semi-purified, hypercholesterolaemic rat diet. The British Journal of Nutrition, 75(4), 557–571. https://doi.org/10.1079/bjn19960159
- Dandachy, S., Mawlawi, H., & Obeid, O. (2019). Effect of processed chickpea flour incorporation on sensory properties of Man koushe Zaatar. Foods (Basel, Switzerland), 8(5), 151. https://doi.org/10.3390/foods8050151
- Day, L. (2013). Proteins from land plants—Potential resources for human nutrition and food security. Trends in Food Science and Technology. 32(1), 25–42. 005 https://doi.org/10.1016/j.tifs.2013.05
- Fahmy, S. R., Soliman, A. M., Sayed, A. A., & Marzouk, M. (2015). Possible antiosteoporotic mechanism of Cicer arietinum extract in ovariectomized rats. Int. J. Clin. Exp. Pathol, 8(4), 3477.
- Food and Agriculture Organization of the United Nations (FAO). (2020). FAOSTAT statistical database, chickpea production data. FAO.
- Frimpong, A., Sinha, A., Tar’an, B., Warkentin, T. D., Gossen, B. D., & Chibbar, R. N. (2009). Genotype and growing environment influence chickpea (Cicer arietinum L.) seed composition. Journal of the Science of Food and Agriculture, 89(12), 2052–2063. https://doi.org/10.1002/jsfa.3690
- Gangola, M. P., Ramadoss, B. R., Jaiswal, S., Fabek, H., Tulbek, M., Anderson, G. H., & Chibbar, R. N. (2022). Nutritional composition and in vitro starch digestibility of crackers supplemented with faba bean whole flour, starch concentrate, protein concentrate and protein isolate. Foods (Basel, Switzerland), 11(5), 645. https://doi.org/10.3390/foods11050645
- Garcia-Valle, D. E., Bello-Pérez, L. A., Agama-Acevedo, E., & Alvarez Ramirez, J. (2021). Structural characteristics and in vitro starch digestibility of pasta made with durum wheat semolina and chickpea flour. LWT, 145, 111347. https://doi.org/10.1016/j.lwt.2021.111347
- Ghribi, A. M., Ben Amira, A., Maklouf Gafsi, I., Lahiani, M., Bejar, M., Triki, M., Zouari, A., Attia, H., & Besbes, S. (2018). Toward the enhancement of sensory profile of sausage “Merguez” with chickpea protein concentrate. Meat Science, 143, 74–80. https://doi.org/10.1016/j.meatsci.2018.04.025
- Ghribi, A. M., Maklouf, I., Blecker, C., Attia, H., & Besbes, S. (2015). Nutritional and compositional study of Desi and Kabuli chickpea (Cicer Arietinum L.) flours from Tunisian cultivars. Advanced in Food Technology and Nutritional Sciences - Open Journal, 1(2), 38–47. https://doi.org/10.17140/AFTNSOJ-1-107
- Ghumman, A., Kaur, A., & Singh, N. (2016). Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocolloids, 61, 843–850. https://doi.org/10.1016/j.foodhyd.2016.07.013
- Goñi, I., & Valentín-Gamazo, C. (2003). Chickpea flour ingredient slows glycemic response to pasta in healthy volunteers. Food Chemistry. 81(4), 511–515. https://doi.org/10.1016/S0308-8146(02)00480-6
- Grasso, N., Bot, F., Roos, Y. H., Crowley, S. V., Arendt, E. K., & O’Mahony, J. A. (2023). Plant-based alternatives to cheese formulated using blends of zein and chickpea protein ingredients. Foods (Basel, Switzerland), 12(7), 1492. https://doi.org/10.3390/foods12071492
- Grasso, N., Lynch, N. L., Arendt, E. K., & O’Mahony, J. A. (2022). Chickpea protein ingredients: A review of composition, functionality, and applications. Comprehensive Reviews in Food Science and Food Safety, 21(1), 435–452. https://doi.org/10.1111/1541-4337.12878
- Hall, C., Hillen, C., & Robinson, J. G. (2017). Composition, nutritional value, and health benefits of pulses. Cereal Chemistry, 94(1), 11–31. https://doi.org/10.1094/CCHEM-03-16-0069-FI
- Hao, X., Li, J., Shi, Q., Zhang, J., He, X., & Ma, H. (2009). Characterization of a novel legumin α-amylase inhibitor from chickpea (Cicer arietinum L.) seeds. Bioscience, Biotechnology, and Biochemistry, 73(5), 1200–1202. https://doi.org/10.1271/bbb.80776
- Idate, A., Shah, R., Gaikwad, V., Kumathekar, S., & Temgire, S. (2021). A comprehensive review on antinutritional factors of chickpea (Cicer arietinum L.). The Pharma Innovation, 10(5), 816–823. https://doi.org/10.22271/tpi.2021.v10.i5k.6306
- Iqbal, A., Ateeq, N., Khalil, I. A., Perveen, S., & Saleemullah, S. (2006). Physicochemical characteristics and amino acid profile of chickpea cultivars grown in Pakistan. Journal of Foodservice, 17(2), 94–101. https://doi.org/10.1111/j.1745-4506.2006.00024.x
- Jukanti, A. K., Gaur, P. M., Gowda, C. L. L., & Chibbar, R. N. (2012). Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. The British Journal of Nutrition, 108 Suppl 1(S1), S11–S26. https://doi.org/10.1017/S0007114512000797
- Karaca, A. C., Nickerson, M., & Low, N. H. (2013). Microcapsule production employing chickpea or lentil protein isolates and maltodextrin: Physicochemical properties and oxidative protection of encapsulated flaxseed oil. Food Chemistry, 139(1–4), 448–457. https://doi.org/10.1016/j.foodchem.2013.01.040
- Kaur, R., & Prasad, K. (2021). Technological, processing and nutritional aspects of chickpea (Cicer arietinum)-A review. Trends in Food Science and Technology. 109, 448–463. https://doi.org/10.1016/j.tifs.2021.01.044
- Knights, E. J., & Hobson, K. B. (2016). Chickpea: Overview. In Colin W. Wrigley, Harold Corke, Koushik Seetharaman, & Jonathan Faubion (Eds.), Encyclopedia of food grains (2nd ed., pp. 316–323). Academic Press. https://doi.org/10.1016/B978-0-12-394437-5.00035-8
- Korbu, L., Tafes, B., Kassa, G., Mola, T., & Fikre, A. (2020). Unlocking the genetic potential of chickpea through improved crop management practices in Ethiopia. A review. Agronomy for Sustainable Development, 40(2), 1–20. https://doi.org/10.1007/s13593-020-00618-3
- Kumar, S., Kapoor, V., Gill, K., Singh, K., Xess, I., Das, S. N., & Dey, S. (2014). Antifungal and antiproliferative protein from Cicer arietinum: A bioactive compound against emerging pathogens. BioMed Research International, 2014, 387203–387209. https://doi.org/10.1155/2014/387203
- Kurek, M. A., Onopiuk, A., Pogorzelska-Nowicka, E., Szpicer, A., Zalewska, M., & Półtorak, A. (2022). Novel protein sources for applications in meat-alternative products—Insight and challenges. Foods (Basel, Switzerland), 11(7), 957. https://doi.org/10.3390/foods11070957
- Kyriakopoulou, K., Keppler, J. K., & van der Goot, A. J. (2021). Functionality of ingredients and additives in plant-based meat analogues. Foods (Basel, Switzerland), 10(3), 600. https://doi.org/10.3390/foods10030600
- Laxmi, V. C (2022). larification of non communicable diseases their types and risk factor and some preventive action to reduce the chronic diseases.
- Malunga, L. N., Bar-El Dadon, S., Zinal, E., Berkovich, Z., Abbo, S., & Reifen, R. (2014). The potential use of chickpeas in development of infant follow-on formula. Nutrition Journal, 13(1), 8. https://doi.org/10.1186/1475-2891-13-8
- Mark, K. S., & Davis, T. P. (2000). Stroke: Development, prevention and treatment with peptidase inhibitors. Peptides, 21(12), 1965–1973. https://doi.org/10.1016/s0196-9781(00)00346-6
- Maryniak, N. Z., Sancho, A. I., Hansen, E. B., & Bøgh, K. L. (2022). Alternatives to cow’s milk-based infant formulas in the prevention and management of cow’s milk allergy. Foods (Basel, Switzerland), 11(7), 926. https://doi.org/10.3390/foods11070926
- Matemu, A., Nakamura, S., & Katayama, S. (2021). Health benefits of antioxidative peptides derived from legume proteins with a high amino acid score. Antioxidants, 10(2), 316. https://doi.org/10.3390/antiox10020316
- Mohammed Mubarak, V. (2009). Development and analysis of transgenic chickpea for resistant to Helicoverpa armigera (Hubner) [Doctoral dissertation, Tamil Nadu Agricultural University].
- Monnet, A. F., Laleg, K., Michon, C., & Micard, V. (2019). Legume enriched cereal products: A generic approach derived from material science to predict their structuring by the process and their final properties. Trends in Food Science and Technology. 86, 131–143. https://doi.org/10.1016/j.tifs.2019.02.027
- Nartea, A., Kuhalskaya, A., Fanesi, B., Orhotohwo, O. L., Susek, K., Rocchetti, L., & Papa, R. (2023). Legume byproducts as ingredients for food applications: Preparation, nutrition, bioactivity, and techno‐functional properties. Comprehensive Reviews in Food Science and Food Safety, 22(3), 1953–1985.
- Nasir, M., & Sidhu, J. S. (2012). Common pulses: Chickpea, lentil, mungbean, black gram, pigeon pea and Indian vetch. In M. Siddiq & M. A. Uebersax (Eds.), Dry beans and pulses production, processing and nutrition (pp. 283–309).
- Okagu, O. D., & Udenigwe, C. C. (2022). Molecular interactions of pea globulin, albumin and glutelin with curcumin: Formation and gastric release mechanisms of curcumin-loaded bio-nanocomplexes. Food Biophysics, 17(1), 10–25. https://doi.org/10.1007/s11483-021-09697-5
- Ouazib, M., Garzon, R., Zaidi, F., & Rosell, C. M. (2016). Germinated, toasted and cooked chickpea as ingredients for bread making. Journal of Food Science and Technology, 53(6), 2664–2672. https://doi.org/10.1007/s13197-016-2238-4
- Özer, S., Karaköy, T., Toklu, F., Baloch, F. S., Kilian, B., & Özkan, H. (2010). Nutritional and physicochemical variation in Turkish kabuli chickpea (Cicer arietinum L.) landraces. Euphytica, 175(2), 237–249. https://doi.org/10.1007/s10681-010-0174-3
- Papalamprou, E. M., Doxastakis, G. I., Biliaderis, C. G., & Kiosseoglou, V. (2009). Influence of preparation methods on physicochemical and gelation properties of chickpea protein isolates. Food Hydrocolloids, 23(2), 337–343. 10.1016/j.foodhyd.2008.03.006
- Pathania, S., & Kaur, N. (2022). Utilization of fruits and vegetable by-products for isolation of dietary fibres and its potential application as functional ingredients. Bioactive Carbohydrates and Dietary Fibre, 27, 100295. https://doi.org/10.1016/j.bcdf.2021.100295
- Patil, N. (2023). Chickpea protein: A comprehensive review on nutritional properties, processing, functionality, applications, and sustainable impact.
- Pedroche, J., Yust, M. M., Girón‐Calle, J., Alaiz, M., Millán, F., & Vioque, J. (2002). Utilisation of chickpea protein isolates for production of peptides with angiotensin I‐converting enzyme (ACE)‐inhibitory activity. Journal of the Science of Food and Agriculture, 82(9), 960–965. https://doi.org/10.1002/jsfa.1126
- Rachwa-Rosiak, D., Nebesny, E., & Budryn, G. (2015). Chickpeas—Composition, nutritional value, health benefits, application to bread and snacks: A review. Critical Reviews in Food Science and Nutrition, 55(8), 1137–1145. https://doi.org/10.1080/10408398.2012687418
- Redden, R. J., & Berger, J. D. (2007). Chickpea breeding and management: History and origin of chickpea (pp. 1–13). CAB International.
- Sánchez-Chino, X. M., Jiménez Martínez, C., León-Espinosa, E. B., Garduño-Siciliano, L., Álvarez-González, I., Madrigal-Bujaidar, E., Vásquez-Garzón, V. R., Baltiérrez-Hoyos, R., & Dávila-Ortiz, G. (2019). Protective effect of chickpea protein hydrolysates on colon carcinogenesis associated with a hypercaloric diet. Journal of the American College of Nutrition, 38(2), 162–170. https://doi.org/10.1080/07315724.2018.1487809
- Serrano, C., Carbas, B., Castanho, A., Soares, A., Patto, M. C. V., & Brites, C. (2017). Characterisation of nutritional quality traits of a chickpea (Cicer arietinum) germplasm collection exploited in chickpea breeding in Europe. Crop and Pasture Science, 68(11), 1031–1040. https://doi.org/10.1071/CP17129
- Serrano-Sandoval, S. N., Guardado-Félix, D., & Gutiérrez-Uribe, J. A. (2019). Changes in digestibility of proteins from chickpeas (Cicer arietinum L.) germinated in presence of selenium and antioxidant capacity of hydrolysates. Food Chemistry, 285, 290–295. https://doi.org/10.1016/j.foodchem.2019.01.137
- Sharma, S., Upadhyaya, H. D., Roorkiwal, M., Varshney, R. K., & Gowda, C. L. L. (2013). Chickpea. In M. Singh, H. D. Upadhyaya, & I. S. Bisht Eds.), Genetic and genomic resources of grain legume improvement (pp. 81–111). Elsevier Inc.
- Shevkani, K., Singh, N., Chen, Y., Kaur, A., & Yu, L. (2019). Pulse proteins: Secondary structure, functionality and applications. Journal of Food Science and Technology, 56(6), 2787–2798. https://doi.org/10.1007/s13197-019-03723-8
- Sim, S. Y. J., Srv, A., Chiang, J. H., & Henry, C. J. (2021). Plant proteins for future foods: A roadmap. Foods (Basel, Switzerland), 10(8), 1967. https://doi.org/10.3390/foods10081967
- Singh, N., Sandhu, K. S., & Kaur, M. (2004). Characterization of starches separated from Indian chickpea (CicerarietinumL.) cultivars. Journal of Food Engineering. 63(4), 441–449. https://doi.org/10.1016/j.jfoodeng.2003.09.003
- Singh, U. (1985). Nutritional quality of chickpea (Cicer arietinum L.): Current status and future research needs. Qualitas Plantarum Plant Foods for Human Nutrition, 35(4), 339–351. https://doi.org/10.1007/BF01091779
- Singh, U., & Jambunathan, R. (1982). Distribution of seed protein fractions and amino acids in different anatomical parts of chickpea (Cicerarietinum L.) and pigeon pea (Cajanuscajan L. Qualitas Plantarum Plant Foods for Human Nutrition, 31(4), 347–354.) https://doi.org/10.1007/BF01094046
- Singhal, A., Karaca, A. C., Tyler, R., & Nickerson, M. (2016). Pulse proteins: From processing to structure-function relationships. In A. K. Goyal (Ed.), Grain legumes (pp. 55–78). Intech Open. https://doi.org/10.5772/64020
- Sofi, S. A., Singh, J., Chhikara, N., & Panghal, A. (2020). Effect of incorporation of germinated flour and protein isolate from chickpea on different quality characteristics of rice‐based noodle. Cereal Chemistry, 97(1), 85–94. https://doi.org/10.1002/cche.10192
- Sotelo, A., Hernández, M., Larracilla, J., Arenas, M. L., & Palapa, E. (1987). Use of chickpea (Cicer arietinum L.) in non-dairy formulas. II. Nitrogen balance in children with lactose intolerance, fed with a formula based on chickpea and a commercial soybean product. Archivos Latinoamericanos de Nutricion, 37(3), 468–479.
- Stewart, J., McCallin, T., Martinez, J., Chacko, S., & Yusuf, S. (2020). Hyperlipidemia. Pediatrics in Review, 41(8), 393–402. https://doi.org/10.1542/pir.2019-0053
- Stone, A. K., Nosworthy, M. G., Chiremba, C., House, J. D., & Nickerson, M. T. (2019). A comparative study of the functionality and protein quality of a variety of legume and cereal flours. Cereal Chemistry, 96(6), 1159–1169. https://doi.org/10.1002/cche.10226
- Summo, C., De Angelis, D., Ricciardi, L., Caponio, F., Lotti, C., Pavan, S., & Pasqualone, A. (2019). Nutritional, physico-chemical and functional characterization of a global chickpea collection. Journal of Food Composition and Analysis, 84, 103306. https://doi.org/10.1016/j.jfca.2019.103306
- Tak, Y., Kaur, M., Amarowicz, R., Bhatia, S., & Gautam, C. (2021). Pulse derived bioactive peptides as novel nutraceuticals: A review. International Journal of Peptide Research and Therapeutics, 27(3), 2057–2068. https://doi.org/10.1007/s10989-021-10234-8
- Tosh, S. M., & Yada, S. (2010). Dietary fibre sin pulse seeds and fractions: Characterization, functional attributes, and applications. Food Research International. 43(2), 450–460. https://doi.org/10.1016/j.foodres.2009.09.005
- Tripathi, A., Iswarya, V., Rawson, A., Singh, N., Oomah, B. D., & Patras, A. (2021). Chemistry of pulses—macronutrients. In Brijesh K. Tiwari, Aoife Gowen & Brian McKenna (Eds.), Pulse foods (pp. 31–59). Academic Press.
- Valencia, M. E., Troncoso, R., & Higuera, I. (1988). Linear programming formulation and biological evaluation of chickpea-based infant foods. Cereal Chemistry. 65(2), 101–104.
- Wali, A., Mijiti, Y., Yanhua, G., Yili, A., Aisa, H. A., & Kawuli, A. (2021). Isolation and identification of a novel antioxidant peptide from chickpea (Cicer arietinum L.) sprout protein hydrolysates. International Journal of Peptide Research and Therapeutics, 27(1), 219–227. https://doi.org/10.1007/s10989-020-10070-2
- Wallace, T., Murray, R., & Zelman, K. (2016). The nutritional value and health benefits of chickpeas and hummus. Nutrients, 8(12), 766. https://doi.org/10.3390/nu8120766
- Wang, Y. H., & McIntosh, G. H. (1996). Extrusion and boiling improve rat body weight gain and plasma cholesterol lowering ability of peas and chickpeas. The Journal of Nutrition, 126(12), 3054–3062. https://doi.org/10.1093/jn/126.12.3054
- Wang, Y., Tuccillo, F., Lampi, A.-M., Knaapila, A., Pulkkinen, M., Kariluoto, S., Coda, R., Edelmann, M., Jouppila, K., Sandell, M., Piironen, V., & Katina, K. (2022). Flavor challenges in extruded plant‐based meat alternatives: A review. Comprehensive Reviews in Food Science and Food Safety, 21(3), 2898–2929. https://doi.org/10.1111/1541-4337.12964
- Wang, Y., Yuan, J. J., Li, K., Chen, X., Wang, Y. T., & Bai, Y. H. (2023). Evaluation of chickpea protein isolate as a partial replacement for phosphate in pork meat batters: Techno-functional properties and molecular characteristic modifications. Food Chemistry, 404(Pt A), 134585. https://doi.org/10.1016/j.foodchem.2022.134585
- Wangorsch, A., Kulkarni, A., Jamin, A., Spiric, J., Bräcker, J., Brockmeyer, J., Mahler, V., Blanca-Lopez, N., Ferrer, M., Blanca, M., Torres, M., Gomez, P., Bartra, J., García-Moral, A., Goikoetxea, M. J., Vieths, S., Toda, M., Zoccatelli, G., & Scheurer, S. (2020). Identification and characterization of IgE-reactive proteins and a new allergen (Cica1.01) from chickpea (Cicerarietinum). Molecular Nutrition & Food Research. 64(19), 2000560. https://doi.org/10.1002/mnfr.202000560
- Whiting, D. R., Guariguata, L., Weil, C., & Shaw, J. (2011). IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Research and Clinical Practice, 94(3), 311–321. https://doi.org/10.1016/j.diabres.2011.10.029
- Withana-Gamage, T. S., Wanasundara, J. P., Pietrasik, Z., & Shand, P. J. (2011). Physicochemical, thermal and functional characterization of protein isolates from Kabuli and Desi chickpea (Cicerarietinum L.): A comparative study with soy (Glycine max) and pea (Pisumsativum L.). Journal of the Science of Food and Agriculture, 91(6), 1022–1031. https://doi.org/10.1002/jsfa.4277
- World Health Organization of the United Nations (WHO). (2022). Surveillance data regarding the occurrence of cancer worldwide. https://www.iarc.who.int/featured-news/colorectal-cancer-awareness-month-2022/
- Xing, Q., Dekker, S., Kyriakopoulou, K., Boom, R. M., Smid, E. J., & Schutyser, M. A. (2020). Enhanced nutritional value of chickpea protein concentrate by dry separation and solid state fermentation. Innovative Food Science & Emerging Technologies, 59, 102269. https://doi.org/10.1016/j.ifset.2019.102269
- Xu, B., & Chang, S. K. C. (2009). Phytochemical profiles and health promoting effects of cool-season food legumes as influenced by thermal processing. Journal of Agricultural and Food Chemistry, 57(22), 10718–10731. https://doi.org/10.1021/jf902594m
- Xu, Y., Thomas, M., & Bhardwaj, H. L. (2014). Chemical composition, functional properties and microstructural characteristics of three kabuli chickpea (C icer arietinum L.) as affected by different cooking methods. International Journal of Food Science & Technology, 49(4), 1215–1223. https://doi.org/10.1111/ijfs.12419
- Yadav, S. S., Redden, R. J., Chen, W., & Sharma, B. (Eds.). (2007). Chickpea breeding and management (pp. 72–100). Cab International.
- Yang, Y., Zhou, L., Gu, Y., Zhang, Y., Tang, J., Li, F., Shang, W., Jiang, B., Yue, X., & Chen, M. (2007). Dietary chickpeas reverse visceral adiposity, dyslipidaemia and insulin resistance in rats induced by a chronic high-fat diet. The British Journal of Nutrition, 98(4), 720–726. https://doi.org/10.1017/S0007114507750870
- Yegrem, L. (2021). Nutritional composition, antinutritional factors, and utilization trends of Ethiopian chickpea (Cicer arietinum L.). International Journal of Food Science. 2021, 1–10. https://doi.org/10.1155/2021/5570753
- Zhao, X., Sun, L., Zhang, X., Wang, M., Liu, H., & Zhu, Y. (2021). Nutritional components, volatile constituents and antioxidant activities of 6 chickpea species. Food Bioscience. 41, 100964. https://doi.org/10.1016/j.fbio.2021.100964
- Zulet, M. A., Macarulla, M. T., Portillo, M. P., Noel-Suberville, C., Higueret, P., & Martínez, J. A. (1999). Lipid and glucose utilization in hypercholesterolemic rats fed a diet containing heated chickpea (Cicer aretinum L.): A potential functional food. International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift Fur Vitamin- Und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition, 69(6), 403–411. https://doi.org/10.1024/0300-9831.69.6.403