Abstract
The loss of the endothelium barrier and vascular leakage play a central role in the pathogenesis of hemorrhagic fever viruses. This can be caused either directly by the viral infection and damage of the vascular endothelium, or indirectly by a dysregulated immune response resulting in an excessive activation of the endothelium. This article briefly reviews our knowledge of the importance of the disruption of the vascular endothelial barrier in two severe disease syndromes, dengue hemorrhagic fever and hantavirus pulmonary syndrome. Both viruses cause changes in vascular permeability without damaging the endothelium. Here we focus on our understanding of the virus interaction with the endothelium, the role of the endothelium in the induced pathogenesis, and the possible mechanisms by which each virus causes vascular leakage. Understanding the dynamics between viral infection and the dysregulation of the endothelial cell barrier will help us to define potential therapeutic targets for reducing disease severity.
Introduction
Viral hemorrhagic fevers (VHFs) are a group of illnesses caused by viruses belonging to 4 different families: Flaviviridae, Bunyaviridae, Arenaviridae, and Filoviridae. VHFs are zoonotic diseases transmitted to humans from nonhuman primates, rodents, bats, or arthropod reservoirs. Viruses causing hemorrhagic fevers are globally distributed. While some hemorrhagic fever viruses can cause relatively mild illnesses, many of these viruses cause severe, life-threatening disease with case fatality rates up to 80%. Although VHFs have diverse clinical presentations, they share common characteristic symptoms including fever, hemorrhage, and shock.
One of main target tissues in VHF infections is the microvascular endothelium. The most common clinical finding in severe VHF cases is endothelial dysregulation leading to increased vascular permeability. Therefore the endothelium plays a key role in the development of the disease. The malfunction of the endothelium can be induced either direct by the virus replication or indirectly by virus induced secreted cellular factors that can cause endothelial activation and increase vascular permeability. For example, some of the VHF-causing viruses, such as Ebola, Marburg, and Rift Valley fever viruses, produce a strong cytopathic effect on the infected endothelium, directly affecting permeability. In contrast, arenaviruses, hantavirus, and dengue viruses (DENV) produce little to no cytopathic effect on endothelium. Although induction of cytokines is a contributing factor in increasing vascular permeability in all hemorrhagic fevers, cytokines play a central role in DENV- and hantavirus-induced endothelial dysfunction. Immune activation by the viruses contributes not only to viral clearance but also to the observed immunopathology.
The causes of increased vascular permeability in VHFs have been investigated in previous studies, particularly involving Ebola and Lassa viruses.Citation1-Citation3 While the mechanisms underlying VHFs in which endothelial cells are directly injured by the viruses are better understood, the mechanisms of endothelial cell dysfunction VHFs in which minimal direct endothelial cell injury are unclear. Although dengue and hantavirus infection are quite distinct in the vascular beds involved in the diseases, they share some pathophysiological features including the lack of overt endothelial injury and the associated complete clinical recovery suggestive of transient functional disturbance of endothelium. Comparing pathology and immunology of these VHFs thus may provide some insights into the underlying disease mechanisms (see ). In this review we will particularly focus on the interaction of these viruses with the vascular endothelium, emphasizing the mechanisms by which they likely cause endothelial cell leakage.
Table 1. Similarities and differences between dengue hemorrhagic fever and hantavirus pulmonary syndrome
Dengue Fever and DENV
Dengue is one of the most important vector-borne diseases worldwide, causing an estimated 5 million cases and over 20 000 fatalities annually. Current endemic areas include Southeast Asia, Central and South America, and parts of Africa.Citation4 Recent reports have described major outbreaks of dengue in South Asia and the Middle East.Citation5-Citation7 A few indigenous dengue cases have been reported in Europe and the United States.Citation8,Citation9 The expansion of dengue, both geographically and demographically, underscores its importance as a major global emerging public health problem.
DENV consist of 4 serologically distinct, positive-strand RNA viruses belonging to the Flaviviridae family: DENV1, DENV2, DENV3, and DENV4. The 10 kilobase viral genome encodes 10 gene products, including structural proteins capsid (C), matrix (prM), and envelope (E), and nonstructural proteins NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5.Citation10 The envelope protein plays an important role in viral binding and entry into host cells. The serologic reactivity to the envelope proteins defines the serotypes of DENV. In addition to functions in genome replication, protein processing, and viral particle assembly, some nonstructural proteins have been reported to possess immune evasion properties that may be relevant to DENV pathogenesis.Citation11,Citation12
Clinical dengue disease
In endemic areas, where multiple DENV co-circulate, primary DENV infection occurs early in childhood, and is usually asymptomatic or causes a mild undifferentiated febrile illness.Citation13 Primary infections in older children or adults, as well as secondary infections in all ages, are associated with more severe symptoms. Following an incubation period of 4–10 d after DENV infected mosquito bites, patients develop a high, sustained fever along with other symptoms including anorexia, nausea, headache, joint pain, and skin rash. Common laboratory findings include leucopenia and decreased platelet counts. The febrile period typically lasts 2–7 d. In the majority of cases, defervescence is followed by an uneventful recovery. In a minority of cases, plasma leakage occurs around the time of defervescence, resulting in pleural effusion or ascites. Plasma leakage is the main risk factor associated with disease severity, and the main feature distinguishing dengue fever (DF) from dengue hemorrhagic fever (DHF).Citation14,Citation15 Hemorrhagic manifestations are common in both DF and DHF, but tend to be more severe in DHF, particularly in cases with severe plasma leakage and shock. Severe hemorrhage occasionally occurs in DF (DF with hemorrhage). Isolated organ involvement in dengue, including encephalopathy, cardiac, and hepatic failure, has also been reported.Citation14-Citation17 However, the real incidence of isolated organ involvement in the absence of plasma leakage among all dengue cases is not known, since severe organ involvement can also be a consequence of poor organ perfusion associated with shock secondary to severe leakage.
Since the 1960s, clinical dengue has been classified into DF and DHF.Citation15 Dengue fever is defined as a febrile illness with 2 or more of the following: myalgia, arthralgia, retro-orbital pain, skin rash, hemorrhagic manifestation, and leukopenia. The diagnosis of DF requires laboratory or epidemiological evidence supportive of a dengue virus infection. The case definition of DHF is a febrile illness with thrombocytopenia (<100 000/mm3), hemorrhagic tendency, and plasma leakage. This classification scheme has been criticized for its applicability, usefulness, and reflection of dengue’s natural history. A new classification scheme has been suggested in the 2009 WHO guidelines based on a multicenter study conducted in Asia and the Americas.Citation18 In this new scheme, dengue is classified as non-severe dengue and severe dengue. The 2009 classification is currently being evaluated for its applicability. Since most studies have classified dengue based on the DF/DHF classification scheme until recently, this review will be based on the earlier classification.
Currently there are no specific treatments for dengue. Supportive treatment including fluid management to correct volume depletion and blood transfusion are the primary treatments. Since plasma leakage is the major cause of disease severity close monitoring for signs of plasma leakage and prompt fluid replacement is key to dengue care. Corticosteroids have been used without clear benefits in most studies in children.Citation19-Citation21
Risk factors for severe disease
Several epidemiological studies have identified previous exposure to DENV as an important risk factor for DHF when an individual experiences a secondary infection.Citation13,Citation22,Citation23 It has been postulated that antibodies specific to one DENV serotype fail to provide protection against the second DENV serotype. Rather, they enhance viral uptake and replication though an Fc receptor mediated mechanism. This notion is supported by in vitro and in vivo studies: the levels of virus in tissue culture supernatant and in circulation increase when infection occurs in the presence of DENV-specific antibodies.Citation24,Citation25 In addition, exaggerated, cross-reactive, cell-mediated immunity during a secondary infection has been documented, and likely contributes to disease severity.Citation4
Several host genetic polymorphisms in loci implicated in pathogen entry and host immune responses have been reported. These include DC-SIGN (CD209), HLA class I and class II, transporters associated with antigen presentation (TAPs), cytotoxic lymphocyte antigen 4 (CTLA4), Fc receptor, and soluble factors including TNF-α, TGF-β, and mannose binding lectin-2 (MBL-2).Citation26,Citation27 It is likely that each genetic locus may be associated with distinct aspects of dengue pathogenesis and the relevance of these polymorphisms to disease severity may vary in different populations.
Although there are currently no studies that precisely delineate viral factors that confer virulence, several outbreaks occurred when a new strain of DENV had been introduced into a new geographical area or when there was an emergence of a new viral strain.Citation28,Citation29 These outbreaks might have been the result of the lack of host immunity to the virus as well as the intrinsic virulence of the pathogen. Southeast Asian DENV2 that caused a major outbreak in the Americas in 1980–1990 replicated more efficiently in vitro compared with the preexisting American DENV2, and this has been attributed to the differences in the 3′ UTR and the coding regions of the viral genomes.Citation30 Specific mutations in various non-structural genes, such as NS-1 and NS4B, have also been associated with changes in viral replication in vitro and virulence in vivo.Citation31
Pathology and pathogenesis
DENV enters the host through the bites of infected mosquitoes. Experimental infection in non-human primates and mice, as well as ex vivo infection of human skin explants, has shown that different types of cells may be infected near the sites of inoculation, including keratinocytes, Langerhans cells, and dendritic cells (DC).Citation32-Citation34 Various host molecules that are involved in viral binding and uptake have been reported to bind DENV. In particular, C-type lectins, DC-sign (CD209), and CLEC 5A have been shown to interact with the envelope protein and play a role in viral entry.Citation35-Citation37 Human autopsy studies have demonstrated that the cells infected by DENV (as defined by detectable DENV antigen and/or genome) are mostly cells of the lymphoid and reticuloendothelial system, including monocytes, tissue macrophages, and lymphocytes.Citation38,Citation39 Other cells reported to express DENV antigen include lung endothelial cells, liver and spleen sinusoidal endothelial cells, and hepatocytes.Citation38,Citation39
There have been few human autopsy studies that provide systemic, detailed analyses of tissue pathology.Citation40 The most common pathological findings in DENV infection are tissue edema and mild perivascular mononuclear infiltration. Hemorrhage is a common autopsy finding in fatal cases, ranging from superficial petechiae of the skin, serosal, and mucosal surfaces, to focal hemorrhage of the gastrointestinal tract, lungs, and central nervous system. Perivascular edema and red blood cell diapedesis are observed, but structural changes of capillaries and postcapillary venules are not observed under routine histology.Citation40 Endothelial cell apoptosis has been detected using a molecular technique to detect DNA breakage.Citation41 However, the extent of the apoptosis appears to be quite limited. Fatty changes of the liver parenchyma to various extent have been reported, but studies are conflicting regarding infection of hepatocytes by DENV.Citation39,Citation41,Citation42 Periportal inflammation and focal areas of necrosis and apoptosis of liver cells have also been reported.Citation40,Citation41,Citation43 These findings are consistent with elevated liver enzyme levels commonly observed in dengue patients.Citation44
The roles of adaptive and innate immunity
The increased risk of DHF during secondary DENV infection underscores the important role of pre-existing DENV-specific immunity in disease severity. Antibodies specific to DENV from a previous exposure may enhance the uptake and replication of DENV of another serotype, leading to higher viral burden and severity. Consistent with this hypothesis, studies have shown higher viral burden (as measured by levels of viremia, viral RNA, or NS-1 antigen) in plasma of DHF cases compared with DF.Citation45-Citation47 These enhancing antibodies were recently shown to be largely specific to DENV prM protein.Citation48 During an acute secondary DENV infection, a massive expansion of DENV-specific B cells occurs at the time of defervescence; B cells have been shown to harbor the highest levels of DENV among peripheral blood mononuclear cells. Thus, DENV-specific B cells may be an important viral target during secondary infection.Citation49 DENV-specific antibodies may also enhance viral replication by an FcR-mediated immune suppression. A monocyte cell line, THP-1, produced IL-10 when infected with DENV immune complexes.Citation50 In addition, DENV immune complexes also disrupted RIG-I, MDA-5, and TLR signaling in these cells.Citation50,Citation51 Overall, B cells and antibodies likely participate in DENV pathogenesis through various mechanisms that lead to increased viral replication and viral burden.
Several studies have demonstrated more intense cellular immune activation in DHF compared with DF cases.Citation4,Citation52 Increased expression of activation markers and higher frequencies of DENV-specific T cells quantified using tetramer staining and functional assays have been demonstrated in DHF. Similar to B cell responses, memory T cells specific to the previously exposed DENV are preferentially expanded during a secondary infection.Citation4 A number of peptide epitopes have been identified and analyses of the responses to these epitopes have shown functional differences in cytolytic activity and patterns of cytokines produced by DENV specific T cells activated with peptide variants.Citation53,Citation54 Ex vivo analyses of T cells from dengue patients revealed decreased degranulation marker expression (a marker for cytolytic activity) and increased cytokine production when cross-reactive memory T cells were stimulated with epitope peptides of the infecting DENV serotype.Citation55 The enhanced cytokine response may play a critical role in the loss of vascular integrity, leading to plasma leakage.
DENV activates innate immunity and induces a cytokine cascade that likely plays both protective and pathogenic roles. Early cytokine response in DENV infection is characterized by the production of type I interferon (IFN). Multiple receptors, including TLR3, MDA5, and RIG-I, are involved in DENV activation of type I IFN response.Citation56 Several non-structural DENV proteins interfere with type I IFN signaling through various mechanisms, including degrading STAT2 by NS5, interfering with STAT1 phosphorylation by NS4B, and inhibiting type I IFN production by NS2B3 complex through STING degradation.Citation11,Citation57-Citation60 The impaired initial control of viral replication may lead to the higher viral burden observed in DHF cases.
Cellular mediators involved in disease severity
Several pro-inflammatory cytokines are produced by DENV-infected cells. DENV-infected monocytes and DC produce a number of mediators, such as interleukin 6 (IL-6), IL-8, and IFN-γ-inducible chemokines CXCL9, 10, and 11, with permeability-enhancing and chemoattractant properties.Citation61,Citation62 Elevated levels of these mediators have been documented in plasma/serum or pleural effusions of DHF cases.Citation63,Citation64 Immunomodulatory cytokine IL-10 can be produced by DENV infected monocytes/DC, and elevated IL-10 levels have been reported in DHF patients.Citation50,Citation52 TNF-α, a permeability-enhancing and pro-coagulation factor, has been extensively studied, but reports on TNF-α levels in severe DENV infection are conflicting.Citation65,Citation66 Enhanced activation of DENV-specific T cells likely contributes to the elevated cytokine levels in DHF. Multiple cytokines are secreted by DENV-specific T cells, including Th1 type cytokines IL-2, IFN-γ, TNF-α, and various chemokines.Citation53,Citation67 Elevated levels of both Th1- (IFN-γ) and Th2-type cytokines (IL-4, IL-13, and IL-10) have been reported in DHF.Citation68,Citation69 The potential contribution of these cytokines in the pathogenesis of DHF can only be speculated from their known biological activities.
The difficulties in interpreting cytokine level data in DENV infection are due to the heterogeneity in study designs, times of sample collection, differences in patient populations, and clinical classification. Further, the lack of animal models that completely reproduce the full spectrum of clinical DENV infection has been a major obstacle in understanding the relative roles of various mediators in DENV pathogenesis. Recent progress has been made using immunocompromised mice and/or mouse-adapted DENV. Some of these models exhibit clinical features similar to human disease, including fever, thrombocytopenia, rash, hemorrhage, and vascular leakage.Citation70,Citation71 However, pattern of plasma leakage in these models differ from human DHF. Studies using these models have implicated TNF-α in mediating plasma leakage and hemorrhage, possibly through the production of reactive oxygen species.Citation71,Citation72
Endothelial cells in severe DENV infection
Since plasma leakage is the central feature of severe DENV infection, endothelial cells have been speculated to play a key role in DENV pathogenesis. Autopsy studies in humans have reported perivascular edema with some mononuclear cell infiltration. Changes in endothelial cells, such as edema and pyknosis, were also found.Citation40 However, the degree and extent of these changes appeared to be limited. Although DENV antigen has been identified in endothelial cells in the lungs, liver, and spleen in humans and experimental mouse models, conclusive evidence of DENV infection in human endothelial cells is still lacking.Citation38,Citation39 In vitro, endothelial cells can be infected with DENV, leading to the release of chemokines, including IL-8, RANTES, and monocyte chemoattractant protein-1 (MCP-1).Citation61,Citation64 Increased cell surface expression of intercellular adhesion molecule-1 (ICAM-1) and β3-integrin, and cytokine receptor VEGF-R2 has been reported.Citation73,Citation74 Notably, the increase in VEGF-R2 expression was associated with an enhanced response to VEGF by DENV infected endothelial cells.Citation73 In vivo evidence supporting endothelial cell activation includes elevated circulating levels of soluble surface molecules expressed by endothelial cells, such as soluble intercellular adhesion molecule (sICAM-1) and vascular cell adhesion molecule (sVCAM-1).Citation75,Citation76 Changes in circulating levels of angiogenic factors, including VEGF, its soluble receptors VEGF-R1 and VEGF-R2, and angiopoietin-1 and angiopoietin-2 have been reported to correlate with DENV infection severity.Citation73,Citation77 In support of this notion, similar “pro-leakage” angiogenic profile; low levels of angiopoietin-1 and elevated levels of angiopoietin-2, have been shown in other conditions with plasma leakage such as sepsis and have been found to correlate with severity and outcomes.Citation78,Citation79 These changes may reflect the effects of DENV or the immune response on vascular and hemopoietic cells, such as endothelial cells and platelets, which are important sources of these factors. A number of studies have shown that endothelial cell permeability could be altered by interaction with DENV-infected cells, particularly monocytes. In these models, TNF-α, IL-8, and MCP-1 released from monocytes have been implicated as the key mediator in some studies.Citation80-Citation82 DENV-infected DCs have been demonstrated to secrete matrix metalloproteases (MMPs) that disrupt endothelial cell integrity and enhance permeability.Citation83 Taken together, endothelial cells may be directly affected by DENV or indirectly through the interaction with DENV-infected cells and/or activated immune cells, resulting in the loss in endothelial integrity. A proposed model for dengue pathogenesis is shown in .
Figure 1. A model for dengue pathogenesis. Infection of target cells (monocyte, dendritic cells) is enhanced in the presence of non-neutralizing, cross-reactive antibodies. The output viruses and viral proteins such as NS-1 may binds to DENV-specific antibodies and activate complement system leading the release of vasoactive complement fragments resulting functional and structural changes in endothelial cells (EC). Infected cells and activated T lymphocytes release various cytokines with permeability enhancing activities such as IL-6, IL-8, TNF-α, MCP-1, and VEGF. It is controversial whether EC are infected with DENV in vivo. However, in vitro infected EC have been shown to upregulate VEGF-R 2 expression and secrete various cytokines. Activation of EC by VEGF and other cytokines leads to disruption of adherens junctions, resulting in increased permeability.
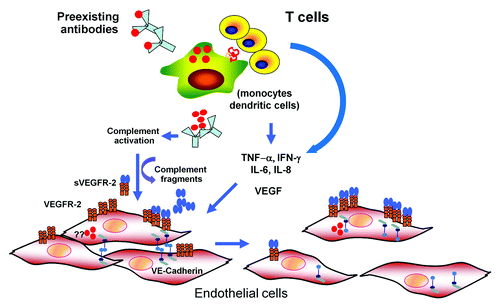
Many aspects of the coagulation pathway are affected during DENV infection which may predispose to bleeding tendency. Prolonged prothrombin and partial thromboplastin time, elevated levels of tissue factor, thrombomodulin, and decreased levels of Protein C and Protein S have been reported in severe dengue cases.Citation84,Citation85 These changes, in conjunction with reported increase in levels of active form of von Willebrand factor (VWF) and D-dimer, are consistent with an abnormal activation of the coagulation and fibrinolysis system.Citation84,Citation86 The mechanisms underlying these changes are complex and multiple. DENV-infected HUVEC upregulated tissue factor expression and downregulated thrombomodulin expression and protein C activity.Citation87-Citation89 Activation of endothelial cells either by DENV or by mediators such as TNF-α is likely the mechanism associated with the changes in coagulation in dengue.
A series of studies have demonstrated that DENV induces autoantibodies against endothelial cells, platelets, and molecules in the coagulation and fibrinolytic pathways in humans and experimental animals.Citation90-Citation92 The majority of these antibodies react to NS1 antigen, although some were E- and prM-specific.Citation93 These antibodies induce abnormal activation and function of platelets and endothelial cells, leading to endothelial cell apoptosis in vitro, and to mortality in experimental animals.Citation90,Citation92 It is unclear how long these antibodies persist after an acute DENV infection and how clinically important these antibodies are in DENV pathogenesis, since patients usually have a complete recovery without autoimmune manifestations as long-term complications, particularly in children. More definitive studies will be needed to clarify the role of autoantibodies in DENV pathogenesis.
Hantaviruses
Another group of hemorrhagic fever viruses associated with endothelial dysfunction is the hantaviruses. Hantaviruses are distributed worldwide, and are broadly split into Old World hantaviruses, which cause hemorrhagic fever with renal syndrome (HFRS), and the New World hantaviruses, which cause HPS. HFRS-causing hantaviruses are mainly present in Asia and Europe, and have a case fatality of 0.1–15%. HPS-causing hantaviruses are found throughout the Americas, and have much higher case fatality rates of 35–40%.Citation94 Old World hantaviruses were discovered to cause HFRS around the time of the Korean War, although the first hantavirus was not isolated until 1978. However, hantaviruses were not known to cause human disease in the Americas until Sin Nombre virus—which means “no name virus” in Spanish—was first identified as the cause of an outbreak of acute respiratory distress syndrome in the southwestern United States in 1993.Citation95 Since then, HPS has been an emerging infectious disease in the Americas, and more than 20 different HPS-associated hantaviruses have been identified.Citation96 The virus causing the highest burden of disease in South America is Andes virus (ANDV), the only known hantavirus implicated in human-to-human transmission.Citation97,Citation98 Hantaviruses are transmitted to humans by inhalation of infectious aerosols from rodent excreta.Citation99 Although a rare disease with a total of a few thousand cases reported, HPS remains in the spotlight because of the high case fatality rates. For example, a recent HPS outbreak in Yosemite park in California, in the summer of 2012, although modest in size with 10 confirmed cases and 3 deaths, caused international alarm, and hundreds of thousands of international visitors had to be notified by the National Park Services.Citation100 Currently, no FDA-approved immunotherapeutics, antivirals, or vaccines are available for use against HPS.Citation101
The hallmarks of both HFRS and HPS are increased vascular permeability and thrombocytopenia.Citation96,Citation102-Citation104 In both syndromes, patients may have pulmonary and/or renal involvement, though pulmonary involvement dominates in HPS, and renal involvement in HFRS. For the rest of the review, we will mainly focus on HPS-causing hantaviruses.
Hantaviruses have a single-stranded, negative-sense RNA genome consisting of 3 RNA segments: small (S), medium (M), and large (L). The term “negative-sense” refers to an RNA genome complementary to the mRNA encoding the viral proteins. The S segment varies from 1600–2004 nucleotides in length and encodes the viral nucleoprotein (N). The M RNA segment of hantaviruses is ~3600 nucleotides long, and encodes the mature Gn and Gc glycoproteins. The hantavirus L segment is approximately 6500 nucleotides, and encodes the virus L polymerase protein.Citation94,Citation105 The naked RNA genome is not infectious; an infectious virus unit requires the viral RNA to be encapsidated by the N protein, forming the nucleocapsid core associated with the viral L polymerase protein. To form a mature virus, this unit is then enveloped by the host cell membrane. Embedded in the membrane via the cytoplasmic tail of Gn are the two viral surface glycoproteins, Gn and Gc, which mediate viral attachment to cells.Citation106,Citation107
Hantavirus pulmonary syndrome
Like many acute viral infections, the initial symptoms for HPS are reported as a flu-like illness, with fever, myalgia, and headache, followed by severe gastrointestinal symptoms, such as abdominal pain, vomiting and diarrhea. This prodromal phase of HPS lasts around 5 d, during which these cases are difficult to distinguish from other viral infections. The second phase of the disease is very rapid and can progress over a few hours to acute pulmonary edema, severe respiratory syndrome, and shock. Patients require mechanical ventilation within the first 24 h. Extracorporeal membrane oxygenation support has been shown to improve the survival rates for patients with severe HPS.Citation108
The severe clinical impact of HPS is on the lungs, and is typically manifested as rapidly developing, diffuse non-cardiogenic pulmonary edema, followed in fatal cases by cardiogenic shock.Citation102,Citation109 The pulmonary manifestations are similar among hantaviruses, but differ subtly in presentation among diseases caused by the North and South American hantaviruses.Citation102,Citation110-Citation114 In addition to the pulmonary symptoms, South American hantaviruses often present with some hemorrhagic and renal manifistations,Citation111,Citation112,Citation114-Citation116 and viral antigens and infectious virus can also be detected in urine of HPS patients infected with ANDV.Citation117 In contrast to HPS caused by ANDV, however, hemorrhagic and renal symptoms are not a common characteristic of HPS caused by Sin Nombre virus.
Immune modulation in hantavirus pulmonary syndrome
In general, increased vascular permeability is an important component of severe disease progression in VHFs.Citation118 A number of studies have investigated the cause of increased vascular permeability in VHFs induced by viruses such as DENV or Ebola virus.Citation2,Citation3,Citation119-Citation123 The major hantavirus target is the microvascular endothelium, and severe human disease has been attributed to microvascular leakage. Initially, the innate immune system recognizes the virus, and proinflammatory cytokines and chemokines are induced. This is followed by the adaptive immune system, which consists of cytotoxic T cells and B cells that produce virus-specific antibodies. Although these responses are crucial for viral clearance, they can also damage the host if not properly regulated. It is a long-standing belief that the induction of an uncontrolled immune response to hantavirus infection and the generation of a cytokine storm, rather than the viral infection per se, causes microvascular leakage and HPS.Citation103,Citation124-Citation126 Evidence supporting the hypothesis that HPS is an immune-modulated disease is outlined below.
Histopathological examination of multiple organs has revealed the presence of small and enlarged mononuclear cell infiltrates, but no evidence of cell destruction despite prominent accumulation of viral antigen in the infected vascular endothelium. The major histopathological features in fatal HPS cases are observed mainly in the lung, and the infected lung macrovascular endothelium is uniformly infected with high viral titers.Citation103 Hantaviruses infect endothelial cells in various tissues throughout the body in addition to the lung endothelium.Citation95,Citation103 Large immunoblasts are in circulation at the onset of HPS, and while they are primarily concentrated in the lungs, they are also observed within the red pulp of the spleen and other lymphoid organs.Citation103,Citation127 Hantaviruses can also infect other human cell types, including monocytes and macrophagesCitation128,Citation129 and DCCitation130 without apparent cytopathic effects in vitro.
Hantaviruses induce a long-lasting humoral immune response. Initially, antibodies against hantavirus antigens are present in HPS patient sera at the time of disease onset, followed by neutralizing antibodies directed against the viral Gn glycoprotein.Citation131,Citation132 Production of IgM-specific antibodies occurs early during the clinical course of the disease followed by an increase in IgG antibody titers. However, unlike in DENV infection, the presence of anti-hantavirus antibodies does not correlate with an increased risk of severe disease. On the contrary, the presence of high-titer neutralizing antibodies in patient sera correlates with favorable disease outcomes.Citation133,Citation134
High levels of cytokines have been detected in HPS patients.Citation126,Citation135 Based on early immunocytochemical studies, several cytokines are detected, especially in lung tissues from patients with fatal HPS,Citation135 including those potentially produced by T cells, such as TNF-α, IL-2, IL-6, and IFN-γ. Both TNF-α and IL-2 can increase vascular permeability. In vitro studies have also shown the induction of chemokines, such as MCP-1, RANTES, and IP-10 in infected vascular endothelium.Citation136-Citation139 These chemokines can recruit immune cell infiltrates into the lung and other organs. Primary human DC infected with hantaviruses in vitro are quickly activated and secrete pro-inflammatory cytokines like TNF-α and an active form of MMP9.Citation140 These immune cells can then release more cytokines, intensifying the cytokine storm, further increasing vascular permeability, and inducing pulmonary edema.
Virus-specific CD8+ T cells correlate with disease severity. A robust T cell response is generated in humans during hantavirus infection, and is followed by a long-lived memory T cell response.Citation141 Several CD4+ and CD8+ T cell clones obtained from the blood of acute and convalescent hantavirus HPS patients recognize epitopes identified in hantavirus N, Gn, and Gc proteins.Citation141-Citation143 Surprisingly, the most immunodominant epitopes are located in the Gn glycoprotein.Citation141 Higher frequencies of virus-specific CD8+ T cells were detected in PBMCs from HPS patients than in patients with other virus infections, like DENV or influenza virus. Most importantly, these frequencies were higher in patients with severe HPS (hospitalized patients that required mechanical ventilation) than in patients with moderate disease (hospitalized patients that did not require mechanical ventilation).Citation143 These are strong indications that virus-specific CD8+ T cells play a role in virus-induced immunopathology.
Genetic factors associated with clinical outcomes in hantavirus infection have also been investigated in a few studies. Specific HLA alleles have been correlated with either mild or severe disease. In HPS patients infected with Sin Nombre virus, the HLA-B*3501 allele has been associated with severe disease.Citation143 However, in a more recent study of ANDV-infected patients, the HLA-B*35 allele was detected more frequently in patients with rather mild disease than those with severe HPS.Citation141 In addition, the polymorphic TNF-2 allele (308G/A) has been associated as a high-risk factor for developing HPS, but no connection has been found so far regarding this allele and disease severity.Citation144
Direct effects of hantavirus infection on the vascular endothelium
So far we have highlighted that hantavirus-infected vascular endothelium orchestrates the induced immune pathology by secreting chemokines that attract the monocytes, macrophages, and T cells to the vicinity of the virus. However, more recent studies show that hantavirus replication alone can compromise the integrity of the endothelial cell barrier.Citation145 Most importantly, hantavirus infection can sensitize the endothelium and cause vascular hyper-permeability in response to permeability factors like vascular endothelial growth factor (VEGF).Citation146-Citation148
Endothelial cell permeability is a highly regulated process and is maintained by both tight and adherens junctions. A transient disruption of adherens junctions is sufficient to disturb endothelial barrier function and increase permeability, leading to edema.Citation149-Citation151 Vascular endothelial cadherin (VE-cadherin) is an adherens junction adhesion protein that plays a central role in maintaining the vascular barrier.Citation152 In order to maintain the endothelial cell barrier, VE-cadherin interacts with the tyrosine kinase receptor VEGF-R2. However, when VEGF, a potent inducer of vascular permeability, is present, it binds to VEGF-R2, disassociating VE-cadherin and VEGF-R2.Citation153,Citation154 This can initiate a junction-dependent increase in permeability by a clathrin-dependent internalization and degradation of VE-cadherin and disruption of the adherens junctions. Infection of human primary lung endothelial cells with HPS-associated hantaviruses revealed a moderate early increase in secreted VEGF and a concomitant decrease in VE-cadherin.Citation145 This resulted in increased vascular permeability and loss of integrity of the endothelial cell barrier. Consistent with this process, antibody blockage of VEGF-R2 activation inhibited hantavirus-induced VE-cadherin reduction.Citation145
The most prominent cellular response to hantavirus infection, however, is the induction of the hyper-permeability of the microvascular endothelium. In vitro experiments have shown that adding VEGF to primary endothelial cell monolayers infected with pathogenic hantaviruses activated VEGF-R2 and Src kinase, and led to VE-cadherin internalization and degradation.Citation147,Citation155,Citation156 Adding factors such as Angiopoietin-1 and S1P, which inhibit VEGF-R2 induced permeability, blocked VE-cadherin internalization in response to VEGF.Citation147,Citation155-Citation157 In vitro studies have also shown that small molecule inhibitors which block the VEGFR2 or Src kinase, such as pazopanib and dasatinib, can block ANDV-induced hyper-permeability.Citation156 Increased amounts of VEGF in the serum of HPS patients have been reported.Citation145,Citation148
The results of the above studies suggest that agents that can block the activation of VEGF-R2 and Src kinases may be efficacious HPS treatments. Currently, FDA-approved small molecule inhibitors, particularly tyrosine kinase inhibitors such as vandetanib, are being used in studies utilizing the Syrian golden hamster HPS disease model to test such hypotheses.Citation101
A model of the activation of vascular endothelium by hantavirus infection
Increased vascular permeability observed in infections with pathogenic hantaviruses is certainly a multifactorial event. A strong immune response and release of permeability factors can contribute to increased vascular permeability and HPS induction. Viral infection of the endothelium can also initiate vascular leakage and the cascade of cell signaling events leading to hyper-permeability and disease. The following is our current model of what happens in the lung of an HPS patient based on the synthesis of our current knowledge of in vitro and animal studies (see ).
Figure 2. Proposed model for HPS pathogenesis. The exact means of entry of hantaviruses into the vascular endothelium is not known, but likely is via infected dendritic cells and/or infected alveolar macrophages (A). Infection of endothelial cells by hantavirus causes secretion of VEGF, triggering the disruption of adherens junctions and downregulation of VE-cadherin (see details in [D]). Hantavirus-infected endothelial cells also produce proinflammatory cytokines and chemokines, such as IP-10 and RANTES, and upregulate adhesion molecules on their cell surface, attracting monocytes, macrophages, and T cells. Accumulation of hantavirus infected monocytes and macrophages in the vicinity of the endothelium results in a “cytokine storm” by secreting additional chemokines/cytokines. Additional VEGF is secreted by hantavirus activated T cells, platelets, and macrophages. At this point VEGF could achieve high concentrations in the microvasculature of the lung, resulting in vascular hyper permeability and leakage (B). (C) Diagram of intact adherens junctions. (D) Diagram of disrupted adherens junctions.
![Figure 2. Proposed model for HPS pathogenesis. The exact means of entry of hantaviruses into the vascular endothelium is not known, but likely is via infected dendritic cells and/or infected alveolar macrophages (A). Infection of endothelial cells by hantavirus causes secretion of VEGF, triggering the disruption of adherens junctions and downregulation of VE-cadherin (see details in [D]). Hantavirus-infected endothelial cells also produce proinflammatory cytokines and chemokines, such as IP-10 and RANTES, and upregulate adhesion molecules on their cell surface, attracting monocytes, macrophages, and T cells. Accumulation of hantavirus infected monocytes and macrophages in the vicinity of the endothelium results in a “cytokine storm” by secreting additional chemokines/cytokines. Additional VEGF is secreted by hantavirus activated T cells, platelets, and macrophages. At this point VEGF could achieve high concentrations in the microvasculature of the lung, resulting in vascular hyper permeability and leakage (B). (C) Diagram of intact adherens junctions. (D) Diagram of disrupted adherens junctions.](/cms/asset/d6f5f350-5425-4d49-8440-fe66ad102735/kvir_a_10925569_f0002.gif)
First, hantaviruses infect the respiratory epithelium and/or the DC residing in the respiratory epithelium.Citation130 This is followed by viral dissemination, via infected DC, to monocytes and macrophages in the lymph nodes, and via the respiratory epithelium to the respiratory endothelium.Citation130,Citation158 Efficient viral replication and spread may occur early after infection due to viral inhibition of innate immune responses.Citation159,Citation160 Hantavirus-infected endothelial cells produce proinflammatory chemokines and upregulate adhesion molecules on their cell surface, attracting monocytes, macrophages, and T cells.Citation136,Citation137,Citation139 Infected monocytes and macrophages, in turn, produce additional chemokines, cytokines, and other proinflammatory molecules, like TNF-α and reactive oxygen/nitrogen species.Citation126,Citation128,Citation161-Citation164 Viral infection of the vascular endothelium and early VEGF secretion triggers the disruption of adherens junctions and downregulation of VE-cadherin. VEGF is secreted by virus-activated T cells, platelets, and macrophages in vivo.Citation124,Citation165-Citation167 Under these conditions, permeability factors (TNF-α and/or VEGF) from all these sources reach high concentrations in the microvasculature of the lung, resulting in vascular hyper-permeability and disease ().
Problems and Future Directions
Although DENV and hantaviruses are very distinct, they both cause VHF with notable similarities. The first similarity is the dysregulation the vascular endothelium, leading to increased macrovascular permeability and plasma extravasation. The increased vascular permeability is not based on a strong cytopathic effect on endothelial cells. Second, cell-mediated immunity contributes to disease severity; particularly, CD8+ T cells are likely play an important role in the pathogenesis of both infections. Finally, pro-inflammatory and permeability-promoting factors are produced secondary to a strong immune activation in infection with both viruses.
The continued expansion of the geographic and demographic range of dengue fever and HPS highlights the important global health threat posed by these viruses. The lack of specific treatments for these diseases underscores the urgent need for better insights into mechanisms underlying severe disease and protection. Additional in vitro studies are needed to identify the precise steps leading to virus-induced endothelial cell leakage. Although much progress has been made using animal studies, the applicability of the findings from these models to human disease is uncertain. In the case of DENV, better animal models that closely mimic human disease are still needed. For HPS, the Syrian golden hamster model recapitulates human disease quite well, but the disease is uniformly lethal, as is only seen with ANDV and not with other HPS viruses. Prospective studies which include well-characterized patients encompassing different genetic and demographic backgrounds are a prerequisite for further understanding the pathogenesis of both viruses. The application of novel multi-parametric assays and novel molecular platforms will facilitate more comprehensive and global analysis of the contribution of viral and host factors to disease development. Insights gained from such studies will be critical in developing vaccines and therapeutic interventions for these diseases.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention. The authors thank Dr T Klimova and Dr In-Kyu Yoon for critical reading of the manuscript, and Craig Manning for help with the illustration. Part of this work was supported by National Institutes of Health Grant NIH-P01AI34533. The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the view of the US Government.
Submitted
04/15/13
Revised
06/25/13
Accepted
06/27/13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Moraz ML, Kunz S. Pathogenesis of arenavirus hemorrhagic fevers. Expert Rev Anti Infect Ther 2011; 9:49 - 59; http://dx.doi.org/10.1586/eri.10.142; PMID: 21171877
- Wahl-Jensen VM, Afanasieva TA, Seebach J, Ströher U, Feldmann H, Schnittler HJ. Effects of Ebola virus glycoproteins on endothelial cell activation and barrier function. J Virol 2005; 79:10442 - 50; http://dx.doi.org/10.1128/JVI.79.16.10442-10450.2005; PMID: 16051836
- Yang ZY, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat Med 2000; 6:886 - 9; http://dx.doi.org/10.1038/78645; PMID: 10932225
- Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 2003; 9:921 - 7; http://dx.doi.org/10.1038/nm887; PMID: 12808447
- Amarasinghe A, Letson GW. Dengue in the Middle East: a neglected, emerging disease of importance. Trans R Soc Trop Med Hyg 2012; 106:1 - 2; http://dx.doi.org/10.1016/j.trstmh.2011.08.014; PMID: 22137535
- Baruah K, Singh PK, Mohalia MM, Dhariwal AC. A study on dengue outbreak during 2009 in Bhopal and Indore districts of Madhya Pradesh, India. J Commun Dis 2010; 42:273 - 9; PMID: 22471197
- Sinha N, Gupta N, Jhamb R, Gulati S, Kulkarni Ajit V. The 2006 dengue outbreak in Delhi, India. J Commun Dis 2008; 40:243 - 8; PMID: 19579715
- Franco C, Hynes NA, Bouri N, Henderson DA. The dengue threat to the United States. Biosecur Bioterror 2010; 8:273 - 6; http://dx.doi.org/10.1089/bsp.2010.0032; PMID: 20718665
- Morens DM, Fauci AS. Dengue and hemorrhagic fever: a potential threat to public health in the United States. JAMA 2008; 299:214 - 6; http://dx.doi.org/10.1001/jama.2007.31-a; PMID: 18182605
- Gubler D, Kuno G, Markoff L. Flavivirus, Field’s Virology. Lippincott Williams & Wilkins, 2007.
- Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, Gutman D, et al. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog 2012; 8:e1002934; http://dx.doi.org/10.1371/journal.ppat.1002934; PMID: 23055924
- Muñoz-Jordán JL, Laurent-Rolle M, Ashour J, Martínez-Sobrido L, Ashok M, Lipkin WI, et al. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol 2005; 79:8004 - 13; http://dx.doi.org/10.1128/JVI.79.13.8004-8013.2005; PMID: 15956546
- Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, et al. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol 2002; 156:40 - 51; http://dx.doi.org/10.1093/aje/kwf005; PMID: 12076887
- Srikiatkhachorn A, Green S. Markers of dengue disease severity. Curr Top Microbiol Immunol 2010; 338:67 - 82; http://dx.doi.org/10.1007/978-3-642-02215-9_6; PMID: 19802579
- WHO. Dengue hemorrhagic fever: diagnosis, treatment, prevention and control. Geneva: WHO, 1997.
- Cam BV, Fonsmark L, Hue NB, Phuong NT, Poulsen A, Heegaard ED. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg 2001; 65:848 - 51; PMID: 11791985
- Marques N, Gan VC, Leo YS. Dengue myocarditis in Singapore: two case reports. Infection 2013; 41:709 - 14; http://dx.doi.org/10.1007/s15010-012-0392-9; PMID: 23277366
- Alexander N, Balmaseda A, Coelho IC, Dimaano E, Hien TT, Hung NT, et al, European Union, World Health Organization (WHO‐TDR) supported DENCO Study Group. Multicentre prospective study on dengue classification in four South-east Asian and three Latin American countries. Trop Med Int Health 2011; 16:936 - 48; http://dx.doi.org/10.1111/j.1365-3156.2011.02793.x; PMID: 21624014
- Sumarmo TW, Talogo W, Asrin A, Isnuhandojo B, Sahudi A. Failure of hydrocortisone to affect outcome in dengue shock syndrome. Pediatrics 1982; 69:45 - 9; PMID: 7054760
- Tam DT, Ngoc TV, Tien NT, Kieu NT, Thuy TT, Thanh LT, et al. Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: a randomized, placebo-controlled trial. Clin Infect Dis 2012; 55:1216 - 24; http://dx.doi.org/10.1093/cid/cis655; PMID: 22865871
- Tassniyom S, Vasanawathana S, Chirawatkul A, Rojanasuphot S. Failure of high-dose methylprednisolone in established dengue shock syndrome: a placebo-controlled, double-blind study. Pediatrics 1993; 92:111 - 5; PMID: 8516054
- Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg 1988; 38:172 - 80; PMID: 3341519
- Bravo JR, Guzmán MG, Kouri GP. Why dengue haemorrhagic fever in Cuba? 1. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS). Trans R Soc Trop Med Hyg 1987; 81:816 - 20; http://dx.doi.org/10.1016/0035-9203(87)90041-1; PMID: 3450004
- Halstead SB. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis 1979; 140:527 - 33; http://dx.doi.org/10.1093/infdis/140.4.527; PMID: 117061
- Halstead SB. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis 1989; 11:Suppl 4 S830 - 9; http://dx.doi.org/10.1093/clinids/11.Supplement_4.S830; PMID: 2665015
- Stephens HA. HLA and other gene associations with dengue disease severity. Curr Top Microbiol Immunol 2010; 338:99 - 114; http://dx.doi.org/10.1007/978-3-642-02215-9_8; PMID: 19802581
- Perez AB, Sierra B, Garcia G, Aguirre E, Babel N, Alvarez M, et al. Tumor necrosis factor-alpha, transforming growth factor-β1, and interleukin-10 gene polymorphisms: implication in protection or susceptibility to dengue hemorrhagic fever. Hum Immunol 2010; 71:1135 - 40; http://dx.doi.org/10.1016/j.humimm.2010.08.004; PMID: 20732366
- OhAinle M, Balmaseda A, Macalalad AR, Tellez Y, Zody MC, Saborío S, et al. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med 2011; 3:ra128; http://dx.doi.org/10.1126/scitranslmed.3003084; PMID: 22190239
- Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, et al. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology 1997; 230:244 - 51; http://dx.doi.org/10.1006/viro.1997.8504; PMID: 9143280
- Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Villalobos I, de Chacon, et al. Dengue virus structural differences that correlate with pathogenesis. J Virol 1999; 73:4738 - 47; PMID: 10233934
- Grant D, Tan GK, Qing M, Ng JK, Yip A, Zou G, et al. A single amino acid in nonstructural protein NS4B confers virulence to dengue virus in AG129 mice through enhancement of viral RNA synthesis. J Virol 2011; 85:7775 - 87; http://dx.doi.org/10.1128/JVI.00665-11; PMID: 21632767
- Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, et al. Human skin Langerhans cells are targets of dengue virus infection. Nat Med 2000; 6:816 - 20; http://dx.doi.org/10.1038/77553; PMID: 10888933
- Taweechaisupapong S, Sriurairatana S, Angsubhakorn S, Yoksan S, Bhamarapravati N. In vivo and in vitro studies on the morphological change in the monkey epidermal Langerhans cells following exposure to dengue 2 (16681) virus. Southeast Asian J Trop Med Public Health 1996; 27:664 - 72; PMID: 9253864
- Limon-Flores AY, Perez-Tapia M, Estrada-Garcia I, Vaughan G, Escobar-Gutierrez A, Calderon-Amador J, et al. Dengue virus inoculation to human skin explants: an effective approach to assess in situ the early infection and the effects on cutaneous dendritic cells. Int J Exp Pathol 2005; 86:323 - 34; http://dx.doi.org/10.1111/j.0959-9673.2005.00445.x; PMID: 16191104
- Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med 2003; 197:823 - 9; http://dx.doi.org/10.1084/jem.20021840; PMID: 12682107
- Chen ST, Lin YL, Huang MT, Wu MF, Cheng SC, Lei HY, et al. CLEC5A is critical for dengue-virus-induced lethal disease. Nature 2008; 453:672 - 6; http://dx.doi.org/10.1038/nature07013; PMID: 18496526
- Watson AA, Lebedev AA, Hall BA, Fenton-May AE, Vagin AA, Dejnirattisai W, et al. Structural flexibility of the macrophage dengue virus receptor CLEC5A: implications for ligand binding and signaling. J Biol Chem 2011; 286:24208 - 18; http://dx.doi.org/10.1074/jbc.M111.226142; PMID: 21566123
- Balsitis SJ, Coloma J, Castro G, Alava A, Flores D, McKerrow JH, et al. Tropism of dengue virus in mice and humans defined by viral nonstructural protein 3-specific immunostaining. Am J Trop Med Hyg 2009; 80:416 - 24; PMID: 19270292
- Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis 2004; 189:1411 - 8; http://dx.doi.org/10.1086/383043; PMID: 15073678
- Bhamarapravati N, Tuchinda P, Boonyapaknavik V. Pathology of Thailand haemorrhagic fever: a study of 100 autopsy cases. Ann Trop Med Parasitol 1967; 61:500 - 10; PMID: 5634140
- Limonta D, Capó V, Torres G, Pérez AB, Guzmán MG. Apoptosis in tissues from fatal dengue shock syndrome. J Clin Virol 2007; 40:50 - 4; http://dx.doi.org/10.1016/j.jcv.2007.04.024; PMID: 17693133
- Kangwanpong D, Bhamarapravati N, Lucia HL. Diagnosing dengue virus infection in archived autopsy tissues by means of the in situ PCR method: a case report. Clin Diagn Virol 1995; 3:165 - 72; http://dx.doi.org/10.1016/0928-0197(94)00032-P; PMID: 15566798
- Couvelard A, Marianneau P, Bedel C, Drouet MT, Vachon F, Hénin D, et al. Report of a fatal case of dengue infection with hepatitis: demonstration of dengue antigens in hepatocytes and liver apoptosis. Hum Pathol 1999; 30:1106 - 10; http://dx.doi.org/10.1016/S0046-8177(99)90230-7; PMID: 10492047
- Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, et al. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis 1997; 176:313 - 21; http://dx.doi.org/10.1086/514047; PMID: 9237695
- Libraty DH, Endy TP, Houng HS, Green S, Kalayanarooj S, Suntayakorn S, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis 2002; 185:1213 - 21; http://dx.doi.org/10.1086/340365; PMID: 12001037
- Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 2002; 186:1165 - 8; http://dx.doi.org/10.1086/343813; PMID: 12355369
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 2000; 181:2 - 9; http://dx.doi.org/10.1086/315215; PMID: 10608744
- Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010; 328:745 - 8; http://dx.doi.org/10.1126/science.1185181; PMID: 20448183
- Srikiatkhachorn A, Wichit S, Gibbons RV, Green S, Libraty DH, Endy TP, et al. Dengue viral RNA levels in peripheral blood mononuclear cells are associated with disease severity and preexisting dengue immune status. PLoS One 2012; 7:e51335; http://dx.doi.org/10.1371/journal.pone.0051335; PMID: 23284680
- Ubol S, Phuklia W, Kalayanarooj S, Modhiran N. Mechanisms of immune evasion induced by a complex of dengue virus and preexisting enhancing antibodies. J Infect Dis 2010; 201:923 - 35; http://dx.doi.org/10.1086/651018; PMID: 20158392
- Modhiran N, Kalayanarooj S, Ubol S. Subversion of innate defenses by the interplay between DENV and pre-existing enhancing antibodies: TLRs signaling collapse. PLoS Negl Trop Dis 2010; 4:e924; http://dx.doi.org/10.1371/journal.pntd.0000924; PMID: 21200427
- Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, et al. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. J Med Virol 1999; 59:329 - 34; http://dx.doi.org/10.1002/(SICI)1096-9071(199911)59:3<329::AID-JMV12>3.0.CO;2-G; PMID: 10502265
- Mangada MM, Rothman AL. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J Immunol 2005; 175:2676 - 83; PMID: 16081844
- Friberg H, Burns L, Woda M, Kalayanarooj S, Endy TP, Stephens HA, et al. Memory CD8+ T cells from naturally acquired primary dengue virus infection are highly cross-reactive. Immunol Cell Biol 2011; 89:122 - 9; http://dx.doi.org/10.1038/icb.2010.61; PMID: 20421879
- Mongkolsapaya J, Duangchinda T, Dejnirattisai W, Vasanawathana S, Avirutnan P, Jairungsri A, et al. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal?. J Immunol 2006; 176:3821 - 9; PMID: 16517753
- Nasirudeen AM, Wong HH, Thien P, Xu S, Lam KP, Liu DX. RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLoS Negl Trop Dis 2011; 5:e926; http://dx.doi.org/10.1371/journal.pntd.0000926; PMID: 21245912
- Jones M, Davidson A, Hibbert L, Gruenwald P, Schlaak J, Ball S, et al. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J Virol 2005; 79:5414 - 20; http://dx.doi.org/10.1128/JVI.79.9.5414-5420.2005; PMID: 15827155
- Yu CY, Chang TH, Liang JJ, Chiang RL, Lee YL, Liao CL, et al. Dengue virus targets the adaptor protein MITA to subvert host innate immunity. PLoS Pathog 2012; 8:e1002780; http://dx.doi.org/10.1371/journal.ppat.1002780; PMID: 22761576
- Ashour J, Morrison J, Laurent-Rolle M, Belicha-Villanueva A, Plumlee CR, Bernal-Rubio D, et al. Mouse STAT2 restricts early dengue virus replication. Cell Host Microbe 2010; 8:410 - 21; http://dx.doi.org/10.1016/j.chom.2010.10.007; PMID: 21075352
- Rodriguez-Madoz JR, Belicha-Villanueva A, Bernal-Rubio D, Ashour J, Ayllon J, Fernandez-Sesma A. Inhibition of the type I interferon response in human dendritic cells by dengue virus infection requires a catalytically active NS2B3 complex. J Virol 2010; 84:9760 - 74; http://dx.doi.org/10.1128/JVI.01051-10; PMID: 20660196
- Bosch I, Xhaja K, Estevez L, Raines G, Melichar H, Warke RV, et al. Increased production of interleukin-8 in primary human monocytes and in human epithelial and endothelial cell lines after dengue virus challenge. J Virol 2002; 76:5588 - 97; http://dx.doi.org/10.1128/JVI.76.11.5588-5597.2002; PMID: 11991987
- Dejnirattisai W, Duangchinda T, Lin CL, Vasanawathana S, Jones M, Jacobs M, et al. A complex interplay among virus, dendritic cells, T cells, and cytokines in dengue virus infections. J Immunol 2008; 181:5865 - 74; PMID: 18941175
- Huang KJ, Li SY, Chen SC, Liu HS, Lin YS, Yeh TM, et al. Manifestation of thrombocytopenia in dengue-2-virus-infected mice. J Gen Virol 2000; 81:2177 - 82; PMID: 10950974
- Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol 1998; 161:6338 - 46; PMID: 9834124
- Azeredo EL, Zagne SM, Alvarenga AR, Nogueira RM, Kubelka CF, de Oliveira-Pinto LM. Activated peripheral lymphocytes with increased expression of cell adhesion molecules and cytotoxic markers are associated with dengue fever disease. Mem Inst Oswaldo Cruz 2006; 101:437 - 49; http://dx.doi.org/10.1590/S0074-02762006000400016; PMID: 16951817
- Braga EL, Moura P, Pinto LM, Ignácio SR, Oliveira MJ, Cordeiro MT, et al. Detection of circulant tumor necrosis factor-alpha, soluble tumor necrosis factor p75 and interferon-gamma in Brazilian patients with dengue fever and dengue hemorrhagic fever. Mem Inst Oswaldo Cruz 2001; 96:229 - 32; http://dx.doi.org/10.1590/S0074-02762001000200015; PMID: 11285501
- Bashyam HS, Green S, Rothman AL. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J Immunol 2006; 176:2817 - 24; PMID: 16493038
- Bozza FA, Cruz OG, Zagne SM, Azeredo EL, Nogueira RM, Assis EF, et al. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect Dis 2008; 8:86; http://dx.doi.org/10.1186/1471-2334-8-86; PMID: 18578883
- Butthep P, Chunhakan S, Yoksan S, Tangnararatchakit K, Chuansumrit A. Alteration of cytokines and chemokines during febrile episodes associated with endothelial cell damage and plasma leakage in dengue hemorrhagic fever. Pediatr Infect Dis J 2012; 31:e232 - 8; http://dx.doi.org/10.1097/INF.0b013e31826fd456; PMID: 22926216
- Mota J, Rico-Hesse R. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J Virol 2009; 83:8638 - 45; http://dx.doi.org/10.1128/JVI.00581-09; PMID: 19535452
- Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol 2006; 80:10208 - 17; http://dx.doi.org/10.1128/JVI.00062-06; PMID: 17005698
- Wu-Hsieh BA, Yen YT, Chen HC. Dengue hemorrhage in a mouse model. Ann N Y Acad Sci 2009; 1171:Suppl 1 E42 - 7; http://dx.doi.org/10.1111/j.1749-6632.2009.05053.x; PMID: 19751401
- Srikiatkhachorn A, Ajariyakhajorn C, Endy TP, Kalayanarooj S, Libraty DH, Green S, et al. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic Fever. J Virol 2007; 81:1592 - 600; http://dx.doi.org/10.1128/JVI.01642-06; PMID: 17151115
- Zhang JL, Wang JL, Gao N, Chen ZT, Tian YP, An J. Up-regulated expression of beta3 integrin induced by dengue virus serotype 2 infection associated with virus entry into human dermal microvascular endothelial cells. Biochem Biophys Res Commun 2007; 356:763 - 8; http://dx.doi.org/10.1016/j.bbrc.2007.03.051; PMID: 17382900
- Cardier JE, Rivas B, Romano E, Rothman AL, Perez-Perez C, Ochoa M, et al. Evidence of vascular damage in dengue disease: demonstration of high levels of soluble cell adhesion molecules and circulating endothelial cells. Endothelium 2006; 13:335 - 40; http://dx.doi.org/10.1080/10623320600972135; PMID: 17090406
- Koraka P, Murgue B, Deparis X, Van Gorp EC, Setiati TE, Osterhaus AD, et al. Elevation of soluble VCAM-1 plasma levels in children with acute dengue virus infection of varying severity. J Med Virol 2004; 72:445 - 50; http://dx.doi.org/10.1002/jmv.20007; PMID: 14748068
- Michels M, van der Ven AJ, Djamiatun K, Fijnheer R, de Groot PG, Griffioen AW, et al. Imbalance of angiopoietin-1 and angiopoetin-2 in severe dengue and relationship with thrombocytopenia, endothelial activation, and vascular stability. Am J Trop Med Hyg 2012; 87:943 - 6; http://dx.doi.org/10.4269/ajtmh.2012.12-0020; PMID: 22949515
- Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 2006; 3:e46; http://dx.doi.org/10.1371/journal.pmed.0030046; PMID: 16417407
- Ricciuto DR, dos Santos CC, Hawkes M, Toltl LJ, Conroy AL, Rajwans N, et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med 2011; 39:702 - 10; http://dx.doi.org/10.1097/CCM.0b013e318206d285; PMID: 21242795
- Dewi BE, Takasaki T, Kurane I. In vitro assessment of human endothelial cell permeability: effects of inflammatory cytokines and dengue virus infection. J Virol Methods 2004; 121:171 - 80; http://dx.doi.org/10.1016/j.jviromet.2004.06.013; PMID: 15381354
- Kelley JF, Kaufusi PH, Nerurkar VR. Dengue hemorrhagic fever-associated immunomediators induced via maturation of dengue virus nonstructural 4B protein in monocytes modulate endothelial cell adhesion molecules and human microvascular endothelial cells permeability. Virology 2012; 422:326 - 37; http://dx.doi.org/10.1016/j.virol.2011.10.030; PMID: 22129847
- Lee YR, Liu MT, Lei HY, Liu CC, Wu JM, Tung YC, et al. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J Gen Virol 2006; 87:3623 - 30; http://dx.doi.org/10.1099/vir.0.82093-0; PMID: 17098977
- Luplertlop N, Missé D, Bray D, Deleuze V, Gonzalez JP, Leardkamolkarn V, et al. Dengue-virus-infected dendritic cells trigger vascular leakage through metalloproteinase overproduction. EMBO Rep 2006; 7:1176 - 81; http://dx.doi.org/10.1038/sj.embor.7400814; PMID: 17028575
- Sosothikul D, Seksarn P, Pongsewalak S, Thisyakorn U, Lusher J. Activation of endothelial cells, coagulation and fibrinolysis in children with Dengue virus infection. Thromb Haemost 2007; 97:627 - 34; PMID: 17393026
- Wills BA, Oragui EE, Stephens AC, Daramola OA, Dung NM, Loan HT, et al. Coagulation abnormalities in dengue hemorrhagic Fever: serial investigations in 167 Vietnamese children with Dengue shock syndrome. Clin Infect Dis 2002; 35:277 - 85; http://dx.doi.org/10.1086/341410; PMID: 12115093
- Djamiatun K, van der Ven AJ, de Groot PG, Faradz SM, Hapsari D, Dolmans WM, et al. Severe dengue is associated with consumption of von Willebrand factor and its cleaving enzyme ADAMTS-13. PLoS Negl Trop Dis 2012; 6:e1628; http://dx.doi.org/10.1371/journal.pntd.0001628; PMID: 22563509
- Cabello-Gutiérrez C, Manjarrez-Zavala ME, Huerta-Zepeda A, Cime-Castillo J, Monroy-Martínez V, Correa BB, et al. Modification of the cytoprotective protein C pathway during Dengue virus infection of human endothelial vascular cells. Thromb Haemost 2009; 101:916 - 28; PMID: 19404546
- Huerta-Zepeda A, Cabello-Gutiérrez C, Cime-Castillo J, Monroy-Martínez V, Manjarrez-Zavala ME, Gutiérrez-Rodríguez M, et al. Crosstalk between coagulation and inflammation during Dengue virus infection. Thromb Haemost 2008; 99:936 - 43; PMID: 18449425
- Jiang Z, Tang X, Xiao R, Jiang L, Chen X. Dengue virus regulates the expression of hemostasis-related molecules in human vein endothelial cells. J Infect 2007; 55:e23 - 8; http://dx.doi.org/10.1016/j.jinf.2007.04.351; PMID: 17573116
- Chuang YC, Lei HY, Lin YS, Liu HS, Wu HL, Yeh TM. Dengue virus-induced autoantibodies bind to plasminogen and enhance its activation. J Immunol 2011; 187:6483 - 90; http://dx.doi.org/10.4049/jimmunol.1102218; PMID: 22079981
- Lin CF, Lei HY, Shiau AL, Liu CC, Liu HS, Yeh TM, et al. Antibodies from dengue patient sera cross-react with endothelial cells and induce damage. J Med Virol 2003; 69:82 - 90; http://dx.doi.org/10.1002/jmv.10261; PMID: 12436482
- Lin CF, Lei HY, Shiau AL, Liu HS, Yeh TM, Chen SH, et al. Endothelial cell apoptosis induced by antibodies against dengue virus nonstructural protein 1 via production of nitric oxide. J Immunol 2002; 169:657 - 64; PMID: 12097367
- Lin YS, Yeh TM, Lin CF, Wan SW, Chuang YC, Hsu TK, et al. Molecular mimicry between virus and host and its implications for dengue disease pathogenesis. Exp Biol Med (Maywood) 2011; 236:515 - 23; http://dx.doi.org/10.1258/ebm.2011.010339; PMID: 21502191
- Schmaljohn CS, Nichol ST. Bunyaviridae. In: Knipe DM, Howley PM, eds. Fields Virology. 5th ed. Philadelphia, Pa: Lippincott-Raven, 2007:1741-89.
- Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, et al. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science 1993; 262:914 - 7; http://dx.doi.org/10.1126/science.8235615; PMID: 8235615
- Hjelle B, Torres-Pérez F. Hantaviruses in the americas and their role as emerging pathogens. Viruses 2010; 2:2559 - 86; http://dx.doi.org/10.3390/v2122559; PMID: 21994631
- Jonsson CB, Hooper J, Mertz G. Treatment of hantavirus pulmonary syndrome. Antiviral Res 2008; 78:162 - 9; http://dx.doi.org/10.1016/j.antiviral.2007.10.012; PMID: 18093668
- Martinez VP, Bellomo C, San Juan J, Pinna D, Forlenza R, Elder M, et al. Person-to-person transmission of Andes virus. Emerg Infect Dis 2005; 11:1848 - 53; http://dx.doi.org/10.3201/eid1112.050501; PMID: 16485469
- Schmaljohn C, Hjelle B. Hantaviruses: a global disease problem. Emerg Infect Dis 1997; 3:95 - 104; http://dx.doi.org/10.3201/eid0302.970202; PMID: 9204290
- Centers for Disease Control and Prevention (CDC). Hantavirus pulmonary syndrome in visitors to a national park--Yosemite Valley, California, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:952; PMID: 23169317
- Dolgin E. Hantavirus treatments advance amidst outbreak in US park. Nat Med 2012; 18:1448; http://dx.doi.org/10.1038/nm1012-1448a; PMID: 23042337
- Duchin JS, Koster FT, Peters CJ, Simpson GL, Tempest B, Zaki SR, et al, The Hantavirus Study Group. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N Engl J Med 1994; 330:949 - 55; http://dx.doi.org/10.1056/NEJM199404073301401; PMID: 8121458
- Zaki SR, Greer PW, Coffield LM, Goldsmith CS, Nolte KB, Foucar K, et al. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am J Pathol 1995; 146:552 - 79; PMID: 7887439
- Macneil A, Nichol ST, Spiropoulou CF. Hantavirus pulmonary syndrome. Virus Res 2011; 162:138 - 47; http://dx.doi.org/10.1016/j.virusres.2011.09.017; PMID: 21945215
- Spiropoulou CF, Morzunov S, Feldmann H, Sanchez A, Peters CJ, Nichol ST. Genome structure and variability of a virus causing hantavirus pulmonary syndrome. Virology 1994; 200:715 - 23; http://dx.doi.org/10.1006/viro.1994.1235; PMID: 8178455
- Spiropoulou CF. Hantavirus maturation. Curr Top Microbiol Immunol 2001; 256:33 - 46; http://dx.doi.org/10.1007/978-3-642-56753-7_3; PMID: 11217405
- Spiropoulou CF, Goldsmith CS, Shoemaker TR, Peters CJ, Compans RW. Sin Nombre virus glycoprotein trafficking. Virology 2003; 308:48 - 63; http://dx.doi.org/10.1016/S0042-6822(02)00092-2; PMID: 12706089
- Dietl CA, Wernly JA, Pett SB, Yassin SF, Sterling JP, Dragan R, et al. Extracorporeal membrane oxygenation support improves survival of patients with severe Hantavirus cardiopulmonary syndrome. J Thorac Cardiovasc Surg 2008; 135:579 - 84; http://dx.doi.org/10.1016/j.jtcvs.2007.11.020; PMID: 18329474
- Khan AS, Khabbaz RF, Armstrong LR, Holman RC, Bauer SP, Graber J, et al. Hantavirus pulmonary syndrome: the first 100 US cases. J Infect Dis 1996; 173:1297 - 303; http://dx.doi.org/10.1093/infdis/173.6.1297; PMID: 8648200
- Ketai LH, Williamson MR, Telepak RJ, Levy H, Koster FT, Nolte KB, et al. Hantavirus pulmonary syndrome: radiographic findings in 16 patients. Radiology 1994; 191:665 - 8; PMID: 8184043
- Williams RJ, Bryan RT, Mills JN, Palma RE, Vera I, De Velasquez F, et al. An outbreak of hantavirus pulmonary syndrome in western Paraguay. Am J Trop Med Hyg 1997; 57:274 - 82; PMID: 9311636
- Castillo C, Naranjo J, Sepúlveda A, Ossa G, Levy H. Hantavirus pulmonary syndrome due to Andes virus in Temuco, Chile: clinical experience with 16 adults. Chest 2001; 120:548 - 54; http://dx.doi.org/10.1378/chest.120.2.548; PMID: 11502657
- Boroja M, Barrie JR, Raymond GS. Radiographic findings in 20 patients with Hantavirus pulmonary syndrome correlated with clinical outcome. AJR Am J Roentgenol 2002; 178:159 - 63; http://dx.doi.org/10.2214/ajr.178.1.1780159; PMID: 11756112
- Riquelme R, Riquelme M, Torres A, Rioseco ML, Vergara JA, Scholz L, et al. Hantavirus pulmonary syndrome, southern Chile. Emerg Infect Dis 2003; 9:1438 - 43; http://dx.doi.org/10.3201/eid0911.020798; PMID: 14718088
- Toro J, Vega JD, Khan AS, Mills JN, Padula P, Terry W, et al. An outbreak of hantavirus pulmonary syndrome, Chile, 1997. Emerg Infect Dis 1998; 4:687 - 94; http://dx.doi.org/10.3201/eid0404.980425; PMID: 9866751
- Padula P, Martinez VP, Bellomo C, Maidana S, San Juan J, Tagliaferri P, et al. Pathogenic hantaviruses, northeastern Argentina and eastern Paraguay. Emerg Infect Dis 2007; 13:1211 - 4; http://dx.doi.org/10.3201/eid1308.061090; PMID: 17953094
- Godoy P, Marsac D, Stefas E, Ferrer P, Tischler ND, Pino K, et al. Andes virus antigens are shed in urine of patients with acute hantavirus cardiopulmonary syndrome. J Virol 2009; 83:5046 - 55; http://dx.doi.org/10.1128/JVI.02409-08; PMID: 19279096
- Peters CJ, Zaki SR. Role of the endothelium in viral hemorrhagic fevers. Crit Care Med 2002; 30:Suppl S268 - 73; http://dx.doi.org/10.1097/00003246-200205001-00016; PMID: 12004247
- Srikiatkhachorn A, Krautrachue A, Ratanaprakarn W, Wongtapradit L, Nithipanya N, Kalayanarooj S, et al. Natural history of plasma leakage in dengue hemorrhagic fever: a serial ultrasonographic study. Pediatr Infect Dis J 2007; 26:283 - 90, discussion 291-2; http://dx.doi.org/10.1097/01.inf.0000258612.26743.10; PMID: 17414388
- Srikiatkhachorn A. Plasma leakage in dengue haemorrhagic fever. Thromb Haemost 2009; 102:1042 - 9; PMID: 19967133
- Tseng CS, Lo HW, Teng HC, Lo WC, Ker CG. Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol 2005; 43:99 - 102; http://dx.doi.org/10.1016/j.femsim.2004.10.004; PMID: 15607642
- Dalrymple NA, Mackow ER. Endothelial cells elicit immune-enhancing responses to dengue virus infection. J Virol 2012; 86:6408 - 15; http://dx.doi.org/10.1128/JVI.00213-12; PMID: 22496214
- Schnittler HJ, Feldmann H. Viral hemorrhagic fever--a vascular disease?. Thromb Haemost 2003; 89:967 - 72; PMID: 12783108
- Terajima M, Hayasaka D, Maeda K, Ennis FA. Immunopathogenesis of hantavirus pulmonary syndrome and hemorrhagic fever with renal syndrome: Do CD8+ T cells trigger capillary leakage in viral hemorrhagic fevers?. Immunol Lett 2007; 113:117 - 20; http://dx.doi.org/10.1016/j.imlet.2007.08.003; PMID: 17897725
- Borges AA, Campos GM, Moreli ML, Souza RL, Aquino VH, Saggioro FP, et al. Hantavirus cardiopulmonary syndrome: immune response and pathogenesis. Microbes Infect 2006; 8:2324 - 30; http://dx.doi.org/10.1016/j.micinf.2006.04.019; PMID: 16793309
- Borges AA, Campos GM, Moreli ML, Moro Souza RL, Saggioro FP, Figueiredo GG, et al. Role of mixed Th1 and Th2 serum cytokines on pathogenesis and prognosis of hantavirus pulmonary syndrome. Microbes Infect 2008; 10:1150 - 7; http://dx.doi.org/10.1016/j.micinf.2008.06.006; PMID: 18606242
- Koster F, Foucar K, Hjelle B, Scott A, Chong YY, Larson R, et al. Rapid presumptive diagnosis of hantavirus cardiopulmonary syndrome by peripheral blood smear review. Am J Clin Pathol 2001; 116:665 - 72; http://dx.doi.org/10.1309/CNWF-DC72-QYMR-M8DA; PMID: 11710682
- Markotić A, Hensley L, Daddario K, Spik K, Anderson K, Schmaljohn C. Pathogenic hantaviruses elicit different immunoreactions in THP-1 cells and primary monocytes and induce differentiation of human monocytes to dendritic-like cells. Coll Antropol 2007; 31:1159 - 67; PMID: 18217475
- Temonen M, Vapalahti O, Holthöfer H, Brummer-Korvenkontio M, Vaheri A, Lankinen H. Susceptibility of human cells to Puumala virus infection. J Gen Virol 1993; 74:515 - 8; http://dx.doi.org/10.1099/0022-1317-74-3-515; PMID: 8445370
- Raftery MJ, Kraus AA, Ulrich R, Krüger DH, Schönrich G. Hantavirus infection of dendritic cells. J Virol 2002; 76:10724 - 33; http://dx.doi.org/10.1128/JVI.76.21.10724-10733.2002; PMID: 12368315
- Jenison S, Yamada T, Morris C, Anderson B, Torrez-Martinez N, Keller N, et al. Characterization of human antibody responses to four corners hantavirus infections among patients with hantavirus pulmonary syndrome. J Virol 1994; 68:3000 - 6; PMID: 7512156
- Valdivieso F, Vial P, Ferres M, Ye C, Goade D, Cuiza A, et al. Neutralizing antibodies in survivors of Sin Nombre and Andes hantavirus infection. Emerg Infect Dis 2006; 12:166 - 8; http://dx.doi.org/10.3201/eid1201.050930; PMID: 16494739
- Bharadwaj M, Nofchissey R, Goade D, Koster F, Hjelle B. Humoral immune responses in the hantavirus cardiopulmonary syndrome. J Infect Dis 2000; 182:43 - 8; http://dx.doi.org/10.1086/315657; PMID: 10882580
- MacNeil A, Comer JA, Ksiazek TG, Rollin PE. Sin Nombre virus-specific immunoglobulin M and G kinetics in hantavirus pulmonary syndrome and the role played by serologic responses in predicting disease outcome. J Infect Dis 2010; 202:242 - 6; http://dx.doi.org/10.1086/653482; PMID: 20521946
- Mori M, Rothman AL, Kurane I, Montoya JM, Nolte KB, Norman JE, et al. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J Infect Dis 1999; 179:295 - 302; http://dx.doi.org/10.1086/314597; PMID: 9878011
- Sundstrom JB, McMullan LK, Spiropoulou CF, Hooper WC, Ansari AA, Peters CJ, et al. Hantavirus infection induces the expression of RANTES and IP-10 without causing increased permeability in human lung microvascular endothelial cells. J Virol 2001; 75:6070 - 85; http://dx.doi.org/10.1128/JVI.75.13.6070-6085.2001; PMID: 11390609
- Khaiboullina SF, St Jeor SC. Hantavirus immunology. Viral Immunol 2002; 15:609 - 25; http://dx.doi.org/10.1089/088282402320914548; PMID: 12513931
- Khaiboullina SF, Rizvanov AA, Deyde VM, St Jeor SC. Andes virus stimulates interferon-inducible MxA protein expression in endothelial cells. J Med Virol 2005; 75:267 - 75; http://dx.doi.org/10.1002/jmv.20266; PMID: 15602733
- Geimonen E, Neff S, Raymond T, Kocer SS, Gavrilovskaya IN, Mackow ER. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc Natl Acad Sci U S A 2002; 99:13837 - 42; http://dx.doi.org/10.1073/pnas.192298899; PMID: 12368479
- Marsac D, García S, Fournet A, Aguirre A, Pino K, Ferres M, et al. Infection of human monocyte-derived dendritic cells by ANDES Hantavirus enhances pro-inflammatory state, the secretion of active MMP-9 and indirectly enhances endothelial permeability. Virol J 2011; 8:223; http://dx.doi.org/10.1186/1743-422X-8-223; PMID: 21569520
- Manigold T, Mori A, Graumann R, Llop E, Simon V, Ferrés M, et al. Highly differentiated, resting gn-specific memory CD8+ T cells persist years after infection by andes hantavirus. PLoS Pathog 2010; 6:e1000779; http://dx.doi.org/10.1371/journal.ppat.1000779; PMID: 20174562
- Ennis FA, Cruz J, Spiropoulou CF, Waite D, Peters CJ, Nichol ST, et al. Hantavirus pulmonary syndrome: CD8+ and CD4+ cytotoxic T lymphocytes to epitopes on Sin Nombre virus nucleocapsid protein isolated during acute illness. Virology 1997; 238:380 - 90; http://dx.doi.org/10.1006/viro.1997.8827; PMID: 9400611
- Kilpatrick ED, Terajima M, Koster FT, Catalina MD, Cruz J, Ennis FA. Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J Immunol 2004; 172:3297 - 304; PMID: 14978138
- Borges AA, Donadi EA, Campos GM, Moreli ML, de Sousa RL, Saggioro FP, et al. Association of -308G/A polymorphism in the tumor necrosis factor-alpha gene promoter with susceptibility to development of hantavirus cardiopulmonary syndrome in the Ribeirão Preto region, Brazil. Arch Virol 2010; 155:971 - 5; http://dx.doi.org/10.1007/s00705-010-0655-7; PMID: 20372945
- Shrivastava-Ranjan P, Rollin PE, Spiropoulou CF. Andes virus disrupts the endothelial cell barrier by induction of vascular endothelial growth factor and downregulation of VE-cadherin. J Virol 2010; 84:11227 - 34; http://dx.doi.org/10.1128/JVI.01405-10; PMID: 20810734
- Gavrilovskaya IN, Gorbunova EE, Mackow ER. Pathogenic hantaviruses direct the adherence of quiescent platelets to infected endothelial cells. J Virol 2010; 84:4832 - 9; http://dx.doi.org/10.1128/JVI.02405-09; PMID: 20181715
- Gavrilovskaya IN, Gorbunova EE, Mackow NA, Mackow ER. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J Virol 2008; 82:5797 - 806; http://dx.doi.org/10.1128/JVI.02397-07; PMID: 18367532
- Gavrilovskaya I, Gorbunova E, Koster F, Mackow E. Elevated VEGF Levels in Pulmonary Edema Fluid and PBMCs from Patients with Acute Hantavirus Pulmonary Syndrome. Adv Virol 2012; 2012:674360; http://dx.doi.org/10.1155/2012/674360; PMID: 22956954
- Lampugnani MG, Dejana E. The control of endothelial cell functions by adherens junctions. Novartis Found Symp 2007; 283:4 - 13, discussion 13-7, 238-41; http://dx.doi.org/10.1002/9780470319413.ch2; PMID: 18300410
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol 2008; 10:923 - 34; http://dx.doi.org/10.1038/ncb1752; PMID: 18604199
- Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 2009; 16:209 - 21; http://dx.doi.org/10.1016/j.devcel.2009.01.004; PMID: 19217423
- Gavard J. Breaking the VE-cadherin bonds. FEBS Lett 2009; 583:1 - 6; http://dx.doi.org/10.1016/j.febslet.2008.11.032; PMID: 19059243
- Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 2006; 8:1223 - 34; http://dx.doi.org/10.1038/ncb1486; PMID: 17060906
- Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 2008; 121:2115 - 22; http://dx.doi.org/10.1242/jcs.017897; PMID: 18565824
- Gorbunova E, Gavrilovskaya IN, Mackow ER. Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells. J Virol 2010; 84:7405 - 11; http://dx.doi.org/10.1128/JVI.00576-10; PMID: 20463083
- Gorbunova EE, Gavrilovskaya IN, Pepini T, Mackow ER. VEGFR2 and Src kinase inhibitors suppress Andes virus-induced endothelial cell permeability. J Virol 2011; 85:2296 - 303; http://dx.doi.org/10.1128/JVI.02319-10; PMID: 21177802
- Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 2008; 14:25 - 36; http://dx.doi.org/10.1016/j.devcel.2007.10.019; PMID: 18194650
- Rowe RK, Pekosz A. Bidirectional virus secretion and nonciliated cell tropism following Andes virus infection of primary airway epithelial cell cultures. J Virol 2006; 80:1087 - 97; http://dx.doi.org/10.1128/JVI.80.3.1087-1097.2006; PMID: 16414986
- Spiropoulou CF, Albariño CG, Ksiazek TG, Rollin PE. Andes and Prospect Hill hantaviruses differ in early induction of interferon although both can downregulate interferon signaling. J Virol 2007; 81:2769 - 76; http://dx.doi.org/10.1128/JVI.02402-06; PMID: 17202220
- Levine JR, Prescott J, Brown KS, Best SM, Ebihara H, Feldmann H. Antagonism of type I interferon responses by new world hantaviruses. J Virol 2010; 84:11790 - 801; http://dx.doi.org/10.1128/JVI.00916-10; PMID: 20844031
- Temonen M, Lankinen H, Vapalahti O, Ronni T, Julkunen I, Vaheri A. Effect of interferon-alpha and cell differentiation on Puumala virus infection in human monocyte/macrophages. Virology 1995; 206:8 - 15; http://dx.doi.org/10.1016/S0042-6822(95)80014-X; PMID: 7831843
- Temonen M, Mustonen J, Helin H, Pasternack A, Vaheri A, Holthöfer H. Cytokines, adhesion molecules, and cellular infiltration in nephropathia epidemica kidneys: an immunohistochemical study. Clin Immunol Immunopathol 1996; 78:47 - 55; http://dx.doi.org/10.1006/clin.1996.0007; PMID: 8599883
- Markotic A, Hensley L, Geisbert T, Spik K, Schmaljohn C. Hantaviruses induce cytopathic effects and apoptosis in continuous human embryonic kidney cells. J Gen Virol 2003; 84:2197 - 202; http://dx.doi.org/10.1099/vir.0.19090-0; PMID: 12867652
- Cebalo L, Markotić A. Chemokine production predominates in human monocytes infected with Tula virus. Viral Immunol 2007; 20:206 - 13; http://dx.doi.org/10.1089/vim.2006.0039; PMID: 17425435
- Bambace NM, Levis JE, Holmes CE. The effect of P2Y-mediated platelet activation on the release of VEGF and endostatin from platelets. Platelets 2010; 21:85 - 93; http://dx.doi.org/10.3109/09537100903470298; PMID: 20063989
- Terajima M, Vapalahti O, Van Epps HL, Vaheri A, Ennis FA. Immune responses to Puumala virus infection and the pathogenesis of nephropathia epidemica. Microbes Infect 2004; 6:238 - 45; http://dx.doi.org/10.1016/j.micinf.2003.10.017; PMID: 15049335
- Suidan GL, Dickerson JW, Chen Y, McDole JR, Tripathi P, Pirko I, et al. CD8 T cell-initiated vascular endothelial growth factor expression promotes central nervous system vascular permeability under neuroinflammatory conditions. J Immunol 2010; 184:1031 - 40; http://dx.doi.org/10.4049/jimmunol.0902773; PMID: 20008293
