Figures & data
Figure 1. Reversible phosphorylation of SrtA and Co-IP experiments. (A) Recombinant SrtAΔN24 was incubated with the recombinant Stk1KD in the presence of [γ-32P]ATP. Stp1 proteins were added subsequent to the in vitro phosphorylation reaction in order to dephosphorylate SrtAΔN24 as indicated. Recombinant SrtAΔN24 and Stp1 have similar molecular weights (~27 KD) and migrate similarly in SDS-PAGE. (B) Reversible phosphorylation of SrtA by Stk1 and Stp1. (C) Phosphorylation of SrtAΔN24 in the context of a bacterial lysate. WT, cell lysates from wild-type Newman strain (harbouring pYJ335); Δstp1, cell lysates from a Δstp1 strain (harbouring pYJ335); stk1+++, cell lysates from S. auerus strain overexpressing the stk1 (Newman/pYJ335:stk1). (D and E) Dephosphorylation of recombinant SrtAΔN59 expressed and purified from either Δstp1 or WT Newman strain, band intensities from Pro-Q Diamond chemiluminescence were normalized against the corresponding coomassie brilliant blue stained band, which were set to 1.0 for purified recombinant SrtAΔN59 from the Δstp1 strain. In A-E, Reactions were stopped by addition of SDS – PAGE loading buffer (without DTT), samples were separated by SDS-PAGE, visualized by either autoradiography (32P) or pro-Q diamond stained gel (Pro-Q), and stained with coomassie blue. (F) Co-IP between Stk1 and SrtA. IP was performed with anti-Stk1 antibody followed by immunoblotting with anti-SrtA antibody (SrtA) and anti-Stk1 antibody (Stk1). The beads control was obtained in the absence of Stk1 antibody. (G) Co-IP between Stp1 and SrtA. IP was performed with anti-Stp1 antibody followed by immunoblotting with anti-SrtA antibody (SrtA) and anti-Stp1 antibody (Stp1). The beads control was obtained in the absence of Stp1 antibody. (H) Co-IP between SrtA and Stk1 or Stp1. IP was performed with anti-SrtA antibody followed by immunoblotting with anti-Stk1 antibody (Stk1), anti-Stp1 antibody (Stp1), and anti-SrtA antibody (SrtA). The beads control was obtained in the absence of SrtA antibody. In F-H, WT denotes WT S. aureus Newman strain, Δstp1 denotes stp1 deletion mutant, stk1-I denotes S. aureus Newman mutant with transposon insertion in stk1 gene, ΔsrtA denotes srtA deletion mutant.
![Figure 1. Reversible phosphorylation of SrtA and Co-IP experiments. (A) Recombinant SrtAΔN24 was incubated with the recombinant Stk1KD in the presence of [γ-32P]ATP. Stp1 proteins were added subsequent to the in vitro phosphorylation reaction in order to dephosphorylate SrtAΔN24 as indicated. Recombinant SrtAΔN24 and Stp1 have similar molecular weights (~27 KD) and migrate similarly in SDS-PAGE. (B) Reversible phosphorylation of SrtA by Stk1 and Stp1. (C) Phosphorylation of SrtAΔN24 in the context of a bacterial lysate. WT, cell lysates from wild-type Newman strain (harbouring pYJ335); Δstp1, cell lysates from a Δstp1 strain (harbouring pYJ335); stk1+++, cell lysates from S. auerus strain overexpressing the stk1 (Newman/pYJ335:stk1). (D and E) Dephosphorylation of recombinant SrtAΔN59 expressed and purified from either Δstp1 or WT Newman strain, band intensities from Pro-Q Diamond chemiluminescence were normalized against the corresponding coomassie brilliant blue stained band, which were set to 1.0 for purified recombinant SrtAΔN59 from the Δstp1 strain. In A-E, Reactions were stopped by addition of SDS – PAGE loading buffer (without DTT), samples were separated by SDS-PAGE, visualized by either autoradiography (32P) or pro-Q diamond stained gel (Pro-Q), and stained with coomassie blue. (F) Co-IP between Stk1 and SrtA. IP was performed with anti-Stk1 antibody followed by immunoblotting with anti-SrtA antibody (SrtA) and anti-Stk1 antibody (Stk1). The beads control was obtained in the absence of Stk1 antibody. (G) Co-IP between Stp1 and SrtA. IP was performed with anti-Stp1 antibody followed by immunoblotting with anti-SrtA antibody (SrtA) and anti-Stp1 antibody (Stp1). The beads control was obtained in the absence of Stp1 antibody. (H) Co-IP between SrtA and Stk1 or Stp1. IP was performed with anti-SrtA antibody followed by immunoblotting with anti-Stk1 antibody (Stk1), anti-Stp1 antibody (Stp1), and anti-SrtA antibody (SrtA). The beads control was obtained in the absence of SrtA antibody. In F-H, WT denotes WT S. aureus Newman strain, Δstp1 denotes stp1 deletion mutant, stk1-I denotes S. aureus Newman mutant with transposon insertion in stk1 gene, ΔsrtA denotes srtA deletion mutant.](/cms/asset/8299ad45-1ff7-40b9-aa3c-48800f38d16b/kvir_a_2171641_f0001_oc.jpg)
Figure 2. Different phosphorylation events on SrtA. (A) Phosphorylation sites of SrtA revealed by mass spectrometry (MS/MS). Phosphorylation sites are highlighted with asterisk, the catalytic residues of SrtA (Cys184, His120, and Arg197) are highlighted with triangle, and some secondary structural elements are indicated according to the structure of S. aureus SrtA (PDB ID 1T2P). (B) A representative MS/MS spectrum of phosphorylation on the Cys184 of SrtA. The phosphor-peptide QLTLITpCDDYNEK was observed at m/z 818.3611 Da obtained after trypsin digestion of SrtA that has been phosphorylated by the bacterial cell lysates of stk1+++ strain. Individual fragments are labelled based on the b- or y-ion nomenclature. (C) In vitro phosphorylation of SrtAΔN24 and its variant by bacterial cell lysates of stk1+++ strain in the presence of [γ-32P]ATP. stk1+++, stk1-overexpressing strain (Newman/pYJ335:stk1). SrtAΔN24 C184A, variant of SrtAΔN24 harbouring a Cys to Ala substitution at C184. (D) Effect of DTT on the phosphorylation of SrtA that has been purified from Δstp1 strain. (E) Effect of AcP (25 mM) on the phosphorylation of SrtAΔN24 and ClpP in vitro. (F) Effect of sample heating or Stp1-treatment on the AcP-mediated phosphorylation of SrtAΔN24. (G) SrtAΔN59 C184A could be phosphorylated by acetyl phosphate (25 mM). (H) Phosphorylation of SrtAΔN24 C184A by Stk1. SrtAΔN24 and SrtAΔN24 C184A were respectively incubated with recombinant Stk1KD in the presence of [γ-32P]ATP; Stp1 was added subsequent to in vitro phosphorylation reaction in order to dephosphorylate SrtAΔN24 and SrtAΔN24 C184A. The reactions were stopped by addition of SDS – PAGE loading buffer (without DTT). Samples were separated by SDS-PAGE, visualized by autoradiography (32P) or Pro-Q diamond stained gel (Pro-Q), and stained with coomassie blue (Coomassie). recombinant SrtAΔN24 and Stp1 have similar molecular weights (~27 KD) and migrate similarly. SrtAΔN24 C184A, SrtAΔN24 variant harbouring a Cys to Ala substitution at C184.
![Figure 2. Different phosphorylation events on SrtA. (A) Phosphorylation sites of SrtA revealed by mass spectrometry (MS/MS). Phosphorylation sites are highlighted with asterisk, the catalytic residues of SrtA (Cys184, His120, and Arg197) are highlighted with triangle, and some secondary structural elements are indicated according to the structure of S. aureus SrtA (PDB ID 1T2P). (B) A representative MS/MS spectrum of phosphorylation on the Cys184 of SrtA. The phosphor-peptide QLTLITpCDDYNEK was observed at m/z 818.3611 Da obtained after trypsin digestion of SrtA that has been phosphorylated by the bacterial cell lysates of stk1+++ strain. Individual fragments are labelled based on the b- or y-ion nomenclature. (C) In vitro phosphorylation of SrtAΔN24 and its variant by bacterial cell lysates of stk1+++ strain in the presence of [γ-32P]ATP. stk1+++, stk1-overexpressing strain (Newman/pYJ335:stk1). SrtAΔN24 C184A, variant of SrtAΔN24 harbouring a Cys to Ala substitution at C184. (D) Effect of DTT on the phosphorylation of SrtA that has been purified from Δstp1 strain. (E) Effect of AcP (25 mM) on the phosphorylation of SrtAΔN24 and ClpP in vitro. (F) Effect of sample heating or Stp1-treatment on the AcP-mediated phosphorylation of SrtAΔN24. (G) SrtAΔN59 C184A could be phosphorylated by acetyl phosphate (25 mM). (H) Phosphorylation of SrtAΔN24 C184A by Stk1. SrtAΔN24 and SrtAΔN24 C184A were respectively incubated with recombinant Stk1KD in the presence of [γ-32P]ATP; Stp1 was added subsequent to in vitro phosphorylation reaction in order to dephosphorylate SrtAΔN24 and SrtAΔN24 C184A. The reactions were stopped by addition of SDS – PAGE loading buffer (without DTT). Samples were separated by SDS-PAGE, visualized by autoradiography (32P) or Pro-Q diamond stained gel (Pro-Q), and stained with coomassie blue (Coomassie). recombinant SrtAΔN24 and Stp1 have similar molecular weights (~27 KD) and migrate similarly. SrtAΔN24 C184A, SrtAΔN24 variant harbouring a Cys to Ala substitution at C184.](/cms/asset/adefc2b9-0a4b-44fc-847e-16ba43ead92b/kvir_a_2171641_f0002_oc.jpg)
Figure 3. In vitro phosphorylation of SrtAΔN24. (A) Effect of DTT on Stk1-mediated phosphorylation of SrtAΔN24. The reaction was stopped by addition of SDS-PAGE loading buffer containing without or with DTT at a final concentration of 50 mM. samples were separated by SDS – PAGE, visualized by pro-Q diamond stained gel (left panel), and stained with Coomassie Blue (right panel). (B) Effect of sample heating on Stk1-mediated phosphorylation of SrtAΔN24. The reaction was stopped by addition of SDS – PAGE loading buffer (with DTT at a final concentration of 50 mM) without or with sample heating (95 °C, 10 min). samples were separated by SDS – PAGE, visualized by pro-Q diamond stained gel (left panel), and stained with coomassie blue (right panel).
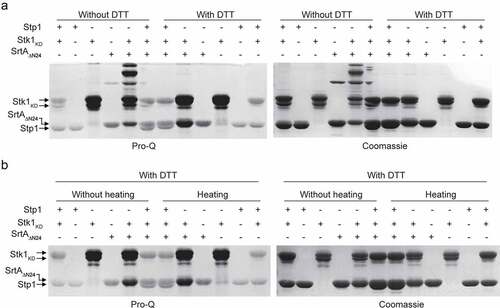
Figure 4. Phosphorylation inhibits the enzymatic activity of SrtA in vitro. (A) Effect of Stp1 and DTT on the enzyme activity of phosphorylated SrtAΔN24 (P~SrtAΔN24) in vitro. P~SrtAΔN24 was purified from E. coli-expressed SrtAΔN24 that has been phosphorylated by the cell lysates of Δstp1 strain. Stp1 and DTT were respectively added to the reaction mixture in order to dephosphorylate P~SrtAΔN24. (B) Effect of AcP-treatment on the in vitro enzyme activity of SrtAΔN24. Purified SrtAΔN24 expressed in E. coli was treated by AcP (25 mM) as described in materials and methods. Stp1 was added to the reaction mixture in order to dephosphorylate the AcP-mediated phosphorylation of SrtAΔN24. (C) Effect of Stk1KD and Stp1 on the enzyme activity of SrtAΔN24 in vitro. Recombinant Stk1KD was added to the reaction mixture in order to phosphorylate SrtAΔN24 while Stp1 was added in order to dephosphorylate SrtAΔN24. In (A) to (C), a fluorescently self-quenched peptide, Abz-LPETG-Dnp, was used to mimic the surface protein substrate, which contains a LPETG sorting signal. When Abz-LPETG-Dnp is cleaved by SrtA, the fluorophore Abz group is released from the quencher Dnp group, and an enhanced fluorescence signal was detected. RFI, relative fluorescence intensity.
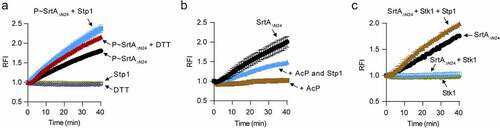
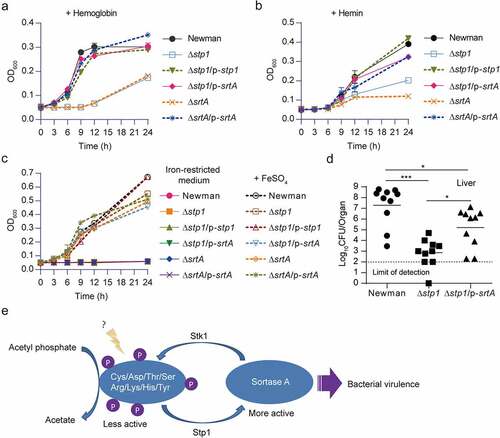
Figure 5. Overexpression of srtA alleviates the defects of stp1 deletion mutant. (A and B) Effect of constitutive expression of either stp1 or srtA on the bacterial growth of Δstp1 mutant in iron-restricted RPMI medium (Chelex 100 resin-treated RPMI) supplemented with either haemoglobin (2.5 μg/ml) or haem (2 μM) as the sole iron source. data shown represent the mean ± SD from triplicate experiments. (C) Growth curves for S. aureus in iron-restricted RPMI medium (Chelex 100 resin-treated RPMI) supplemented with or without FeSO4 (300 μM). Data shown represent the mean ± SD (n = three biological replicates). (D) Bacterial virulence of S. aureus Newman and its variants in mouse infection models. BALB/c female mice (n = 10) were retro-orbitally injected with 4 × 106 CFU S. aureus bacteria; S. aureus colonization in murine liver was measured after 5 days of infection; each symbol represents the value for an individual mouse; horizontal bars indicate observation means and dashed lines mark limits of detection; *p<0.05, ***p<0.001 (Mann–Whitney Test, two-tailed). S. aureus WT Newman harbours vector pYJ335 (Newman); stp1 deletion mutant harbours plasmid pYJ335 alone (Δstp1), pYJ335:stp1(Δstp1/p-stp1) or pYJ335:srtA (Δstp1/p-srtA); srtA deletion mutant (ΔsrtA) harbours plasmid pYJ335 alone (ΔsrtA) or (ΔsrtA/p-srtA). (E) A proposed mechanism of SrtA phosphorylation. SrtA may act as a mediator in a variety of signalling pathways in S. aureus through integrating diverse phosphorylation events.
Supplemental Material
Download MS Word (2.6 MB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.

