Figures & data
Figure 1. Growth characteristics of LSDV/RCH/P50. Vero cells, in triplicates, were infected with LSDV/RCH/P50 at MOI of 0.1 for 2 h, followed by washing with PBS and addition of fresh DMEM. Supernatant was collected from the infected cells at indicated time points and quantified by determination of TCID50 in Vero cells. Values are means ± SD and representative of the result of at least 3 independent experiments.
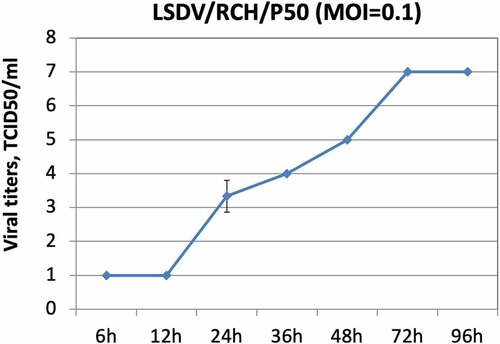
Table 1. Observation of various safety parameters following vaccination with Lumpi-ProVacInd (Experimental trial).
Figure 2. Safety of Lumpi-ProVacInd in field animals. a total of 26,940 animals across six Indian states (a) comprising of 26,527 cattle, 413 buffaloes (2889 pregnant cattle/buffaloes and 10,108 lactating buffaloes) (b) were included in the study. All the animals were injected with 1 ml of Lumpi-ProVacInd (containing 103.5 TCID50/dose) by subcutaneous route and monitored for swelling/skin nodules at the site of injection, generalized skin nodules, abortions in pregnant animals and efficacy of the vaccine (c).
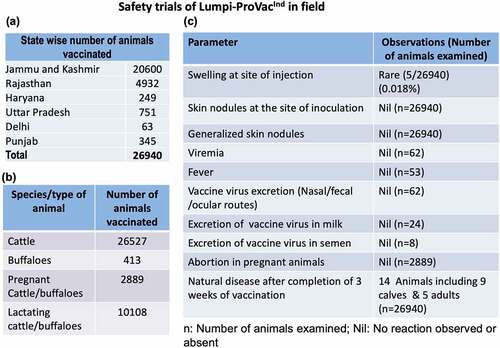
Table 2. Safety and efficacy of Lumpi-ProVacInd in field animals.
Table 3. Effect of Lumpi-ProVacInd on milk production.
Figure 3. Immunogenicity of Lumpi-ProVacInd. Immunogenicity was evaluated in experimental animals (n=10) and selected field animals (n=22). (a) Antibody titers. Percentage of animals (experimental and field trials) that revealed detectable anti-LSDV antibodies in serum by virus neutralization assay is shown. (b) Lymphocyte proliferation assay (experimental trial). PBMCs were separated from the blood collected from vaccinated (n=10) or unvaccinated (n=3) calves at day 30 pv. PBMCs were cultured in RPMI and stimulated with UV-inactivated LSDV. Relative proliferation of lymphocyte from vaccinated as compared to unvaccinated animals is shown. (c) Lymphocyte proliferation assay (field trial). PBMCs were separated from the blood collected from vaccinated (n=22) or unvaccinated (n=10) animals (all age groups) at day 30 pv and stimulated with UV-inactivated LSDV. Relative proliferation of lymphocyte from vaccinated as compared to the unvaccinated animals is shown. (d) IFN-ϒ levels following vaccination. Sera from experimental calves, separated at indicated times post-vaccination were subjected for determination of IFN-ϒ by using Bovine IFN-ϒ-ELISA kit. (e) IFN-ϒ levels following challenge infection. Sera from vaccinated, vaccinated-challenged and unvaccinated-challenged were examined for determination of IFN-ϒ. (f) Percentage of animals that developed IFN-ϒ response. Percentage of animals that exhibited IFN-ϒ response at indicated times following LSDV exposureis shown. *=p<0.05, NS=non-significant difference.
Table 4. Efficacy of Lumpi-ProVacInd in cattle (Experimental challenge).
Figure 4. Development and progression of skin nodules following challenge with virulent LSDV. Animals were either unvaccinated or vaccinated with Lumpi-ProVacInd. At day 30 pv, all the animals were challenged with virulent LSDV. Whereas vaccinated animals remained apparently healthy without showing any skin nodules, control (unvaccinated) animals developed the skin nodules following challenge. Primary swelling was seen at day 5–6 pc (a), which progressively increased in size from ~600 mm2 (day 6 pc) to 5000 mm2 (day 16 pc) before becoming stable at day 17 pc (b). The size of the developing skin nodule on various days post-challenge is also shown (c). IVRI 829, IVRI 1492 and IVRI 1525 are unvaccinated-challenged animals.
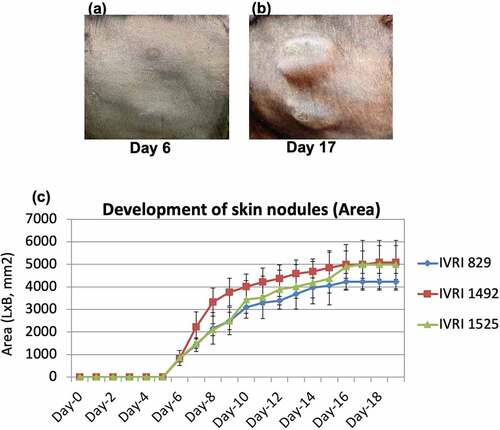
Table 5. Morbidity and mortality in vaccinated animals that succumbed to natural infection before development of a complete immunity.
Table 6. Differentiation of vaccine and field (virulent) strains of LSDV.
Table 7. Case fatality rate in animals that were vaccinated after appearance of the clinical disease.
Figure 5. Evaluation of the excretion of vaccine virus in milk and semen in vaccinated animals under field conditions. Animals were vaccinated with Lumpi-ProVacInd. Milk and semen samples were collected from the lactating cows and bulls respectively, and examined for the presence of LSDV genome by qRT-PCR. –Ve represents absence of LSDV genome.
Table 8. Neutralization of various LSDV strains by sera derived from LSDV/2019.
Supplemental Material
Download MS Word (475.6 KB)Data availability statement
The data that support the findings of this study are openly available in the concerned repositories. LSDV field strains used in this study were isolated by our group and deposited at National Centre for Veterinary Type Cultures, Hisar, India (repository of animal microbes; http://ncvtc.org.in/) with Accession Numbers VTCCAVA288 (LSDV/cattle/2019), VTCCAVA 321 (LSDV/cattle/2021), VTCCAVA 370 (LSDV/cattle/2022) and VTCCAVA 371 (LSDV/camel/2022). The complete nucleotide sequences of field (LSDV/2019/RCH/P0) and vaccine strain (LSDV/2019/RCH/P50) is available with GenBank Accession Number of MW883897.1 and OK422494.1, respectively.

