Figures & data
Figure 1. SDS-PAGE and Western blot analysis of host plasma proteins recruited to the bacterial surface. (a) Bacteria were incubated with normal human plasma (1) or IgG-free human plasma (2). Host proteins bound to the bacterial surface were eluted and subjected to SDS-PAGE and Western blot analysis. SEZ strain C2 but not the mutant C2ΔSzM bound C1q.
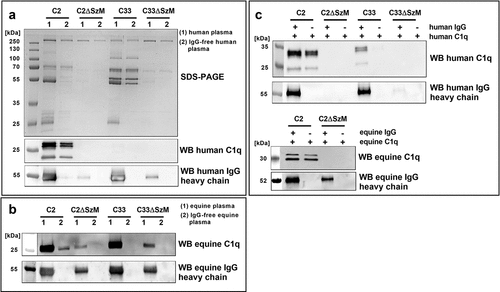
Figure 2. Expression of SzM in SEZ C2 and C33 is crucial for significant reduction of C3b labelling in human but not in equine plasma. Flow cytometry analysis of C3b deposition on bacteria incubated with human (a and b) or equine plasma (c) at 37°C for 30 min. After incubation in plasma, the bacteria were stained with a FITC-labelled goat F(ab)2 anti-human C3 antibody, which also detects equine C3. The deposition of C3 at the bacterial surface is shown as the geomean of the fluorescence intensity of all gated bacteria. EDTA-treated plasma, in which the complement system is inactivated, served as a negative control. The apathogenic bacterium Lactococcus lactis was used as a positive control for complement activation (n = 3).
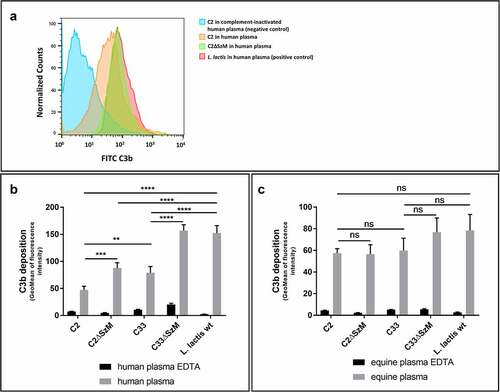
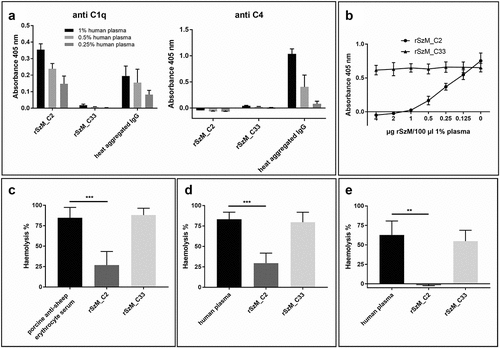
Figure 3. (a) rSzm binds C1q but does not activate the complement system. ELISA plates were coated with rSzM and heat aggregated human IgG (positive control) and incubated with the indicated amounts of human plasma. Bound C1q and C4 were detected with a goat anti-human complement C1q and a goat anti-human complement C4 antibody, respectively. Heat-aggregated human IgG was recognised by C1q and led to an activation of the complement system, shown as C4 deposition. rSzM_C2 bound C1q but did not activate the complement system as no C4 deposition was detected. (b) rSzM_C2 leads to an inhibition of complement activation. One percent human plasma was pre-incubated with the indicated amounts of rSzM at 37°C for 1 h. Then, pre-incubated plasma was incubated in the wells of an ELISA plate, coated with heat-aggregated human IgG. Activation of the complement system was detected by the deposition of C4. rSzM_C2 showed a dose-dependent inhibition of complement activation as shown by detection of C4 deposition, whereas rSzM_C33 did not influence C4 deposition. (c) rSzM_C2 but not rSzM_C33 inhibits activation of the classical complement pathway in porcine serum. Porcine anti-sheep erythrocyte serum (of three different pigs) was pre-incubated with recombinant SzM proteins at 37 °C for 30 min. Pre-incubated serum was mixed with washed sheep erythrocytes and incubated for 2 h at 37 °C. Haemolysis of erythrocytes was determined by measuring the absorbance at 405 nm of the supernatant. (d) rSzM_C2 but not rSzM_C33 inhibits activation of the classical complement pathway in human plasma. Sheep erythrocytes were antibody labelled with EDTA-treated porcine anti-sheep erythrocyte serum. afterwards, antibody-labelled erythrocytes were washed and incubated with human plasma (source of active complement system), which was pre-incubated with the indicated rSzM variants. (n = 3). (e) rSzM_C2 but not rSzM_C33 inhibits IgG-mediated activation of the complement system in human plasma. Sheep erythrocytes were antibody labelled with purified porcine anti-sheep erythrocyte IgG. Afterwards, IgG-labelled erythrocytes were washed and incubated with human plasma (source of active complement system), which was pre-incubated with the indicated rSzM variants. (n = 3).