Figures & data
Table 1. Effects of BPA in the stress axis of rats.
Figure 1. The hypothalamic-pituitary-adrenal axis (HPA) and BPA's effects under basal conditions. Hypothalamic and higher brain centers such as the hippocampus control HPA axis function. Corticotrophin releasing hormone (CRH) from the paraventricular hypothalamic area stimulates the release of adrenocorticotrophin hormone (ACTH) from the anterior pituitary, which in turn triggers the release of glucocorticoids (corticosterone in rodents) from the adrenal cortex. Following stress, the highly secreted glucocorticoids, via their receptors (GR), are responsible for the termination of the axis activation through a negative feedback loop exerted on the central axis components. GR activity is tightly regulated by the co-chaperon FKBP51 that inhibits GR hormone binding affinity and nuclear translocation via an ultra-short feedback loop within the HPA axis. The figure illustrates the reported effects of BPA on the HPA axis under basal conditions. Perinatal BPA exposure appears to affect most HPA axis regulators, in a sex-specific manner, including FKBP51 expression.
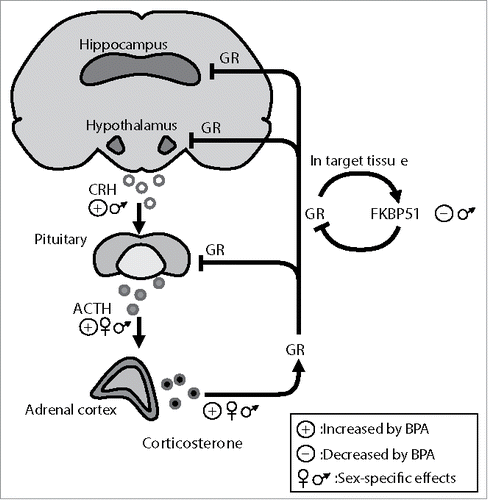
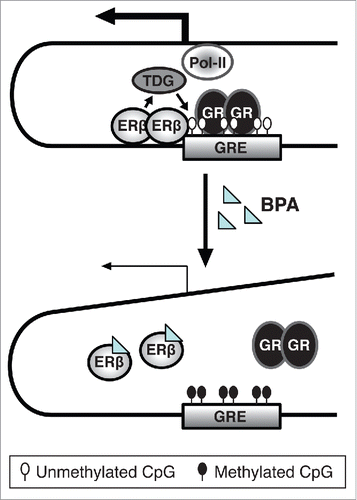
Figure 2. Suggested model for the role of ERβ in the inhibitory effect of BPA on Fkbp5, based on observations in differentiating HT22 cells. In the absence of BPA treatment, ERβ prevents DNA-methylation of CpGs at the glucocorticoid response element (GRE) of the Fkbp5 gene. This might be mediated by ERβ's recruitment of thymine DNA-glycosylase (TDG) that is involved in active DNA demethylation. As a result, a transcriptional complex is formed that can cooperate with upstream promoter regions to mediate Fkbp5 expression by activated GRs.Citation19 However, when the cells are exposed to BPA during differentiation, BPA might disrupt ERβ binding, resulting in no TDG recruitment, DNA hypermethylation and lower Fkbp5 expression. This, translated to the HPA axis, could result in enhanced GR activity.