Figures & data
Figure 1. IVD Development Competing Attributes. IVD device development can be viewed as a balance between meeting a clinical need, the technological innovation, and potential overlap with current offerings. Through this perspective, a developer can know where the product sits relative to competitors and in terms of prioritising the need. The green shaded region indicates the ideal goal of IVD development and where the innovation opportunity can be most impactful.
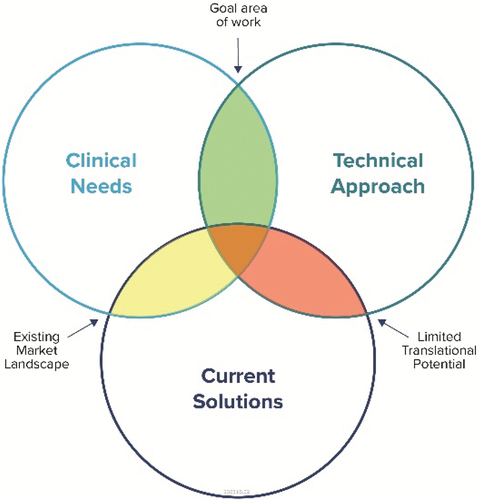
Table 1. Questions (and sample responses) that guide developers in scoping their product development.
Figure 2. A notional TPP development and review cycle. Relevant participant groups are listed at the key stages for the TPP development process who may help in properly defining an IVD product roadmap.
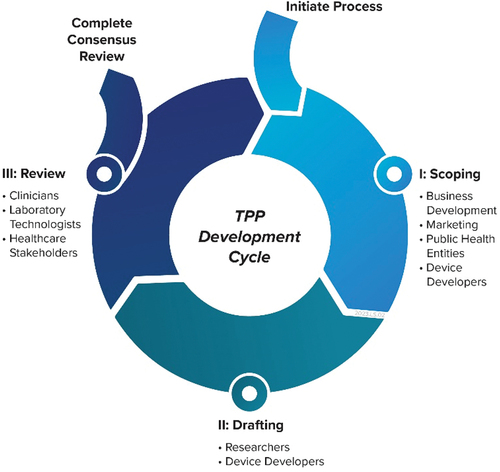
Table 2. IVD development criteria. List of key criteria, corresponding description, and examples for framing a target product profile.
Figure 3. IVD attributes and corresponding configuration options that are commonly considered in the development process. The attributes along the critical development path (i.e. Analyte, Intended Use, etc.) relate to each other and define the development focus areas of an IVD product. The four elements under the Technical attribute outline the common, IVD operational workflow..
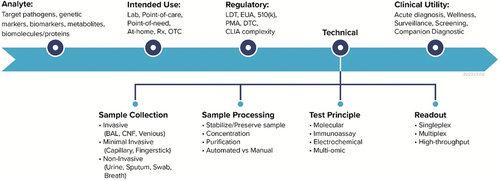
Figure 4. Graphical representation showing generalised natural history of infection and notional patient condition during respiratory disease course. The illustration helps visualise how current diagnostic algorithms involving Nucleic Acid Amplification Tests (NAATs) and serological IVDs are implemented. Actual levels of analyte (i.e. pathogen nucleic acids or antibodies) could vary depending on disease.
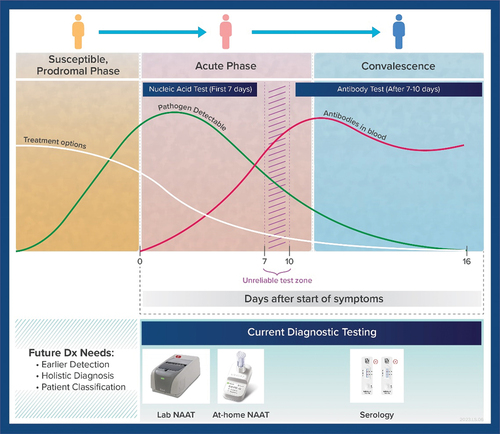
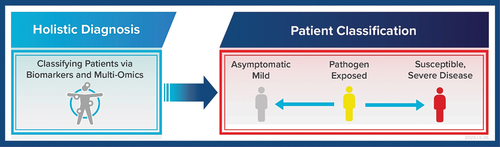
Figure 5. Holistic diagnostic approach in future IVD developments is needed to classify patients and thus focus treatments and care management on individuals susceptible to developing severe disease. This can be considered part of a future precision medicine approach to addressing infectious diseases through novel IVDs that for instance can accomplish multi-omics testing.