Figures & data
Figure 1 Mechanisms of Treg Cells in Female Pregnancy. Estrogen and semen recruit macrophages and DCs, which promotes their polarization towards M2 macrophages and tDC phenotypes. tDCs uptake paternal antigens in semen and transport them to the PALN draining the uterus. Under the stimulation of paternal antigens, Th0 cells in PALN differentiate into pTreg cells. pTreg cells and tTreg cells converge in the peripheral circulation and re-enter the uterine cavity during the implantation stage to exert their functions. Decidual Treg cells restrict Teffs by secreting IL-10 and TGF-β and expressing CD25, CTLA4 and PD-L1, which directly promotes the embryo implantation. Additionally, they work in collaboration with decidual immune cells to promote decidualization and enhance endometrial receptivity. Treg cells not only directly inhibit Teffs and M1 macrophages, but also work in collaboration with M2 macrophages, uNK, and tDC cells to promote trophoblast invasion and vascular remodeling. PALN, para-aortic lymph node. (By Figdraw).
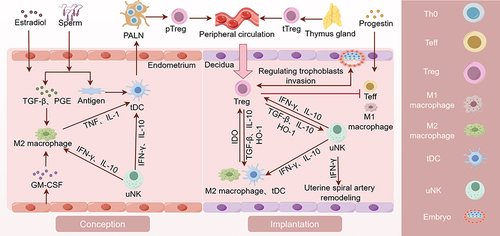
Table 1 Different Phenotypes of Treg Cells During Pregnancy
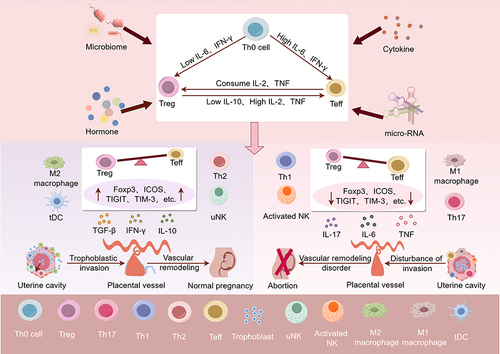
Figure 2 The relationship between Decidual Treg cells and URSA. In human, cytokines, hormones, micro-RNAs, the reproductive tract microbiome, and seminal fluid composition all have the potential to interfere with the immune balance at the maternal–fetal interface. High levels of IL-6 and IFN-γ could skew Th0 differentiation toward Teffs and cause Treg cells to transdifferentiate. Newly generated pTreg cells are susceptible to lineage instability and phenotype switching. High levels of IL-2, TNF and low levels of IL-10 could skew pTreg cells differentiation toward Teffs. If pTreg cells express additional markers associated with functional stability, such as Foxp3, ICOS, TIGIT, and TIM3, it could potentially shift the immune balance at the maternal–fetal interface towards immune tolerance. This may lead to elevated TGF-β, IL-10, M2 macrophages and tDC cells, which promotes trophoblast invasion, vascular remodeling and placental development. Conversely, if the newly generated pTreg cells are unstable, it could shift the immune balance at the maternal–fetal interface towards pro-inflammation. This could result in elevated IL-6, IL-17, TNF, M1 macrophages, Th1 cells and etc, which may hinder trophoblast invasion, vascular remodeling, and ultimately causing placental dysplasia and URSA. (By Figdraw).