Figures & data
Figure 1 The mechanism of chemical exchange saturation transfer (CEST).
Abbreviation: MR, magnetic resonance.
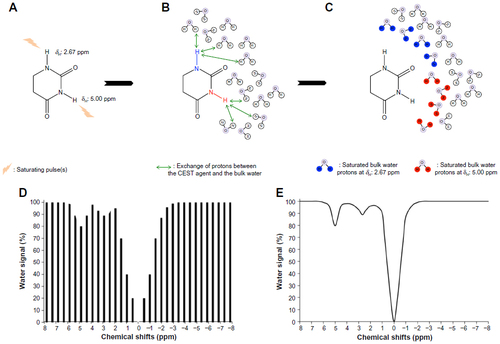
Figure 2 ParaCEST agents that detect enzyme activity.
Abbreviation: ParaCEST, paramagnetic chemical exchange saturation transfer.
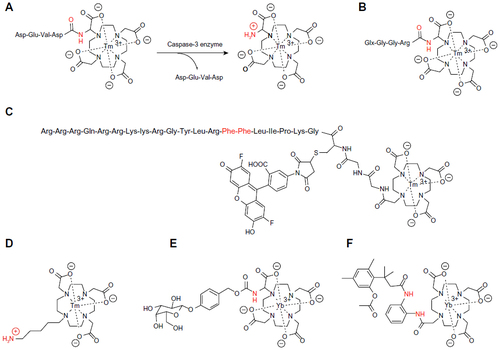
Figure 3 Responsive CEST agents.
Abbreviation: CEST, chemical exchange saturation transfer.
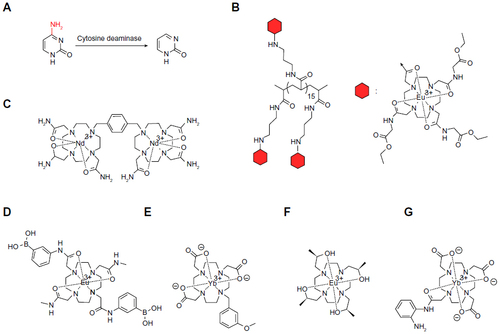
Figure 4 ParaCEST agents that detect ions, redox state, and temperature.
Abbreviation: ParaCEST, paramagnetic chemical exchange saturation transfer.
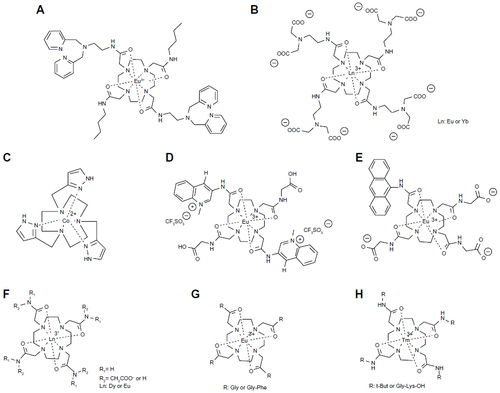
Figure 5 CEST agents that measure pH include (A) Yb-DOTAM-Gly-X, (B) Ln-DOTAM-Gly, (C) Yb(III)-HPDO3A, (D) Co(II)-complex, (E) iopamidol, (F) iopromide, (G) iobitridol, (H) and Tm-DOTAM-Gly-Lys. (I) Deprotonation of a Eu(III) complex changes the coordination around the Eu3+ ion, which changes the CEST effect of this agent.
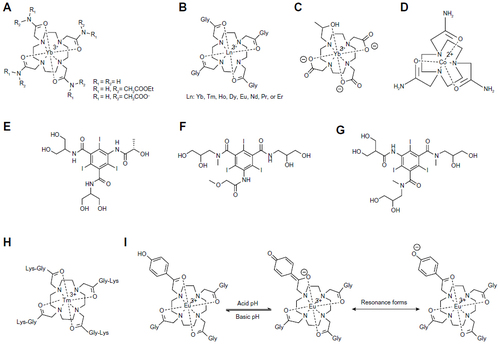
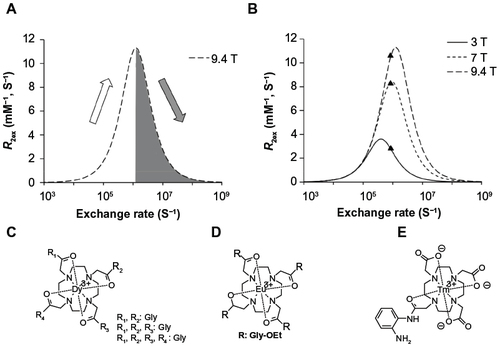
Figure 6 The mechanism of the T2ex process.
Abbreviation: T2ex, T2 exchange.
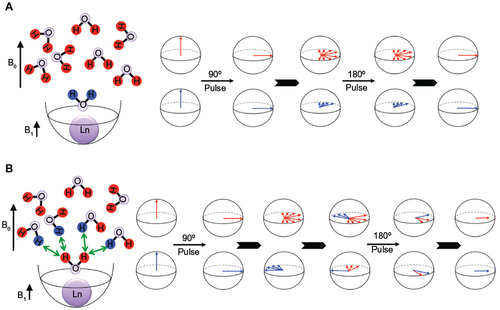
Figure 7 The dependence of T2ex on environmental conditions.
Abbreviation: T2ex, T2 exchange.