Figures & data
Table 1. Similarities and differences between dengue hemorrhagic fever and hantavirus pulmonary syndrome
Figure 1. A model for dengue pathogenesis. Infection of target cells (monocyte, dendritic cells) is enhanced in the presence of non-neutralizing, cross-reactive antibodies. The output viruses and viral proteins such as NS-1 may binds to DENV-specific antibodies and activate complement system leading the release of vasoactive complement fragments resulting functional and structural changes in endothelial cells (EC). Infected cells and activated T lymphocytes release various cytokines with permeability enhancing activities such as IL-6, IL-8, TNF-α, MCP-1, and VEGF. It is controversial whether EC are infected with DENV in vivo. However, in vitro infected EC have been shown to upregulate VEGF-R 2 expression and secrete various cytokines. Activation of EC by VEGF and other cytokines leads to disruption of adherens junctions, resulting in increased permeability.
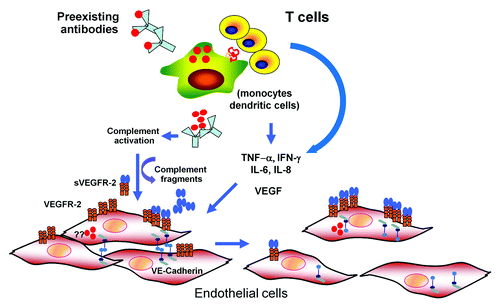
Figure 2. Proposed model for HPS pathogenesis. The exact means of entry of hantaviruses into the vascular endothelium is not known, but likely is via infected dendritic cells and/or infected alveolar macrophages (A). Infection of endothelial cells by hantavirus causes secretion of VEGF, triggering the disruption of adherens junctions and downregulation of VE-cadherin (see details in [D]). Hantavirus-infected endothelial cells also produce proinflammatory cytokines and chemokines, such as IP-10 and RANTES, and upregulate adhesion molecules on their cell surface, attracting monocytes, macrophages, and T cells. Accumulation of hantavirus infected monocytes and macrophages in the vicinity of the endothelium results in a “cytokine storm” by secreting additional chemokines/cytokines. Additional VEGF is secreted by hantavirus activated T cells, platelets, and macrophages. At this point VEGF could achieve high concentrations in the microvasculature of the lung, resulting in vascular hyper permeability and leakage (B). (C) Diagram of intact adherens junctions. (D) Diagram of disrupted adherens junctions.
![Figure 2. Proposed model for HPS pathogenesis. The exact means of entry of hantaviruses into the vascular endothelium is not known, but likely is via infected dendritic cells and/or infected alveolar macrophages (A). Infection of endothelial cells by hantavirus causes secretion of VEGF, triggering the disruption of adherens junctions and downregulation of VE-cadherin (see details in [D]). Hantavirus-infected endothelial cells also produce proinflammatory cytokines and chemokines, such as IP-10 and RANTES, and upregulate adhesion molecules on their cell surface, attracting monocytes, macrophages, and T cells. Accumulation of hantavirus infected monocytes and macrophages in the vicinity of the endothelium results in a “cytokine storm” by secreting additional chemokines/cytokines. Additional VEGF is secreted by hantavirus activated T cells, platelets, and macrophages. At this point VEGF could achieve high concentrations in the microvasculature of the lung, resulting in vascular hyper permeability and leakage (B). (C) Diagram of intact adherens junctions. (D) Diagram of disrupted adherens junctions.](/cms/asset/d6f5f350-5425-4d49-8440-fe66ad102735/kvir_a_10925569_f0002.gif)