ABSTRACT
Increased level of stump harvest on clearcuts is expected due to growing demand for bioenergy. For saproxylic species, this will reduce habitat supply. Negative effects on populations may be buffered by alternative deadwood habitats, especially stumps after forest thinning. We investigated clearcuts and mature forest to see whether such stumps or other standing deadwood could host saproxylic beetles that occur in clear-felling stumps. The study was conducted in boreal Sweden, on clearcuts 3–14 years after harvest and in mature forests. Beetles were sampled from the bark of spruce, pine and birch, from stumps after clear-felling, stumps after thinning, snags within forests and high-stumps on clearcuts. Beta diversity differed significantly between deadwood types, but not tree species. The stumps after thinning had a species poor assemblage compared to other deadwood. Abundance of common species was higher in deadwood on clearcuts compared to forests, and higher in snags than in stumps. We conclude that stumps after thinning and other standing deadwood will not buffer habitat loss from increased stump harvest for species inhabiting stumps on clearcuts, because these communities require sun-exposed habitats. Within mature managed forests, improving the quality and diversity of deadwood will be important for increasing the diversity of saproxylic beetle communities.
Introduction
The transition from fossil fuel-based to renewable energy sources has led to a growing demand for bioenergy, and this demand is expected to increase even further in the near future (Anonymous Citation2016). Logging residues such as stumps, branches and treetops from managed forests constitute an important source of bioenergy (Egnell et al. Citation2007; Swedish Forest Agency Citation2009). In Sweden, the majority of stumps after harvest remain on clearcuts and the extraction is not yet implemented at a large scale, because of legal and economic reasons (Egnell Citation2013). However, as the need for bioenergy continues to grow, we may expect that more of the logging residues will be harvested, particularly the stumps (Egnell Citation2013), with potential consequences for saproxylic species, i.e. species relying on deadwood as habitat (Hjältén et al. Citation2010; Walmsley and Godbold Citation2010; Bouget et al. Citation2012; Victorsson and Jonsell Citation2013; Jonsell and Schroeder Citation2014; Ranius et al., Citation2018).
Loss of deadwood from managed forests has negative effects on forest biodiversity (Esseen et al. Citation1997). In Scandinavia where there is a long history of intensive forest management (Kouki et al. Citation2001; Siitonen Citation2001), both the amount and diversity of deadwood are substantially reduced (Fridman and Walheim Citation2000; Siitonen et al. Citation2000). This has resulted in many saproxylic species being threatened (Stokland et al. Citation2013). In order to mitigate these negative effects, some deadwood is retained and created during forestry operations, which can benefit species associated with natural forest disturbances (Gustafsson and Perhans Citation2010; The Royal Swedish Academy of Agriculture and Forestry Citation2015). Stumps left after harvesting and other residues are sometimes regarded as having a lower value for biodiversity compared to logs and snags, which may have contributed to the limited number of ecological studies (Bouget et al. Citation2012). Yet, these deadwood types have been shown to host diverse saproxylic communities (Caruso et al. Citation2008; Hjältén et al. Citation2010; Jonsell and Hansson Citation2011) and some species have large parts of their populations in slash and stumps (Jonsell and Schroeder Citation2014; Hiron et al. Citation2017; Ranius et al., Citation2018). Consequently, extraction of logging residues can lead to reduced species richness, e.g. beetles and fungi (Toivanen et al. Citation2012; Victorsson and Jonsell Citation2013), and therefore poses further threats to the saproxylic diversity.
Stumps after harvest on clearcuts constitute about 80% of all coarse deadwood in managed forests in Sweden, and are therefore important for supporting large populations of saproxylic species (Egnell et al. Citation2007). Their volume has even increased by about 30% during the last 50 years (de Jong and Dahlberg Citation2017). However, species inhabiting those stumps also use other man-made or natural deadwood types, which may buffer their decline on a landscape scale under increased extraction scenarios (Jonsell and Schroeder Citation2014; Hiron et al. Citation2017). Some studies have found that moderate levels of stump harvest do not necessarily compromise biodiversity values, provided that the harvesting sites and tree species are selected with caution (Dahlberg et al. Citation2011; Lassauce et al. Citation2012; Zolotarjova et al. Citation2016; Hiron et al. Citation2017). Negative effects of loss of one particular habitat on populations may be mitigated by the availability of alternative habitat, e.g. other types of standing deadwood, like natural snags or man-made high-stumps. Even low stumps created during forest thinning operations may serve as habitat, and this deadwood type is particularly interesting because it is abundant in managed forest landscapes, constituting about 25% of all stumps (Swedish Forest Agency Citation2009). They have, however, several differences compared to stumps on clearcuts; most apparently, they are under closed canopy and usually have smaller diameters, which may affect their suitability. Sun exposure affects the occurrence of many saproxylic species that prefer disturbed forest habitats (Kouki et al. Citation2001; Lindhe et al. Citation2005; Vogel et al. Citation2020), and experimental assessments of saproxylic species preferences often indicate microclimate as one of the most important factors determining species occurrence or community composition (Johansson et al. Citation2007; Müller et al. Citation2020; Uhl et al. Citation2022). Therefore, we may expect that different microclimatic conditions would lead to different species assemblages in clearcut stumps compared to forest stumps.
So far, most deadwood studies have focused on logs, snags and branches, while comparatively few have investigated low stumps (but see Abrahamsson and Lindbladh Citation2006; Hjältén et al. Citation2010; Jonsell and Hansson Citation2011; Uhl et al. Citation2022). Low stumps have a close connection to the ground, which provides moisture. Even stumps on clearcuts become partly shaded, as soon as the vegetation has reached some meters height, which may create some similarities between stumps after thinning and stumps after clearcutting. Snags and high-stumps, on the other hand, remain sun-exposed for longer. To understand how different factors influence saproxylic communities, more comparative studies between deadwood types are needed. Knowing to what degree species in clearcut stumps can use other standing deadwood, including stumps after thinning, snags and high-stumps, would help to assess the amount of habitat available for this fauna. The results may also be relevant for assessing how saproxylic beetles might be affected by the implementation of alternative management methods, like different types of continuous cover forestry, which create habitats with varying degrees of sun exposure, in regions previously dominated by clearcutting.
In this study, we compared the beta diversity and abundance of saproxylic beetles from bark samples of stumps and snags/high-stumps, located either within mature forest or on clearcuts, 3–14 years after cutting. We were especially interested in analysing if felling stumps after forest thinning could provide complementary habitats to stumps after clearcuting. The data were collected by Jonsell and Schroeder (Citation2014), who estimated the proportions of saproxylic beetle fauna that use clearcut stumps compared to other deadwood types at a landscape scale. In that study, the relative importance of stumps after thinning for beetle diversity was not formally assessed. Therefore, in the present study, we aimed to evaluate whether stumps from forest thinning provide habitat for beetle communities using stumps on clearcuts. We posed the following questions:
- Is the saproxylic beetle fauna similar between felling stumps on clearcuts and other types of standing deadwood?
- What are the main deadwood characteristics that affect abundance patterns of individual beetle species?
We would consider different dead wood types as fully complementary habitats if they host similar beetle species at similar levels of abundance. If the identities of species are similar, but they vary in abundance, it may indicate that different deadwood types can be used as alternative habitats, but cannot support the same population sizes. Finally, if there are clear differences in both species identities and abundance, it would suggest that the species communities are highly deadwood-specific, and habitat loss of any of the deadwood types is likely to have negative effects on their populations. Our hypothesis was that the thinning stumps would be largely complementary to the clearfelling stumps in terms of species composition, because of similar (man-made) origin, tree species and age. However, we expected that they may have lower species abundances, due to smaller size at cutting and fewer substrates created at the same time.
Methods
Study area
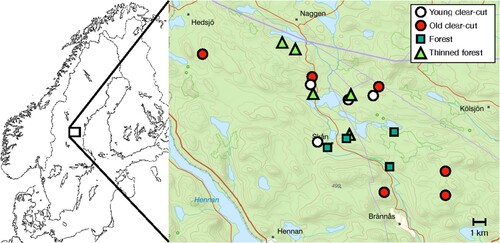
The study landscape was located in central Sweden in the province of Hälsingland (62N, 16E, ). The total area was 24,449 ha, consisting of 83% forest and the rest mainly mires and lakes. The forest is typical of the central boreal region, with Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies L. Karst.) as the dominant tree species. The most common deciduous tree species are birch (Betula pendula Roth. and B. pubescens Ehrh.) and aspen (Populus tremula L.), but they only form a minor part of the stand basal area. The forest land in the area is owned by a single forest company Holmen Skog AB.
Selection of stands
The study was conducted on ten clearcuts, five old forest stands and five thinned stands (Jonsell and Schroeder Citation2014) (). All the clearcut stands were conifer-dominated, with less than 15% (range 1–13%) deciduous trees. The share of spruce was between 62 and 88% in eight of the clearcuts, and 50% and 16% on the two remaining. The last one was thus pine-dominated. The old forest and thinned stands had similar tree species composition as the clear cuts. The aim was to sample bark from low stumps on clearcuts and in thinned stands, the bases of snags ( = dead standing trees) in the forest, and high-stumps on clearcuts. The clearcuts and the old stands (≥ 60 years) were randomly selected from the forest company’s stand database. We included ten 3–14-year-old clearcuts (four 3–5 and six 7–14 year old) that contained stumps of pine, spruce and birch. If a chosen clearcut did not have stumps of all three tree species, we selected the next nearest clearcut that did. The thinned stands that were treated 3–14 years ago were not possible to identify from the stand database. However, two of the old forest stands met this condition and were therefore chosen for the sampling of stumps after thinning, i.e. both stumps and snags were sampled in these stands. The three remaining thinned stands were chosen with the help of company personnel, among stands >60 years old in the landscape.
The field surveys were conducted in 2009, and the field design has been previously described by Jonsell and Schroeder (Citation2014). Overall, our sampling included two stand types in relation to the rotational stage (forest and clearcut) combined with two standing deadwood types (stump and snag) (). We sampled bark from stumps (diameter >10 cm) of spruce, pine and birch on clearcuts and in thinned stands, and from trunk bases on dead standing trees in the old forest stands because the basal part of snags might be similar to low-stumps. Those samples were taken at a height corresponding to the bark surface on a harvest stump. We also sampled bark from high-stumps on clearcuts, but these were sampled at breast height for the purpose of another study (Jonsell and Schroeder Citation2014).
Table 1. The number of sampled CWD objects grouped by stand type and tree species.
Sampling design within stands
We selected 10–12 sampling plots per clearcut by positioning a regular square grid on a map of each selected clearcut, where each node was the center of a circular sampling plot (100 m2, 5.64 m radius) (Jonsell and Schroeder Citation2014). The variation is due to the grid being randomly applied to the area and that we chose to sample all nodes. The sampling plot centers were identified in the field with a GPS.
In each sampling plot on the clearcuts, we sampled beetles on one stump of each of the target tree species (pine, spruce, birch) (). We chose stumps that were closest to the plot center and had remaining bark. The aim was to sample eight stumps per tree species per clearcut and when this limit was reached, we did not sample the remaining plots. In some plots, the stumps of targeted tree species were not found, particularly birch stumps were often lacking. Therefore, we did not find the intended number of birch stumps on some clearcuts, and on three clearcuts, we found no birch stumps at all. On two clearcuts, we could not find the full number of pine stumps. We also sampled bark from spruce high-stumps that were encountered within or between the sampling plots. We sampled between 1 and 5 spruce high-stumps per clearcut, the number depending on how many were present, making a total of 37 high-stumps. Spruce was chosen because earlier sampling programs in the same area focused on spruce (Schroeder et al. Citation2006); spruce is also the most common species of which high-stumps are created. In the thinned stands, we sampled the first eight stumps encountered of each tree species with bark remaining. In older forests, we sampled trunk bases ( = the bark area corresponding to what would have been left if the tree was felled) of dead-standing trees in the same way but restricted the sample size to five trunk bases per tree species in each stand. The quadratic network, which we used on the clearcuts, was not possible to use in the mature forests as the stumps were too sparsely distributed. Therefore, a large part of the stand had to be covered in order to find all samples required. Because both sampling methods covered most of the stand area, we think that the difference in approaches between clearcuts and mature forests is of minor importance. For each sampled object (stump or trunk base), we estimated the area of bark sampled and measured the size (circumference in cm) and decay stage (5 classes, based on Siitonen and Saaristo Citation2000) (Table S1).
Beetle collection
The beetle fauna was sampled by sifting, using the same method as reported in earlier studies (Schroeder et al. Citation2006; McGeoch et al. Citation2007). The sifts consisted of a textile bag, which has a grid with an 8 mm mesh size in the middle. The peeled bark was broken into smaller pieces and sifted through the mesh, whereafter the fine fraction at the bottom of the bag was brought to the lab for beetle extraction in Tullgren funnels. The coarse material that did not pass through the grid was discarded in the forest. Sampling was conducted during the summer (23 June–28 July 2009) in all stands, as the time of sifting may influence the results (Wikars et al. Citation2005). Where possible, we sifted 0.5 m2 bark per stump/trunk base. If stumps had less bark than that, all available bark was sifted. The average bark sample size was 0.29 m2, with a range from 0.02 m2 to 0.5 m2. All beetles were determined to species according to the nomenclature in Löbl and Smetana (Citation2003–2012) by taxonomic experts Joel Hallqvist and Vitezslav Manak. We defined as saproxylic all species that are mainly recorded from various kinds of dead wood, according to Hansen (Citation1964), Koch (Citation1992) and Palm (Citation1959). Only saproxylic species were included in the subsequent analyses.
Statistical analyses
Community dissimilarity
As a measure of community dissimilarity, we used beta diversity. We used packages betdiver and vegan (Oksanen Citation2017) in R (R Core Team Citation2022) to visualize community composition and calculate total beta diversity, nestedness and turnover. We compared beta diversity for the four different dead wood types: stumps on clearcuts, high-stumps on clearcuts, stumps after thinning, and snags in the forest, as well as for the three tree species: spruce, pine and birch. For these analyses, the species community data were summed for each dead wood type per stand (n = 31), or for each tree species per stand (n = 46). The analysis is based on PCoA (principal coordinates analysis) of community composition where beta diversity is estimated as the distance to centroid in each group by the betadisper function, which uses information from all PCoA axes. We added Lingoes correction to the data, to avoid axes with negative eigenvalues. We calculated beta diversity measures based on presence–absence data (Jaccard index) as well as abundance data (Bray–Curtis index), and used ANOVA to analyze differences in beta diversity (separately for the total beta diversity, nestedness, and turnover) between the deadwood types and between tree species. Eigenvalues from all PCoA axes for analyses of total beta diversity are presented in Table S2.
Abundance of common species
We used negative binomial generalized linear mixed models (GLMM) in R package MASS to analyze whether stump properties had effects on abundance patterns of the most common beetle species. As common species, we defined those represented by a minimum of 20 individuals and occurring in at least 10 stumps or snags. In total, this encompassed 20 species. Due to a large number of zeros in the data, for each species, we removed categories of stand type and stand ID, deadwood type or tree species where that species was completely absent, in order to avoid variable categories with only zero values. Data for additional sub-categories with only zeros were removed only if they caused problems with model convergence or model fit.
As the response variable, we used the number of individuals found in the bark sample of each stump/snag, and we used stand ID as a random factor. As fixed explanatory variables we used stand type (clearcut or forest) and deadwood type (stump or snag), tree species (spruce, pine, birch) and decay stage (classes 1–5). In addition, we included deadwood size (circumference in cm) and sampled bark area (m2) as covariates to control for the effects of deadwood size and sampling effort; these variables were scaled prior to analysis. We also included geographical coordinates for each stand, to account for possible spatial dependence. The original x- and y-coordinates (SWEREF99TM coordinate system) were normalized by scaling and centering + 0.01, in order to obtain positive data on comparable scales (). The distribution of the sites within the landscape lay along North-West to South-East and the coordinates were highly correlated (>0.7). Therefore, we did not test both coordinates together in the same model. We tested two-way interactions between tree species, stand type, deadwood type and size, when possible (if all sub-categories contained data).
We assessed the model fit by inspecting residual plots and testing for overdispersion and zero-inflation using the diagnostic functions in the DHARMa package in R. Models were simplified based on Akaike`s Information Criterion (AIC), by removing variables if the removal reduced the AIC value of the model. We selected the model with the lowest AIC as the final model for each species. In addition, we compared the AIC values of the final models to the null models (only intercept and random factor), using ΔAIC ≥ 2 as cutoff, in order to assess whether the fixed factors contributed substantially to explaining the variation in the data.
Results
In total, 2669 saproxylic beetles belonging to 145 species were collected from the bark samples. Of these, 1205 individuals belonging to 91 species were collected from clearcut stumps (193 surveyed objects), 318 individuals of 41 species from high-stumps (38 objects), 555 individuals of 42 species from stumps after thinning (112 objects) and 591 individuals of 71 species from forest snags (80 objects). The collected numbers of beetles were distributed similarly among tree species, with 993 beetles found in spruce bark, followed by pine (904 beetles) and birch (782 beetles).
The most abundant species were Pteryx suturalis (582 individuals), Leptusa pulchella (172 individuals), Cerylon histeroides (125 individuals) and Corticaria longicollis (120 individuals); 49 species were represented by singletons (Table S3). Ten species were unique to high-stumps and stumps from forest thinning, while 29 and 32 species were uniquely collected from snags and stumps after clearcutting, respectively.
Community dissimilarity
We found differences in beta diversity between the samples collected from different dead wood types (). The total beta diversity and the species turnover were significantly different, while the nestedness component was only significant for species abundance-based data. The first two axes of the PCoA showed a community overlap between clearcut stumps and forest snags, particularly when considering species abundance data (), while the assemblages of high-stumps and forest stumps were widely separated. The lowest beta diversity was observed for forest stumps compared to the other deadwood types ().
Figure 2. Total beta diversity (abundance-based) of beetle communities collected from four dead wood types: high-stumps on clearcuts, clearcut stumps, forest snags and forest stumps. A – compositional differences illustrated by PCoA; B – beta diversity measured as distance to centroid in each dead wood type; median values (black line), 25th and 75th percentile (boxes), and minimum/maximum values (whiskers) are shown. Beta diversity ( = distance to centroid) differed significantly between dead wood types (ANOVA: F = 8.97, p = 0.004).
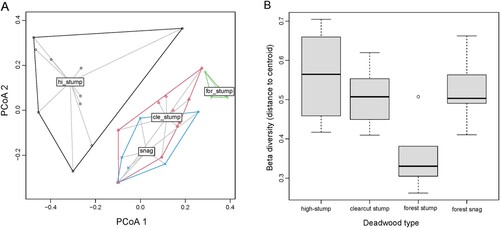
Table 2. Results from ANOVA, comparing beta diversity between beetle assemblages in different dead wood types, and among different tree species.
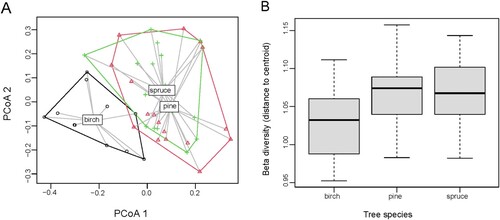
We found no differences in beta diversity between tree species for most beta diversity types (). The single exception was presence–absence-based turnover. In the PCoA, however, there was a clear community separation between birch and the coniferous species, spruce and pine, along the first two axes (). Yet it was also apparent from the PCoA plot that the variation within communities of each tree species, i.e. beta diversity (the average distance to centroid), was similar for all groups ().
Figure 3. Total beta diversity (presence-absence-based) of beetle communities collected from three tree species: spruce, pine and birch. A – compositional differences illustrated by PCoA; B – beta diversity measured as distance to centroid in each dead wood type; median values (black line), 25th and 75th percentile (boxes), and minimum/maximum values (whiskers) are shown. Beta diversity ( = distance to centroid) did not differed significantly between tree species (ANOVA: F = 1.11, p = 0.35).
Abundance of common species
Among the 20 species that were analysed individually, stand type was included in the final models for ten of the species (Tables 3, S5). Only two species showed a positive association with forest habitat (Leptusa pulchella and Crypturgus hispidulus), while more species (7) had significant or marginal negative association with forest, indicating that they prefer clearcuts. Also, more species preferred snags compared to stumps (Tables 3, S5). However, for about half of the species the stand or deadwood type were not included in the final models as relevant factors explaining species abundance. Tree species was the factor retained in most models (13 out of 19), and we found a preference for birch or pine substrate for six species each. Decay stage of deadwood or sampled bark area was important for seven species. The abundance of most species was not affected by deadwood size; however, three vs. one species preferred small or large-diameter wood, respectively. Two species, Hadreule elongatula and Cerylon ferrugineum, were more abundant in the eastern part of the study area, while P. suturalis and Atomaria bella were positively associated with the northern part (Tables 3, S5).
It was only possible to test interactions for six of the species, and for three of these, we found significant interactions. Abundance of L. pulchella differed between forest and clearcut (clearcut < forest) only for coniferous deadwood, but was equally high in birch. The abundance of C. hispidulus was influenced by an interaction between stand type and deadwood type, with a higher abundance in stumps compared to snags on clearcuts, but not within the forest. Finally, fewer individuals of P. suturalis were found in small-size deadwood, but only in snags, not stumps.
Discussion
We found that different species communities inhabited stumps retained on clearcuts compared to those left after forest thinning, and that both deadwood type and tree species affected beetle species composition (, ). The patterns of beta diversity largely resembled those of species composition for deadwood types, and we observed a considerably lower species turnover in stumps from forest thinning (). Beta diversity did not differ significantly between beetle communities of different tree species (). Abundance patterns of individual species depended mainly on the tree species, deadwood type and stand type, while e.g. substrate size and geographical gradients were relatively less important (). Our findings are broadly consistent with other studies on species habitat associations, (e.g. Jonsell et al. Citation1998; Abrahamsson and Lindbladh Citation2006; Johansson et al. Citation2007; Hjältén et al. Citation2010; Müller et al. Citation2020; Vogel et al. Citation2020; Burner et al. Citation2021), and confirm that both the properties of the deadwood itself, as well as the conditions of the surrounding habitat, affect the diversity of saproxylic beetles.
Table 3. Structure and results of the final models, GLM/GLMER (negative binomial), explaining abundance of the most common species* per deadwood (CWD) substrate (Table S4).
Contrary to our hypothesis, our results indicate that other types of standing deadwood, and particularly the stumps left after thinning in mature forests, do not function as complementary habitats for beetle species inhabiting felling stumps on clearcuts. This is because the species communities are different both regarding species identity and their abundance (). The stumps after thinning also had lower beta diversity than the other categories showing that these stumps host a species poorer community. Differences in beta diversity also suggest that beetle communities change across space, i.e. across forest stands within the landscape, in a different manner in different deadwood types. Therefore, alternative deadwood habitats may not be able to buffer the negative effects of habitat loss of one particular substrate type.
Different deadwood types as habitat for saproxylic beetles
Different types of standing deadwood hosted different beetle communities. In particular, stumps from forest thinning clearly differed in species composition from the other deadwood types. In addition, we found considerably lower beta diversity in stumps from thinning (B) and they appear to be avoided by most individual species that we analysed. Only one species (Leptusa pulchella) was positively associated and another (Crypturgus hispidulus) marginally associated with both factors “forest” and “stump” (). L. pulchella is a generalist species in relation to most wood properties, as long as fungi have colonised the wood (Palm Citation1959). C. hispidulus live on conifers, but generally seem to thrive in wood of somewhat moister condition (downed wood) than other species in the same genus (Lekander et al. Citation1977). Other species more frequently occurring in the stumps after thinning occurred to a similar or higher extent in stumps on clearcuts (, S3). Thus, the stumps after thinning seem to host rather species-poor communities and they seem to be a favorable habitat for only a few species, while the majority of species do not thrive in this deadwood type.
Lower diversity in stumps after forest thinning might be due to several factors, including (1) comparatively lower diameter than clearcut stumps, (2) shaded microclimate, (3) that they are created with the same method at the same time, or (4) management history of the stands. We think all those factors might be contributing due to the reasons explained below, starting with the first point. Our data showed only limited evidence for a preference for large-size stumps, as also reported by other studies (e.g. Lindhe et al. Citation2005), which suggests that diameter is probably not a strong contributing factor within the size range studied here.
Microclimate is often identified as one of the main factors shaping saproxylic communities (Vogel et al. Citation2020; Burner et al. Citation2021), and its importance has been demonstrated for many saproxylic beetle communities (Lindhe et al. Citation2005; Johansson et al. Citation2007; Müller et al. Citation2020; Vogel et al. Citation2020). Forest thinning stumps experience similar microclimatic conditions as forest snags, yet we observed considerably higher beetle diversity in snags, similar to that of substrates on clearcuts. This suggests that the habitat type with shaded microclimate isn`t the only factor responsible for low diversity in stumps after thinning.
Another likely explanation for the observed diversity patterns is that man-made deadwood may have a more uniform species assemblage compared to natural deadwood because they are created on the same occasion and with the same method; this applies to stumps after thinning, as well as stumps after clearcutting and high-stumps. Such low heterogeneity of man-made substrates in comparison with naturally created was reported for deciduous high-stumps (Jonsell et al. Citation2004). In comparison, forest snags are created by the natural death of the trees and could therefore be expected to have more varying properties and thereby species communities than the stumps. In our study, however, the beta diversity of beetle communities was also high in high-stumps and clear-felling stumps, even though these are man-made substrates. This might be related to higher sun exposure on clearcuts. Forests with high sun exposure have higher microclimatic heterogeneity both on stand level and substrate level (Lettenmaier et al. Citation2022), which could contribute to richer fauna of sun-exposed environments. Additionally, habitat conditions change rapidly over time after clearcutting, which affects saproxylic communities (Hyvärinen et al. Citation2009). Due to the wide range of clearcut ages (3–14 years) surveyed in this study, habitat heterogeneity is likely higher for the deadwood substrates surveyed in this group. Therefore, stumps left after thinning in mature forests might be relatively more homogeneous than the other deadwood types, at least under the Fennoscandian forest management regime where sun exposure usually benefits insect diversity (Kouki et al. Citation2001).
The history of forest management is rather different between the stands in the study area. In boreal Sweden, forestry had a comparatively low impact until the first half of the 1900s (Östlund et al. Citation1997). Cutting occurred in most forests, but only the most valuable trees were harvested. When the clearcutting system was introduced on a large scale, whole stands were replaced by planted trees. Usually, all trees were removed, including small ones, so the new stands became very uniform (Östlund et al. Citation1997). The old forest stands and many of the stands that have been cut close to the shift of the millennia ( = old clearcuts in our data) have never been subjected to stand-replacing clearcutting, whereas the thinning stands originated after the shift to stand-replacing clearcuts. Therefore, it might be so that the thinned stands have a poorer and more uniform habitat quality with smaller species pools of saproxylic beetles that might colonize the stumps after thinning. The management history is thus correlated with stand age, which also might be a contributing factor.
Species composition and abundance
We observed compositional differences among saproxylic beetle communities between the deadwood types; particularly, the stumps after thinning in the forest and high-stumps on clearcuts hosted distinct communities. High-stumps and low-stumps were expected to be different, which has been shown for spruce earlier (Abrahamsson and Lindbladh Citation2006; Hjältén et al. Citation2010). The high-stumps were sampled at breast height, while all other substrates were sampled near ground, which implies that low stumps have closer contact with ground moisture compared to the wood where the high-stumps were sampled. Similarly, logs in close contact with the ground have been shown to differ from high-stumps (Jonsell and Weslien Citation2003). A conspicuous example of a species differentiating due to this is the ciid beetle Hadreule elongata (Table S3) that has a very strong affinity for high-stumps compared to low stumps or logs on the ground (Jonsell and Weslien Citation2003; Schroeder et al. Citation2006). Other studies have shown that different sections of standing deadwood differ in species diversity (Graf et al. Citation2022) and the base of high-stumps hosts different communities than the section at the breast height (Abrahamsson and Lindbladh Citation2006), which suggests that different moisture levels probably contribute to these diversity patterns. Even if previous studies have mostly focused on early colonizing saproxylic communities, and high-stumps in our study were considerably older, it appears that they maintain a distinct beetle fauna for up to 14 years after clearcutting.
Even if we found that different deadwood types host different species assemblages, there was also overlap between them (). Moreover, about half of the species that we analysed individually preferred a specific stand type or deadwood type, while for the rest of the species, these factors were not important, suggesting that a considerable part of the community uses several substrate types.
We found that the deadwood of birch hosted different beetle species assemblage compared to the communities in spruce and pine (). The lack of differences in beta diversity suggests that the communities in deadwood of different tree species vary in a similar way across the landscape. Also, several individual species varied in abundance between tree species (). Here, we mostly found species with a preference for pine or birch wood, while no species preferred spruce over both pine and birch. That saproxylic species discriminate between wood of different tree species and particularly between deciduous and coniferous wood is well known (Jonsell et al. Citation1998; Dahlberg and Stokland Citation2004; Toivanen and Kotiaho Citation2010; Lassauce et al. Citation2012; Vogel et al. Citation2020; Burner et al. Citation2021). This pattern is usually most apparent early in the succession, and as deadwood reaches later decomposition stages, other factors than tree species, e.g. fungal species community, determine which beetles use the deadwood (Jonsell et al. Citation1998; Seibold et al. Citation2023). Our study covered a rather broad age range of the deadwood, but not the first years when the species turnover is most pronounced, probably explaining why the difference in species composition between pine and spruce was not apparent.
Management and conservation implications
The value of thinning stands for biodiversity is rather overlooked (e.g. Klein et al. Citation2022), and here, we showed that the stumps after forest thinning have comparatively low values for the diversity of saproxylic beetles. More generally, our results suggest that the deadwood on clearcuts has an important niche to fill for the saproxylic biodiversity in managed boreal forests. The open sun-exposed stumps are used by an assemblage of species that would not thrive in stumps within mature forests.
Our study is not unique in finding that a majority of saproxylic beetles prefer open and sunny conditions. As large-scale disturbance by forest fires was very common in boreal forests before humans could control the fires (Niklasson and Granström Citation2000; Niklasson and Drakenberg Citation2001), many species have adapted to track these disturbances (Kouki et al. Citation2001). The fires supplied large quantities of sun-exposed wood, and today clear cuts are the major habitat for those species. However, most of the coarse wood is extracted from the clear cut, leaving the felling stumps to be the main source of coarse deadwood there. Harvesting these stumps would imply an even more restricted supply of habitat and our results suggest that the shaded stumps within the forest would not be a good substitute for those species. In addition, during stump harvest, other types of deadwood present on clearcuts risk being damaged or removed (Hautala et al. Citation2004; Rudolphi and Gustafsson Citation2005). Thus, the results of our study support the conclusions of previous research (Bouget et al. Citation2012; Lassauce et al. Citation2012; Toivanen et al. Citation2012; Victorsson and Jonsell Citation2013; Jonsell and Schroeder Citation2014; Zolotarjova et al. Citation2016; Hiron et al. Citation2017), suggesting that logging residue harvest should be limited to residues of certain tree species and managed stands with low biodiversity values. In addition, increased stump harvest on clearcuts needs to be combined with strategies for improved deadwood retention and protection on a landscape scale. Landscape focus is important since a forest stand on its own will usually not be able to support populations in the long term due to its small size and successional character. Our analyses of beta diversity showed that beetle communities vary across space in different ways among deadwood types, regarding both species turnover and nestedness. This means that all deadwood types have their own unique contribution to the regional species pool and all of them need to be considered in conservation management.
Currently, continuous cover forestry is increasingly considered as a potential way to increase multifunctional forest values, such as recreation, biodiversity and improved resilience of managed forests under climate change (Peura et al. Citation2018; Mason et al. Citation2022). Regarding biodiversity, continuous cover forestry increases habitat availability for late-successional forest species (Peura et al. Citation2018; Ekholm et al. Citation2023), because continuous cover forestry generally aims at minimizing tree-less areas, even if smaller open patches are created during, e.g. gap harvest. Our study shows that stumps under tree cover have a lower diversity and a different assemblage of species than stumps on clearcuts, which is at least in part due to low sun exposure. Therefore, continuous cover forestry that maintains a high degree of shading, like single-tree harvest, will not benefit species adapted to disturbed sun-exposed environments, which has also been highlighted by other studies (Ekholm et al. Citation2023).
For the saproxylic species, irrespective of the forestry method, dead wood retention throughout the successional cycle is necessary, including trees older than the felling age in forestry. Refraining from thinning has been suggested as a cost-effective way to increase habitat availability for threatened saproxylic beetles (Mönkkönen et al. Citation2014). Our results provide some support for such an approach, as stumps after thinning were species-poor compared to forest snags, and without thinning the long-term availability of snags would increase throughout the successional cycle. At a landscape scale, a combination of management regimes, including clear cuts, extended rotation times and stands with continuous cover, provides the highest values for ecosystem services and biodiversity (Mönkkönen et al. Citation2014; Peura et al. Citation2018). Thus, the goal of forest policy in Sweden, to establish continuous cover forestry as a complement to clearcutting (Swedish Forest Agency Citation2023), should be favorable for biodiversity, provided that heterogeneous deadwood habitats are created in forests of all successional stages.
Supplemental Material
Download Zip (150.2 KB)Acknowledgements
We thank our taxonomic experts Joel Hallqvist and Vitezslav Manak for assistance with field work and help with species identification. Also, thanks to the personnel at Holmen Skog AB for help with the identification and localization of suitable forest stands for the survey. Thanks to two anonymous reviewers who provided constructive comments that helped to improve the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The original data supporting the findings of this manuscript is included as a supplemental data sheet.
References
- Abrahamsson M, Lindbladh M. 2006. A comparison of saproxylic beetle occurrence between man-made high- and low-stumps of spruce (Picea abies). For Ecol Manag. 226(1–3):230–237. doi:10.1016/j.foreco.2006.01.046.
- Anonymous 2016. Bioenergi från skog och skogsindustri [Bioenergy from forests and forest industry]. Report from Pöyry Management Consulting. https://www.skogsindustrierna.se/siteassets/dokument/rapporter/bioenergi-fran-skog-och-skogsindustri.pdf
- Bouget C, Lassauce A, Jonsell M. 2012. Effects of fuelwood harvesting on biodiversity— a review focused on the situation in Europe1 this article is one of a selection of papers from the international symposium on dynamics and ecological services of deadwood in forest ecosystems. Can J For Res. 42(8):1421–1432. doi:10.1139/x2012-078.
- Burner RC, et al. 2021. Choosy beetles: how host trees and southern boreal forest naturalness may determine dead wood beetle communities. For Ecol Manag. 487:119023. doi:10.1016/j.foreco.2021.119023.
- Caruso A, Rudolphi J, Thor G. 2008. Lichen species diversity and substrate amounts in young planted boreal forests: a comparison between slash and stumps of Picea abies. Biol Conserv. 141(1):47–55. doi:10.1016/j.biocon.2007.08.021.
- Dahlberg A, et al. 2011. Modelled impact of Norway spruce logging residue extraction on biodiversity in Sweden. Can J For Res. 41(6):1220–1232. doi:10.1139/x11-034.
- Dahlberg A, Stokland JN. 2004. Vedlevande arters krav på substrat - sammentällning och analys av 3600 arter’, Skogsstyrelsen Rapport, pp. 1–75.
- de Jong J, Dahlberg A. 2017. Impact on species of conservation interest of forest harvesting for bioenergy purposes. Forest Ecology and Management. 383:37–48. http://dx.doi.org/10.1016/j.foreco.2016.09.016.
- Egnell G, et al. 2007. Miljökonsekvenser av stubbskörd – en sammanställning av kunskap och kunskapsbehov’.
- Egnell, G. (2013) ‘Skogsbränsle’, Skogsskötselserien, (17), pp. 1–69.
- Ekholm A, et al. 2023. Long-term yield and biodiversity in stands managed with the selection system and the rotation forestry system: a qualitative review. For Ecol Manag. 537:120920. doi:10.1016/j.foreco.2023.120920.
- Esseen P. -A., Ehnström B., Ericsson L., Sjöberg K. 1997. Boreal forests. Ecological Bulletins. 46:16–47.
- Fridman J, Walheim M. 2000. Amount, structure, and dynamics of dead wood on managed forestland in Sweden. For Ecol Manag. 131(1–3):23–36. doi:10.1016/S0378-1127(99)00208-X.
- Graf M, et al. 2022. Saproxylic beetles trace deadwood and differentiate between deadwood niches before their arrival on potential hosts. Insect Conservation and Diversity. 15(1):48–60. doi:10.1111/icad.12534.
- Gustafsson L, Perhans K. 2010. Biodiversity conservation in Swedish forests: ways forward for a 30-year-old multi-scaled approach. Ambio. 39(8):546–554. doi:10.1007/s13280-010-0071-y.
- Hansen V. 1964. Fortegnelse over Danmarks biller 1. og 2. del’. Ent Meddr. 33:1–507.
- Hautala H, et al. 2004. Impacts of retention felling on coarse woody debris (CWD) in mature boreal spruce forests in Finland. Biodivers Conserv. 13(8):1541–1554. doi:10.1023/B:BIOC.0000021327.43783.a9.
- Hiron M, et al. 2017. Consequences of bioenergy wood extraction for landscape-level availability of habitat for dead wood-dependent organisms. J Environ Manag. 198:33–42. doi:10.1016/j.jenvman.2017.04.039.
- Hjältén J, Stenbacka F, Andersson J. 2010. Saproxylic beetle assemblages on low stumps, high stumps and logs: implications for environmental effects of stump harvesting. For Ecol Manag. 260(7):1149–1155. doi:10.1016/j.foreco.2010.07.003.
- Hyvärinen E, Kouki J, Martikainen P. 2009. Prescribed fires and retention trees help to conserve beetle diversity in managed boreal forests despite their transient negative effects on some beetle groups. Insect Conserv Divers. 2(2):93–105. doi:10.1111/j.1752-4598.2009.00048.x.
- Johansson T, et al. 2007. Variable response of different functional groups of saproxylic beetles to substrate manipulation and forest management: implications for conservation strategies. For Ecol Manag. 242(2–3):496–510. doi:10.1016/j.foreco.2007.01.062.
- Jonsell M, Hansson J. 2011. Logs and stumps in clearcuts support similar saproxylic beetle diversity: implications for bioenergy harvest. Silva Fenn. 45(5):1053–1064. doi:10.14214/sf.86.
- Jonsell M, Nittérus K, Stighäll K. 2004. Saproxylic beetles in natural and man-made deciduous high stumps retained for conservation. Biol Conserv. 118(2):163–173. doi:10.1016/j.biocon.2003.08.017.
- Jonsell M, Schroeder M. 2014. Proportions of saproxylic beetle populations that utilise clear-cut stumps in a boreal landscape – biodiversity implications for stump harvest. For Ecol Manag. 334:313–320. doi:10.1016/j.foreco.2014.08.042.
- Jonsell M, Weslien J. 2003. Felled or standing retained wood—it makes a difference for saproxylic beetles. For Ecol Manag. 175(1–3):425–435. doi:10.1016/S0378-1127(02)00143-3.
- Jonsell M, Weslien J, Ehnström B. 1998. Substrate requirements of red-listed saproxylic invertebrates in Sweden. Biodivers Conserv. 7(6):749–764. doi:10.1023/A:1008888319031.
- Klein J, Low M, Sjögren J, Eggers S. 2022. Short-term experimental support for bird diversity retention measures during thinning in European boreal forests. For Ecol Manag. 509:120084. doi:10.1016/j.foreco.2022.120084.
- Koch K. 1992. Die Käfer Mitteleuropas. Ökologie. Band 1-3. Krefeld: Goecke & Evers.
- Kouki J, et al. 2001. Forest fragmentation in Fennoscandia: linking habitat requirements of wood-associated threatened species to landscape and habitat changes. Scand J For Res. 16(sup003):27–37. doi:10.1080/028275801300090564.
- Lassauce A, Lieutier F, Bouget C. 2012. Woodfuel harvesting and biodiversity conservation in temperate forests: effects of logging residue characteristics on saproxylic beetle assemblages. Biol Conserv. 147(1):204–212. doi:10.1016/j.biocon.2012.01.001.
- Lekander B., Bejer-Petersen B., Kangas E., Bakke A. 1977. The distribution of bark beetles in the Nordic countries. Acta Entomologica Fennica. 32:1–37.
- Lettenmaier L, et al. 2022. Beetle diversity is higher in sunny forests due to higher microclimatic heterogeneity in deadwood. Oecologia. 198(3):825–834. doi:10.1007/s00442-022-05141-8.
- Lindhe A, Lindelöw Å, Åsenblad N. 2005. Saproxylic beetles in standing dead wood density in relation to substrate sun-exposure and diameter. Biodivers Conserv. 14(12):3033–3053. doi:10.1007/s10531-004-0314-y.
- Löbl I, Smetana A. 2003–2012. Catalogue of palearctic coleoptera, Vol 1-8. Stenstrup, Denmark: Apollo Books.
- Mason WL, et al. 2022. Continuous cover forestry in Europe: usage and the knowledge gaps and challenges to wider adoption. For: Int J For Res. 95(1):1–12. doi:10.1093/forestry/cpab038.
- McGeoch MA, et al. 2007. Saproxylic beetle diversity in a managed boreal forest: importance of stand characteristics and forestry conservation measures. Divers Distrib. 13(4):418–429. doi:10.1111/j.1472-4642.2007.00350.x.
- Mönkkönen M, et al. 2014. Spatially dynamic forest management to sustain biodiversity and economic returns. J Environ Manag. 134:80–89. doi:10.1016/j.jenvman.2013.12.021.
- Müller J, et al. 2020. Primary determinants of communities in deadwood vary among taxa but are regionally consistent. Oikos. 129(10):1579–1588. doi:10.1111/oik.07335.
- Niklasson M, Drakenberg B. 2001. A 600-year tree-ring fire history from Norra Kvills National Park, southern Sweden: implications for conservation strategies in the hemiboreal zone. Biol Conserv. 101(1):63–71. doi:10.1016/S0006-3207(01)00050-7.
- Niklasson M, Granström A. 2000. Numbers and sizes of fires: long-term spatially explicit fire history in a Swedish boreal landscape. Ecology. 81(6):1484–1499. doi:10.1890/0012-9658(2000)081[1484:NASOFL]2.0.CO;2.
- Oksanen J. 2017. ‘Vegan: ecological diversity’, R Package Version 2.4-4, p. 11. Available at: https://cran.r-project.org/package=vegan.
- Östlund L, Zackrisson O, Axelsson AL. 1997. The history and transformation of a Scandinavian boreal forest landscape since the 19th century. Can J For Res. 27(8):1198–1206. doi:10.1139/x97-070.
- Palm T. 1959. Die Holz- und Rindenkäfer der süd- und mittelschwedischen Laubbäume. Opusc Ent Suppl 16:1–374.
- Peura M, et al. 2018. Continuous cover forestry is a cost-efficient tool to increase multifunctionality of boreal production forests in Fennoscandia. Biol Conserv. 217:104–112. doi:10.1016/j.biocon.2017.10.018.
- Ranius T., Hämälainen A., Egnell G., Olsson B., Eklöf K., Stendahl J., Rudolphi J., Stens A., Felton A. 2018. The effects of logging residue extraction for energy on ecosystemservices and biodiversity: A synthesis. Journal of Environmental Management. 209:409–425.
- R Core Team. 2022. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: https://www.r-project.org/.
- Rudolphi J, Gustafsson L. 2005. Effects of forest-fuel harvesting on the amount of deadwood on clear-cuts. Scand J For Res. 20(3):235–242. doi:10.1080/02827580510036201.
- Schroeder LM, et al. 2006. Recruitment of saproxylic beetles in high stumps created for maintaining biodiversity in a boreal forest landscape. Can J For Res. 36(9):2168–2178. doi:10.1139/x06-119.
- Seibold S, et al. 2023. Drivers of community assembly change during succession in wood-decomposing beetle communities. J Anim Ecol. 92(5):965–978. doi:10.1111/1365-2656.13843.
- Siitonen J, et al. 2000. Coarse woody debris and stand characteristics in mature managed and old-growth boreal mesic forests in southern Finland. For Ecol Manag. 128(3):211–225. doi:10.1016/S0378-1127(99)00148-6.
- Siitonen J. 2001. Oikos editorial forest management, coarse woody debris and saproxylic organisms: fennoscandian boreal forests as an example. Ecol Bull. 49:11–41.
- Siitonen J, Saaristo L. 2000. Habitat requirements and conservation of Pytho kolwensis, a beetle species of old-growth boreal forest. Biol Conserv. 94(2):211–220. doi:10.1016/S0006-3207(99)00174-3.
- Stokland JN, Siitonen J, Jonsson B. 2013. Biodiversity in dead wood. New York: Cambridge University Press.
- Swedish Forest Agency. 2009. Handbok - Stubbskörd, pp. 1–48.
- Swedish Forest Agency. 2023. Hyggesfritt skogsbruk, Swedish Forest Agency. Available at: https://www.skogsstyrelsen.se/bruka-skog/olika-satt-att-skota-din-skog/hyggesfritt-skogsbruk/ (Accessed: 12 June 2023).
- The Royal Swedish Academy of Agriculture and Forestry. 2015. Forests and Forestry in Sweden.
- Toivanen T, et al. 2012. The effect of forest fuel harvesting on the fungal diversity of clear-cuts. Biomass Bioenergy. 39:84–93. doi:10.1016/j.biombioe.2011.11.016.
- Toivanen T, Kotiaho JS. 2010. The preferences of saproxylic beetle species for different dead wood types created in forest restoration treatments. Can J For Res. 40(3):445–464. doi:10.1139/X09-205.
- Uhl B, et al. 2022. Snags, logs, stumps, and microclimate as tools optimizing deadwood enrichment for forest biodiversity. Biol Conserv. 270:109569. doi:10.1016/j.biocon.2022.109569.
- Victorsson J, Jonsell M. 2013. Effects of stump extraction on saproxylic beetle diversity in Swedish clear-cuts. Insect Conservation and Diversity. 6(4):483–493. doi:10.1111/icad.12005.
- Vogel S, et al. 2020. Optimizing enrichment of deadwood for biodiversity by varying sun exposure and tree species: An experimental approach. J Appl Ecol. 57(10):2075–2085. doi:10.1111/1365-2664.13648.
- Walmsley JD, Godbold DL. 2010. Stump harvesting for bioenergy – a review of the environmental impacts. Forestry. 83(1):17–38. doi:10.1093/forestry/cpp028.
- Wikars LO, Sahlin E, Ranius T. 2005. A comparison of three methods to estimate species richness of saproxylic beetles (Coleoptera) in logs and high stumps of Norway spruce. Can Entomol. 137(3):304–324. doi:10.4039/n04-104.
- Zolotarjova V, Kraut A, Lõhmus A. 2016. Slash harvesting does not undermine beetle diversity on small clear-cuts containing sufficient legacies. J Insect Conserv. 20(2):285–294. doi:10.1007/s10841-016-9865-y.