ABSTRACT
The aim of this study was to test the impact of replacing vegetable oils commonly used in the feed of Arctic char (Salvelinus alpinus) with biomass of the oleaginous yeast Rhodotorula toruloides. For that, a control feed containing vegetable oils and an experimental feed including yeast biomass at 15% level, were fed to Arctic char. Sensory properties of the fish were tested. The content of undesirable compounds in the new feed ingredient were evaluated. Selected heavy metals and organic pollutants were measured in the yeast biomass and the levels found were below the limits of the regulations defined by the European Union. Moisture and ash contents were similar between the two feeds. Hepatic activity of 7-ethoxyresorufin-O-deethylase was evaluated between the two fish groups and no significant differences were observed. The sensory analysis conducted on heat treated fish fillets with untrained volunteers showed no perceptible sensory difference.
Introduction
Fish is one of the most traded food commodities worldwide. Fisheries and aquaculture represent an important source of food, income, livelihood and nutrition (FAO, Citation2020). The demand for fish has increased during the last decades due to its highly beneficial protein content and lipid composition. Weekly consumption of fish is recommended by different health agencies and institutes as marine products are an essential source of long chain n-3 fatty acids, EPA 20:5(n-3) and DHA 22:6(n-3). DHA cannot be synthesised in sufficient amounts in humans and is a required element of the diet (Swedish Food Agency, Citation2023; Scientific Advisory Committee on Nutrition (SACN), UK Citation2004). Studies have confirmed the beneficial effects of consumption of marine DHA and EPA on health with improvement of brain function (memory and cell development), neuroprotective effects, antitumor activity and anti-inflammatory effects (Swanson et al., Citation2012; Zhang et al., Citation2019).
To respond to the growing fish consumption, aquaculture has steadily developed while wild capture fisheries have stagnated (FAO, Citation2020). There is a need to investigate new aquafeed ingredients for sustainability while ensuring the health of animals and consumers. Fish feeds for predatory species are traditionally composed of fishmeal and fish oil (FO) as essential constituents, to cover the demand for animal protein and long chain polyunsaturated fatty acids (LCPUFA) of the farmed fish (Tacon et al., Citation2011). The aquafeed ingredient fish oil (FO), rich in n-3 LCPUFA, is often a main limiting component in the production of fish feeds and aquaculture consumes around 75% of the total global supply of FO (Tocher, Citation2015). Parts of FO are currently replaced with vegetable oils (VOs), mainly rapeseed, soy, linseed and palm oil, or with terrestrial animal fat, such as poultry oil and tallow without detrimental effects on fish health or growth (Bell et al., Citation2004; Emery et al., Citation2014; Hatlen et al., Citation2015; Salini et al., Citation2015; Castro et al., Citation2016). Finding alternatives to VOs may reduce monocultures, land use changes, and reduce the carbon footprint of aquaculture (Escobar et al., Citation2009; Azócar et al., Citation2010). The oils sourced from terrestrial plants should be replaced as they represent a competition for food production and strain the use of resources. Research on microbial oils is in progress to enable the use of new suitable and sustainable oil sources such as microalgae, bacteria and yeasts. The utilization of oleaginous yeasts as a lipid source can reduce the impact of food production on the environment and climate (Abeln & Chuck, Citation2021) as already shown for biofuel production by oleaginous yeasts (Karlsson et al., Citation2017). Yeast oil is a promising substitute to VOs as its production on agricultural wastes has been shown to be 10-38% lower in fossil primary energy demand than for the common production of rapeseed oil (Sigtryggsson et al., Citation2023). In this study, we focused on the vegetable parts of the fish feed, and substituted these with an oleaginous yeast biomass.
To produce lipids in a sustainable manner for fish feed, oleaginous yeasts are considered as appropriate candidates. These yeasts have the ability to accumulate 20% and more of their dry matter as lipids and can grow rapidly on low-value substrates, as for example, lignocellulose hydrolysate (Abeln & Chuck, Citation2021; Passoth et al., Citation2023). A number of studies on yeast as a protein source in fish feed has been published (Øverland et al., Citation2013; Vidakovic et al., Citation2020), while little is known about incorporating lipids from yeast biomass in fish feed. At present, yeast culture for food/feed lipid production is costly compared to VOs (Abeln & Chuck, Citation2021) due to the high-value carbon source, such as glucose, currently used for the fermentation process (Qin et al., Citation2017). New culture methodologies with the use of waste glycerol, wastewater and lignocellulose hydrolysate offer an opportunity to include low-value substrates for microbial oil production and could reduce the environmental impact of agriculture and forestry waste (Qin et al., Citation2017; Chmielarz et al., Citation2021). Lignocellulosic biomass, as the most abundant and renewable biopolymer on Earth (Sánchez & Cardona, Citation2008), is a relevant carbon source for yeast production. Yeasts are capable of utilizing nutrients from lignocellulose substrate after a pre-treatment of the lignocellulosic biomass (mechanical, thermo-chemical or both) and after an enzyme hydrolysis releasing monosaccharides from the complex polysaccharide structure (Agboola et al., Citation2021).
We have previously demonstrated in a small-scale study that biomass of the oleaginous yeast Lipomyces starkeyi grown on lignocellulose hydrolysate can be used to replace VOs as a lipid portion in the feed of Arctic char (Salvelinus alpinus) without effects on growth or other general indices (Blomqvist et al., Citation2018). Amino acid content and lipid composition of L. starkeyi biomass-based feed were similar to control feed formulated with VOs rich in saturated (SFA) and monounsaturated fatty acids (MUFA) as for example olive oil or palm oil (Brandenburg et al., Citation2016; Passoth, Citation2017). The present experiment used the oleaginous red yeast Rhodotorula toruloides with a higher level of polyunsaturated fatty acids (PUFA), a faster growth and lipid accumulation compared to L. starkeyi (Brandenburg et al., Citation2021; Passoth et al., Citation2023). Moreover, it produces the carotenoids torulene, torularhodin β – and γ-carotene, which have antioxidant activities (Nagaraj et al., Citation2022).
The inclusion of yeast in fish feed has shown beneficial effects on fish health, in particular on the immune system (Navarrete & Tovar-Ramírez, Citation2014; Agboola et al., Citation2021). Yeast cell walls are partly constituted of β-glucans, mannan-oligosaccharides and chitin, depending on the yeast species. The addition of β-glucans in fish feed through yeasts promotes fish growth rate with increased weight gain, feed efficiency as well as reduced feed conversion ratio and feed intake (Agboola et al., Citation2021). Furthermore, the immune response of fish is improved resulting in increased survival rate and pro-inflammatory cytokine production, improved serum biochemistry and decreased anti-inflammatory cytokine production (Agboola et al., Citation2021). Similarly, mannan-oligosaccharides intake in fish has shown to result in improved growth rate with an increased absorption of nutrients, feed efficiency and improved immune function of fish with enhanced gut barrier function (Agboola et al., Citation2021).
Another important aspect of the sustainable production of food is the consumer acceptance of the food products. Sensory properties like structure, texture, taste and odour are important factors for consumer acceptance (Ruiz-Capillas et al., Citation2021). Therefore, there is a necessity to test possible effects of novel feeds on the sensory aspects of the fish. A previous study on Arctic char evaluated consumers´ perception of fish fillet after replacing the proteins from fishmeal partially with the baker´s yeast (Carlberg et al., Citation2018). The sensory evaluation revealed no differences in the fish fillets between the feeding groups. Exploring the fish fillet perception is important with the inclusion of different microbial ingredients, not used for direct human consumption, in fish feed. The replacement of VOs with microbial oil might affect the consumers’ perception of fish fillet as fat content and types of fatty acids determinate flavours (Besnard et al., Citation2016; Jaime-Lara et al., Citation2023).
The aim of this project was to study the oleaginous yeast R. toruloides, produced on agricultural waste products, as a suitable ingredient in fish feed for Arctic char. This was done (i) by investigating the possible presence and levels of heavy metals and organic pollutants, such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and hexachlorobenzene (HCB) in the yeast ingredient and hydrolysate; (ii) by estimating cytochrome P450A1 monooxygenase (CYP1A1) activity in hepatic microsomes of both groups of fish with the measurement of 7-ethoxyresorufin-O-deethylase (EROD) activity; (iii) by performing a sensory analysis of the farmed Arctic char from the control group fed feeds with vegetable oils and from the experimental group fed with yeast biomass, respectively.
Materials and methods
Hydrolysate preparation and yeast culture
Wheat straw hydrolysate, an agricultural by-product used for growing the yeast biomass in this study, was prepared using steam explosion and enzymatic hydrolysis at the Department of Chemical Engineering, Lund University, Sweden, as described in Blomqvist et al. (Citation2018). Briefly, wheat straw was soaked with 1% acetic acid overnight and the collected biomass was steam exploded at 190°C for 10 min. The liquid fraction was collected and hydrolysed at pH 4.8 at 45°C with 8. Cellic CTec3 enzyme cocktail (Novozyme A/S, Bagsværd, Denmark). After several steps of centrifugation and filtration, the hydrolysate was collected (Blomqvist et al., Citation2018).
The yeast strain Rhodotorula toruloides CBS 14 (Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands), selected as yeast candidate for the experimental feed, was retrieved from 50% v/v glycerol stocks at −80°C and maintained on YM-agar plates (Agar 16 g L−1, glucose 10 g L−1, malt extract 3 g L−1, peptone 5 g L−1, yeast extract 3 g L−1) at 25°C. Before cultivation into fermentors, R. toruloides was inoculated as pre-culture using a loopful of yeast cells from the YM-agar plates into a 100 mL YPD-medium (glucose 20 g L−1, peptone 20 g L−1, yeast extract 10 g L−1) in a 500 mL baffled shake flask on a rotary shaker at 150 rpm (25°C) for 2 days (Blomqvist et al., Citation2018; Nagaraj et al., Citation2022). After inoculation, 100 mL of yeast culture was transferred to a new 400 mL YPD medium into a 3 L Erlenmeyer baffled flasks for inoculation (3 days) in the same conditions as describe before. R. toruloides precultures were harvested by centrifugation (4,000 × g, 10 min) and washed twice with saline solution (NaCl, 9 g L−1). The final cell pellet was resuspended in 10 mL of the saline solution to be added to bioreactors at a final optical density of approximately 5.0 (600 nm).
Cultivations of R. toruloides were performed in 8 L Dolly fermentors (Belach Bioteknik, Stockholm, Sweden) at 25°C, in a total volume of 5 L of lignocellulose hydrolysate (wheat straw) consisting of 60% sterile filtered cellulose- and 40% hemicellulose hydrolysate (Blomqvist et al., Citation2018). A mix of nutrients (1.7 g L−1 Yeast Nitrogen Base without amino acids and without ammonium sulphate (Thermofisher), 1 g L−1 MgSO4 and 2 g L−1 (NH4)3PO4) was added to fermenters (Brandenburg et al., Citation2021) and a desired pH of 6 was automatically controlled and maintained by addition of NaOH (25% w/w) or H3PO4 (3M). The aeration was initiated at 1 L min−1 and increased continuously up to 5 L min−1 during the experiment. The dissolved oxygen tension (pO2) was set to 20% and maintained by changing the stirring speed between 200 and 600 rpm. Polypropylene glycol 2000 (1 mL, Alfa Aesar, Karlsruhe, Germany) was added to the mix to prevent foaming.
After completed fermentation, cells were harvested by centrifugation at 5,400 × g for 10 min followed by a washing step with deionized water and disruption of the yeast cell walls using a French press (Constant systems LTD, Daventry, UK) at 40 psi. The disrupted yeast cells were stored at −20°C until their use in the fish feed.
Fish feed production
To test the effect of microbial ingredients on fish, two feeds were formulated. An experimental feed was prepared using 15% of yeast biomass while control feed contained VOs and casein as described previously (Brunel et al., Citation2022).
To reach a comparable fatty acid composition between experimental and control feeds, the VOs ingredient included in control feed was prepared by mixing together palm oil and rapeseed oil (1:1) obtained from a local supermarket. The remaining ingredients were of similar proportions between the two feeds. The control feed was formulated according to former studies in salmonids (Sánchez-Vázquez et al., Citation1999; Pettersson et al., Citation2009).
As described in Brunel et al. (Citation2022), ingredients included in each feed were blended manually and prepared with a kitchen meat grinder. Each feed was cut into 2-4 mm length pellets and dried overnight at room temperature. The pellets were stored at −20°C in airtight plastic bags until the beginning of the feeding trial. The detailed amino and fatty acid composition of each feed has been presented in Brunel et al. (Citation2022).
Feeding trial
As previously described (Brunel et al., Citation2022), Arctic char (n = 126, juveniles, both sexes) were kept in six water tanks of 1 m × 1 m (water depth of 20 cm) in a flow-through freshwater system (10 L min−1) from Lake Ansjön at Kälarne Aquaculture Centre North, Sweden, with 21 fish per tank. Water temperature was on average at 7.1 ± 1.8°C and rearing was carried out in the natural photoperiod. Before the trial, all fish were randomly assigned to different tanks according to the two feeds. Fish were fed using a commercial feed and left to acclimate for 7 days before the start of the feeding trial. When fish had an approximate mean weight across all tanks of 165 g (with 6 fish measured), the feeding trial started. The control group of fish was fed with control feed containing VOs and casein while experimental fish were fed with the experimental feed containing yeast biomass. During the feeding trial, control and experimental feeds were distributed to the respective tanks by band feeders 4 times a day with a feeding ratio of 2% of the actual biomass in the tanks. After 19 days, fish were weighed individually. The feeding period lasted until fish had almost doubled their weight (72 days, without sampling days). At the end of the trial, fish used for sensory analysis (n = 60) were killed after anaesthesia with a blow to the head. Fish not included in the sensory analysis and sampled for chemical analyses (n = 36) were anaesthetised using tricaine methanesulfonate (MS-222, 30 mg L−1, Sigma Chemicals Co. St. Louis, MO, USA) until overdose and received a blow to the head in the same way as described in Cheng et al., Citation2017. The survival rate of the fish during the feeding trial was 100%. Several growth indices such as the total body length (cm), body and liver weights (g) were recorded as described in Brunel et al. (Citation2022).
Fish sampled for sensory analyses were packed and stored separately as one piece in vacuum bags at −20°C while liver samples were stored at −80°C until analyses. The experiment was carried out in compliance with the European legislation (i.e. Directive 2010/63/EU), and was approved by the Ethical Committee for Animal Experiments in Umeå, Sweden.
Lipid extraction and fatty acid analyses
Lipid extraction and gas liquid chromatography (GLC) methods were used to determine total fat content and FA composition of the feeds and of the fish fillet according to Hara and Radin (Citation1978) and Appelqvist (Citation1968), respectively. Briefly, the samples were homogenized with an Ultra-Turrax (Janke and Kunkel, IKA Werke, Germany) in a hexane-isopropanol mix (8 mL, 3:2, v:v) three times 30 sec and separated with Na2SO4 (6 mL, 6.67%, w:v). The lipid fraction in hexane was transferred and dried under nitrogen gas before gravimetric quantification of the total lipid content. Samples were stored in hexane at −80°C until further analysis. Extracted lipids (2 mg) were methylated using boron trifluoride (BF3) and methanol reagents (Appelqvist, Citation1968). Briefly, both NaOH (2 mL, 0.01 M) in dry methanol and BF3 (3 mL, 14%) were added separately to each sample and exclusively incubated at 60°C for 10 min in the respective order. NaCl (2 mL, 20%) and hexane (2 mL) were added simultaneously to each sample after cooling. The upper phase was transferred to a new tube and evaporated under nitrogen gas. FA methyl esters (FAME) generated were stored in hexane at −80°C for further analysis. A CP 3800 GC instrument (Varian AB, Stockholm, Sweden) was used for FAME analysis in the same way as described in Brunel et al. (Citation2022).
Analyses of feed composition
The lipid content quantification of both feeds was performed as described above. Crude protein content was analysed by Eurofins Food & Feed Testing Sweden AB (Lidköping, Sweden) following the methods ISO 16634-1 2008 and ISO 16634 2016. Amino acid composition was evaluated by Eurofins Food & Feed Testing Sweden AB with SS-EN ISO 13903:2005 method.
The moisture and ash contents of each feed were measured in 5 g of feed according to Nielsen (Citation1994). Shortly, 5 g of feed (in triplicates) was dried at 105°C for 16–18 h for moisture determination. To determine ash content, 5 g of feed (in triplicates) was placed in a muffle furnace at 200°C for two hours and at 550°C for 12 h.
Analyses of toxic compounds
PAHs
Both ingredients used for fish feed preparation, wheat straw hydrolysate samples (n = 3, 50 mL) and yeast samples (n = 3, 20 mL), were screened for presence of PAHs. Each sample (dissolved in 50 mL milli-Q water) was spiked with deuterium-labelled PAHs (Table S1, Supplementary materials) before being liquid–liquid extracted three times using normal-hexane (50 + 40 + 40 mL n-hx). The combined organic phase was gently evaporated to 1 mL (hydrolysate) whereas the yeast samples were evaporated to dryness for gravimetrical lipid determination. After lipid determination, potassium hydroxide dissolved in ethanol (3 mL, 1 M KOH) was added to each yeast sample and saponified for 1 h at 60°C, similar to Sess-Tchotch et al. (Citation2018). Next, water (2 mL milli-Q) and n-hx (3 mL) was added and each sample partitioned (10 min) and centrifuged (850 × g, 10 min). The organic phase was transferred to a new test tube and the water phase was re-extracted two times by adding n-hx (3 + 3 mL) and repeating the partitioning and centrifugation steps. Final extracts from wheat straw hydrolysate and yeast samples were gently evaporated to 1 mL before each sample was applied on clean-up column composed of potassium hydroxide-treated silica (KOH-silica, 5 g) and sodium sulphate (Na2SO4, 3 g). The analytes (PAHs) were eluted with dichloromethane (30 mL DCM) (Arp et al., Citation2014). Each sample was gently evaporated to 0.5 mL and the solvent changed to toluene. Isotope-(13C)-labelled recovery standard (CB-97; Table S2, Supplementary materials) was added and the samples were transferred to GC-vials with a final volume of 0.5 mL.
The instrumental analysis of the PAHs was performed on a GC (Agilent Technologies, 7890B) coupled to a mass spectrometer (MS, Agilent Technologies 5977A) using selected ion monitoring (SIM) mode (Lundstedt et al., Citation2014). Samples were injected in splitless-mode on a DB-5MS column (60 m × 0.25 mm, 0.25 µm; Agilent Technologies) with the following oven temperatures 70°C (2 min), 30°C min−1 until 125°C (2 min), 5°C min−1 until 310°C (5 min). Identification and quantification were performed using authentic reference standards (Table S1, Supplementary materials). The software Agilent MassHunter Quantitative Analysis was used for data evaluation.
PCBs and HCB
In a similar way as with PAHs, wheat straw hydrolysate samples (n = 3, 50 mL) and yeast samples (n = 3, 20 mL), were screened for presence of PCBs and HCB. Each sample (dissolved in 50 mL milli-Q water) was spiked with isotope-(13C)-labelled PCBs and HCB (Table S3, Supplementary materials), before liquid–liquid extraction three times using normal-hexane (50 + 40 + 40 mL n-hx). The combined organic phase was gently evaporated to 1 mL and then applied on a multilayer clean-up column composed of silica (SiO2, 3 g), sulphuric acid treated silica (40% H2SO4:SiO2, 6 g and 20% H2SO4:SiO2, 3 g) and sodium sulphate (Na2SO4, 3 g). The analytes (PCBs and HCB) were eluted with n-hx:DCM (60 mL, 4:1 v/v) (Dahlberg et al., Citation2021). The samples were gently evaporated to 0.5 mL and the solvent changed to isooctane. Isotope-(13C)-labelled recovery standard (CB-97, Table S2, Supplementary materials) was added to each sample and the samples were transferred to GC-vials with a final volume of 100 µL.
The instrumental analysis was performed using GC (Agilent technologies 7890A) coupled to a triple quad mass spectrometer (MS, Agilent technologies 7010 GC/MS Triple Quad) using multiple reaction monitoring mode (Dahlberg et al., Citation2020). Samples were injected in splitless-mode on a DB-5 column (60 m × 0.25 mm, 0.25 µm; Agilent Technologies) with the following oven temperatures 190°C (2 min), 3°C min−1–250°C, then 6°C min−1–310°C (held for 1 min). Identification and quantification were performed using authentic reference standards (Table S3, Supplementary materials). The software Agilent MassHunter Quantitative Analysis (for QQQ) was used for data evaluation.
Heavy metals
Measurements of heavy metals in wheat straw hydrolysate and in yeast samples were performed by ALS Scandinavia AB, Sweden.
Arctic char hepatic microsomes preparation and measurement of EROD activity
The fish metabolic response after ingestion of the new yeast biomass-based feed was assessed with the catalytic activity of CYP1A1through evaluation of EROD activity. CYP1A1 was selected for this study, as it is the most studied isoform in fish due to its important role in the metabolism of xenobiotic compounds (Whyte et al., Citation2000).
Hepatic microsomes (0.5 g of liver) were prepared using the Ca2+ aggregation method (Rasmussen et al., Citation2011). The final microsomal pellets were suspended in 50 mM potassium phosphate buffer containing 20% glycerol (pH 7.4) and stored at −80°C until use. The concentration of protein in the microsomal fractions was determined with a commercially available kit (Bio-Rad Laboratories, Inc.) according to the manufacturer´s instructions, using ɣ-globulin as a standard. The hepatic microsomes were diluted to obtain a protein concentration of 5 mg mL−1.
The EROD activity was estimated as previously described in a study developed for pigs (Zamaratskaia & Zlabek, Citation2009) and adjusted for the activities in the fish. Briefly, 0.2 mg of microsomal protein was incubated in a medium consisting of potassium phosphate buffer (50 mM, pH 7.4) and of 7-ethoxyresorufin (2 μM) in a total volume of 0.5 mL. Enzymatic reactions were initiated by adding NADPH (0.5 mM), followed by an incubation time of 7 min at room temperature. The reactions were stopped by adding 500 μL of ice-cold methanol (100%). Microsomal protein was suspended by centrifugation for 10 min at 7,500 × g. The concentrations of resorufin in the samples were estimated with an ultra-high performance liquid chromatography (UHPLC) using resorufin as a standard curve. EROD activity was estimated based on resorufin formation and expressed as pmol of product formed per minute per milligram of microsomal protein.
The resorufin was quantified on a Shimadzu UHPLC-Nexera series (Kyoto, Japan) equipped with autosampler (SIL – 20AC) and quaternary pumps (LC-20AD). The equipment included, connected in series, a column oven CTO-20AC (at 35°C) and a fluorescence detector RF-20Axs (λexc 530 nm and λem 582 nm, sensitivity was set at ‘high’ and response time 1 sec). Separation of resorufin was achieved with a RP C18 Kinetex 100 Å column (100 mm × 4.6 i.d., 2.6 μm particle size; Phenomenex, Aschaffenburg, Germany) equipped with a guard column and an injection volume of 5 μL. The mobile phase consisted of a methanol and water mix (0.1%, v:v) as well as trifluoroacetic acid (1:1) in isocratic mode. The flow rate was 1 mL min−1. The total run time was 7 min with resorufin eluting at 3.2 min. Data acquisition was performed with LabSolutions Software v. 592. Resorufin calibration curve was prepared under same detection conditions in 400–0.2 pmol mL−1 range.
Sensory test
A sensory analysis with the triangle test method (Sinkinson, Citation2017) was conducted at the Swedish University of Agricultural Sciences (SLU) in November 2019 with 34 untrained volunteers. Fish were filleted while keeping the skin intact, and fillets were cut to pieces of approximately 3 cm × 3 cm. The pieces were steamed-cooked separately in aluminium containers and without any additives in a water bath at 70°C until the core temperature reached 60°C. Volunteers received three samples of fillet with randomized numbers and were asked to identify which of the samples tasted different to the other two. The test persons were requested to look at colour, smell and taste difference.
Statistical analyses
Differences in EROD activity between the two fish groups were analyzed using the PROC MIXED function in SAS software (version 9.4, SAS Institute Inc, Cary, North Carolina, USA) with the different fish tanks as a random factor.
Regarding sensory data, interpretation for significance ‘there is no difference between fish fed with vegetable oils and fish fed with yeast biomass’ was based on the number of answers ‘correct distinction between the two fish with different feeds in taste, texture or others’ within the total amount of answers/participants (N) including a significance level at α ≤ 0.05. According to Sinkinson (Citation2017), with N = 34, a minimum of correct responses for α ≤ 0.05 is equal to 17 to conclude that a difference exists and to reject the hypothesis ‘no difference’.
Results
Safety evaluation of yeast biomass feed: organic pollutants and heavy metals
The levels of PAHs, PCBs and HCB were below the limit of quantification and/or detection for the hydrolysate (wheat straw) used during yeast cultivation (). In yeast sample, PCBs were below levels of detection and/or quantification whereas PAHs were present and measured at a concentration of 200 ng g−1 lipid and HCB at a concentration of 0.21 ng g−1 lipid.
Table 1. Concentrations of organic pollutants in hydrolysate and yeast samples (mean ± standard deviation, n = 3). Concentrations are reported as the total sum of single compounds quantified in the sample.
The proportion of yeast oil included in the fish feed was 5.8%, corresponding to a quantity of 11 ng g−1 of PAHs and a quantity of 0.01 ng g−1 of HCB in fish feed.
All evaluated heavy metals were detected below the allowed values for the European legislation for foodstuffs directly consumed (). Mercury was not detected in any sample (hydrolysate and yeast biomass).
Table 2. Heavy metal concentration (mg kg−1 or mg L−1) in hydrolysate and in yeast biomass compared with maximum levels for contaminants in foodstuffs by EU legislation (EU leg.), European Food Safety Authority (EFSA) and FAO & WHO.
Moisture and ash contents of the fish feeds
Moisture and ash contents were evaluated and added to previously published results on the composition of both fish feeds (Brunel et al., Citation2022). The moisture and ash proportions of the control and experimental feeds were similar with 10% of moisture and 10–12% of ash ().
Table 3. Composition of control and experimental yeast feeds (% of dry matter) for fish in duplicates. Table from Brunel et al. (Citation2022), under Creative Commons Attribution License CC BY 4.0. ‘Vitamin mix’ and ‘Mineral mix’ ingredients were provided by NOFIMA (Norway) and ‘Astaxanthin & vitamin mix’ ingredients were provided by Aller Aqua A/S (Denmark). Further details on vitamin and mineral mixes composition can be found as supplementary materials, Table S4.
Fish lipid profiles and liver biomarker
Fish growth parameters were previously evaluated (Brunel et al., Citation2022) and shown to be similar between the two fish groups with the exception of liver weight and hepatosomatic index (HSI), being significantly higher in fish fed with the experimental feed.
To further confirm a similar fish metabolism between the two feed groups, lipid profiles of fillets from large and small fish from the same feeding trial were retrieved from Jacobsson (Citation2022), and compared with results obtained in Brunel et al. (Citation2022). Fatty acid profiles of fish fillet between the control and experimental fish groups and between large and small fish were comparable (). Total fat content of fish fillet from the same experimental study carried out at Aquaculture Center North (Sweden) was comparable between Jacobsson (Citation2022) and Brunel et al. (Citation2022) for the experimental fish groups and varied for the control fish groups.
Table 4a. Total fat content and fatty acid profile (%) of fish fillet from Arctic char fed with yeast biomass (experimental fish group). Data from Jacobsson (Citation2022) and Brunel et al. (Citation2022) under open access or under Creative Commons Attribution License CC BY 4.0. Data are presented as the mean ± standard deviation, with n sample size per group of fish.
Table 4b. Total fat content and fatty acid profile (%) of fish fillet from Arctic char fed with vegetable oil ingredients (control fish group). Data from Jacobsson (Citation2022) and Brunel et al. (Citation2022) under open access or under Creative Commons Attribution License CC BY 4.0. Data are presented as the mean ± standard deviation, with n sample size per group of fish.
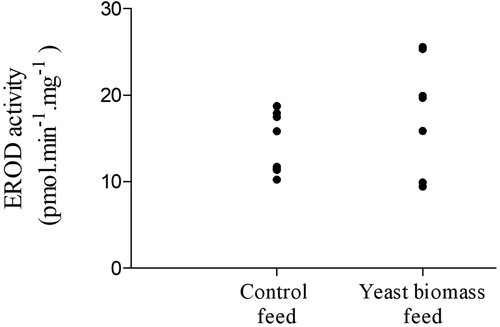
To understand the higher liver weight and HSI observed in Brunel et al. (Citation2022), EROD activity was measured in the livers and was found similar between the fish fed with the experimental feed and the fish fed with the control feed (; p = 0.17 with tank factor; p = 0.11 without tank factor).
Sensory test
A sensory triangle test was included in this study to account for potential effects of the yeast biomass on fish sensory attributes. From the total number of participants attending the triangle test at SLU (34), seven individuals recognized a difference in the sensory characteristics of the fish servings. According to the study by Sinkinson (Citation2017), if the minimum number of correct answers in a triangle test is more than 17 for a total number of participants of 34 and with a risk of 5%, there is a significant difference. In this study, the number of correct answers was less than 17 (), therefore, we can conclude that participants could not identify a perceptible difference in fish from different feeds by appearance, taste, texture and smell.
Table 5. Sensory analysis of fish fillets on untrained volunteers (p < 0.05).Footnote1
Discussion
Oleaginous yeast biomass was tested as a new ingredient in fish feed to improve sustainability of aquaculture. Finding renewable and safe alternatives to the current lipid portion of fish feeds could benefit to the aquaculture fish biodiversity as well as to the human populations relying extensively on fish production, with fish as a source of essential nutrients and as a main source of income (FAO, Citation2020). The modification of fish feed ingredients can lead to a change in flavour, texture and nutritional quality of the fish. In order to encourage consumption of fish and to ensure its attractiveness to consumers, a positive sensory experience is important. Flavour, odour and texture contribute significantly to food choice, appetite and intake (Boesveldt et al., Citation2010; Boesveldt & de Graaf, Citation2017). This makes flavour one of the most important determinants of food choice. Therefore, it is essential to confirm a high sensory quality of nutritional valuable products such as fish and evaluate if taste is affected by novel feed ingredients. The appearance, taste, texture or smell of the fish fed different feeds could affect consumer acceptance of fish fed yeast biomass.
To assess possible effects of differently fed fish on sensory quality for human consumption, a sensory analysis with the discriminative method triangle test was performed in this study with untrained individuals. The majority of the subjects could not identify a difference between fish from the different feeding groups in a large perspective (smell, texture and colour). The lipid levels in fish muscle can affect the sensory attributes of the flesh (Rincón et al., Citation2016). The lipid content of fish fillets from the same experimental trial showed similar levels between the differently fed fish in our previously published results (Brunel et al., Citation2022). When comparing between groups of large and small fish only (based on length and weight) from the same trial (Brunel et al., Citation2022), the total lipid percentages of fish fillet were also found similar between the different fish sizes and between the feeding groups (Jacobsson, Citation2022).
In addition, the fatty acid lipid classes of fillets from large and small fish of the same feeding trial have been previously analysed (Jacobsson, Citation2022). The detection of free fatty acids has been linked to lipid degradation by enzymatic hydrolysis (Andevari & Rezaei, Citation2011). Levels of free fatty acids related to lipid damage tend to increase over time (Refsgaard et al., Citation2000; Andevari & Rezaei, Citation2011) and with higher storage temperature (Refsgaard et al., Citation2000). Free fatty acid levels can be an indicator of fish freshness (Refsgaard et al., Citation2000; Andevari & Rezaei, Citation2011). In small and large fish fillets from Jacobsson (Citation2022), the percentage of free fatty acids was similar between the fish groups, indicating that the addition of yeast biomass to the feed did not alter the fish freshness and confirms our sensory results. Differences in fatty acid profile of fish muscle can lead to organoleptic differences as volatile compounds are produced from the degradation of unsaturated fatty acids (Rincón et al., Citation2016). The similar fatty acid composition of fish fillets between the control and experimental fish earlier published in this trial (Brunel et al., Citation2022; Jacobsson, Citation2022) and between small-sized and large-sized fish groups confirmed that no difference should be observed in the lipid degradation of fish fillets. The low and similar levels of free fatty acids in fish fillets from all groups (Jacobsson, Citation2022) indicate a low lipid degradation, reflecting the sensory outcome. Therefore, no further sensorial evaluation was conducted. A larger scale of fish production based on the feed with yeast included is possible from this point of view.
When novel ingredients are added to feeds, it is important to ensure that they do not harm the organism. Analyses of potential toxic compounds in the yeast biomass and yeast hydrolysate were necessary to assess the safety of using yeast lipids as feed additive since the production of biomass includes thermochemical, chemical and biochemical treatments leading to potential contamination (Ochsenreither et al., Citation2016; Yan et al., Citation2020). The substrate used for growing the yeast as well as the yeast biomass were therefore tested for possible organic pollutants and heavy metals.
No organic pollutants (PAHs, PCBs and HCB) could be quantified in the used wheat straw hydrolysate prepared for yeast growth. Nevertheless, PAHs and HCB were quantified in the lipid fraction of the yeast biomass. In particular, acenapthalene, fluoranthene and pyrene were found in the oil of the yeast biomass at concentrations between 8.4 and 155 ng g−1. As the yeast oil only accounts for 5.8% of the feed, the levels of organic pollutants from the yeast biomass in the feed were below the allowed levels by the European Commission. The Commission Regulation (EC) (Citation2011) sets a maximum of sum PAHs (benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene) in oils and fats intended for direct human consumption or used as an ingredient in food at 10 µg kg−1 or 10 ng g−1. The PAHs listed above and regulated by the European Commission, were not detected in our study. The metabolic excretion of most PAHs has been described earlier as efficient in fish as they can be eliminated within 2–8 days after exposure (Vethaak et al., Citation2016), supporting the safety of adding the yeast biomass to the fish feed. Similar to PAHs levels, the HCB level detected in oil from the yeast biomass (0.21 ng g−1 total detected from 5.8% of lipids in total feed, equivalent to 0.01 ng g−1 in feed) was considered safe as the Commission Directive (Citation2006) allows a maximum of 0.2 µg kg−1 of HCB in products intended for animal feed for fats and oils.
Analysis of heavy metals revealed that all heavy metals detected in the yeast biomass and the wheat straw hydrolysate were below the allowed levels by the European legislation (Commission Regulation (EC) (Citation2006), for most compounds). Because the levels of heavy metals and organic pollutants in the yeast biomass and in the lignocellulose hydrolysate were low or below quantification, these toxicants were not analysed in fish fillets.
The earlier seen higher liver weight and HSI in the experimental fish (Brunel et al., Citation2022) were probably not due to an exposure to chemicals as EROD activity levels were not significantly different between the two fish groups. This result suggests that the detoxification process in the liver from the two differently fed groups of fish was comparable.
Both control and experimental feeds were prepared in the laboratory with the same ingredients with the exception of the yeast, allowing a complete comparison in their compositions. The analysis of the proximate composition of the feed showed a similar content of protein, ash, lipids and moisture, as expected. The yeast biomass was included as whole in the experimental feed after mechanical disruption of yeast cell walls. Therefore, no oil extraction was required and the use of organic solvents for lipid extraction was not necessary. As a result, the sustainability of the new ingredient production was improved. The inclusion of a yeast containing a relatively high content of carotenoids with possible antioxidant properties (Nagaraj et al., Citation2022) into the feed could in addition improve the storage time of the feed. This effect should be further evaluated. A physical disruption of the yeast cell walls was performed by French press to improve the digestibility of the nutrients found in yeast and to release polysaccharides having possibly beneficial effects on the fish immune system (prebiotics) such as β-glucans, chitin or mannoproteins (Navarrete & Tovar-Ramírez, Citation2014). Nevertheless, the composition of yeast R. toruloides is not extensively described in literature, and in particular for the carbohydrate content. Macronutrient composition of R. toruloides is variable as it depends on its growing conditions (carbon and nitrogen availability) with approximately 40% of lipids, 20% carbohydrates and 15-25% proteins (Shen et al., Citation2017; Glencross et al., Citation2020). There is a need to further evaluate possible effects of different substrates on the nutrient composition of R. toruloides and potential effects of the yeast as prebiotics in fish. The higher liver weight observed in the fish as a response to the experimental feed during this study still needs to be further investigated.
Conclusions
Our results indicated that the replacement of VOs with an oleaginous yeast biomass in fish feed did not affect the sensory attributes of fish fillets, therefore no negative outcome on consumer experience can be expected. No environmental contaminants were detected above the limit of legislation in the yeast biomass. EROD levels in the fish liver were similar between the feeding groups, confirming the safety of the feed. Further studies on the effect of novel microbial fish feed sources on fish welfare and metabolism, on fillet quality as well as on feed stability should be conducted and applied to other fish species.
Author contributions
Mathilde Brunel: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Viktoriia Burkina: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Sabine Sampels: Conceptualization, Investigation, Project administration, Writing – review & editing. Anna-Karin Dahlberg: Investigation, Writing – original draft, Writing – review & editing. Volkmar Passoth: Conceptualization, Funding acquisition, Resources, Writing – review & editing. Jana Pickova: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Supplementary_material_Biomass_sensory_Brunel
Download PDF (143.9 KB)Acknowledgements
The authors would like to thank to Albina Bakeeva, Yashaswini Nagavara Nagaraj, Giselle De La Caridad Martín Hernández, Simona Rinaldi, Josef Vaclavik, Mikolaj Chmielarz and Nils Mikkelsen for their help with feed preparation and fish sampling. Special thanks to Galia Zamaratskaia for her help as a consultant in the method development of ethoxyresorufin-O-deethylase activity in hepatic microsomes on (U)HPLC. The authors sincerely thank Karin Wiberg for enabling the use of the instruments to measure organic pollutants. Many thanks to Odd Helge Romarheim from Nofima for providing mineral and vitamin mixes included in the fish feed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of the study are available from the corresponding author upon reasonable request.
Additional information
Funding
Notes
1 Sinkinson (Citation2017).
References
- Abeln, F. & Chuck, C. J. (2021). The history, state of the art and future prospects for oleaginous yeast research. Microbial Cell Factories, 20(1), 221. doi:10.1186/s12934-021-01712-1
- Agboola, J. O., Øverland, M., Skrede, A. & Hansen, JØ. (2021). Yeast as major protein-rich ingredient in aquafeeds: A review of the implications for aquaculture production. Reviews in Aquaculture, 13(2), 949–970. doi:10.1111/raq.12507
- Andevari, G. T. & Rezaei, M. (2011). Effect of gelatin coating incorporated with cinnamon oil on the quality of fresh rainbow trout in cold storage. International Journal of Food Science & Technology, 46(11), 2305–2311. doi:10.1111/j.1365-2621.2011.02750.x
- Appelqvist, L-Å. (1968). Rapid methods of lipid extraction and fatty acid methyl ester preparation for seed and leaf tissue with special remarks on preventing the accumulation of lipid contaminants. Arkiv för Kemi, 28, 551–570.
- Arp, H. P. H., Lundstedt, S., Josefsson, S., Cornelissen, G., Enell, A., Allard, A. S. & Kleja, D. B. (2014). Native Oxy-PAHs, N-PACs, and PAHs in historically contaminated soils from Sweden, Belgium, and France: Their soil-porewater partitioning behavior, bioaccumulation in Enchytraeus crypticus, and bioavailability. Environmental Science & Technology, 48(19), 11187–11195. doi:10.1021/es5034469
- Azócar, L., Ciudad, G., Heipieper, H. J. & Navia, R. (2010). Biotechnological processes for biodiesel production using alternative oils. Applied Microbiology and Biotechnology, 88(3), 621–636. doi:10.1007/s00253-010-2804-z
- Bell, J. G., Henderson, R. J., Tocher, D. R. & Sargent, J. R. (2004). Replacement of dietary fish oil with increasing levels of linseed oil: Modification of flesh fatty acid compositions in Atlantic salmon (Salmo salar) using a fish oil finishing diet. Lipids, 39(3), 223–232. doi:10.1007/s11745-004-1223-5
- Besnard, P., Passilly-Degrace, P. & Khan, N. A. (2016). Taste of fat: A sixth taste modality? Physiological Reviews, 96, 151–176. doi:10.1152/physrev.00002.2015
- Blomqvist, J., Pickova, J., Tilami, S. K., Sampels, S., Mikkelsen, N., Brandenburg, J., Sandgren, M. & Passoth, V. (2018). Oleaginous yeast as a component in fish feed. Scientific Reports, 8(1), 1–8. doi:10.1038/s41598-018-34232-x
- Boesveldt, S. & de Graaf, K. (2017). The differential role of smell and taste for eating behavior. Perception, 46(3–4), 307–319. doi:10.1177/0301006616685576
- Boesveldt, S., Frasnelli, J., Gordon, A. R. & Lundström, J. N. (2010). The fish is bad: Negative food odors elicit faster and more accurate reactions than other odors. Biological Psychology, 84, 313–317. doi:10.1016/j.biopsycho.2010.03.006
- Brandenburg, J., Blomqvist, J., Pickova, J., Bonturi, N., Sandgren, M. & Passoth, V. (2016). Lipid production from hemicellulose with Lipomyces starkeyi in a pH regulated fed-batch cultivation. Yeast, 33(8), 451–462. doi:10.1002/yea.3160
- Brandenburg, J., Blomqvist, J., Shapaval, V., Kohler, A., Sampels, S., Sandgren, M. & Passoth, V. (2021). Oleaginous yeasts respond differently to carbon sources present in lignocellulose hydrolysate. Biotechnology for Biofuels, 14, 1–12. doi:10.1186/s13068-021-01974-2
- Brunel, M., Burkina, V., Pickova, J., Sampels, S. & Moazzami, A. A. (2022). Oleaginous yeast Rhodotorula toruloides biomass effect on the metabolism of Arctic char (Salvelinus alpinus). Frontiers in Molecular Biosciences, 9, 931946. doi:10.3389/fmolb.2022.931946
- Carlberg, H., Lundh, T., Cheng, K., Pickova, J., Langton, M., Gutiérrez, J. L. V., Kiessling, A. & Brännäs, E. (2018). In search for protein sources: Evaluating an alternative to the traditional fish feed for Arctic charr (Salvelinus alpinus L.). Aquaculture, 486, 253–260. doi:10.1016/j.aquaculture.2017.12.027
- Castro, C., Couto, A., Pérez-Jiménez, A., Serra, C. R., Díaz-Rosales, P., Fernandes, R., Corraze, G., Panserat, S. & Oliva-Teles, A. (2016). Effects of fish oil replacement by vegetable oil blend on digestive enzymes and tissue histomorphology of European sea bass (Dicentrarchus labrax) juveniles. Fish Physiology and Biochemistry, 42(1), 203–217. doi:10.1007/s10695-015-0130-1
- Cheng, K., Müllner, E., Moazzami, A. A., Carlberg, H., Brännäs, E. & Pickova, J. (2017). Metabolomics approach to evaluate a Baltic sea sourced diet for cultured Arctic char (Salvelinus alpinus L.). Journal of Agricultural and Food Chemistry, 65(24), 5083–5090. doi:10.1021/acs.jafc.7b00994
- Chmielarz, M., Blomqvist, J., Sampels, S., Sandgren, M. & Passoth, V. (2021). Microbial lipid production from crude glycerol and hemicellulosic hydrolysate with oleaginous yeasts. Biotechnology for Biofuels, 14(1), 1–11. doi:10.1186/s13068-021-01916-y
- Commission Directive. (2006). 2006/77/EC amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for organochlorine compounds in animal feed. OJ L, 271, 53–55; OJ L 314M;254-256. http://data.europa.eu/eli/dir/2006/77/oj
- Commission Regulation (EC). (2006). No 1881/2006. Setting maximum levels for certain contaminants in foodstuffs. OJ L, 364, 5–24; OJ L 314M;558-577. http://data.europa.eu/eli/reg/2006/1881/oj
- Commission Regulation (EC). (2011). No 835/2011. Regulation (EC) (2011). Amending No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. OJ L, 215, 4–8. http://data.europa.eu/eli/reg/2011/835/oj.
- Dahlberg, A. K., Apler, A., Frogner-Kockum, P., Göransson, G., Snowball, I., Wiberg, K. & Josefsson, S. (2021). Dispersal of persistent organic pollutants from fiber-contaminated sediments: biotic and abiotic pathways. Journal of Soils and Sediments, 21(4), 1852–1865. doi:10.1007/s11368-020-02871-1
- Dahlberg, A. K., Apler, A., Vogel, L., Wiberg, K. & Josefsson, S. (2020). Persistent organic pollutants in wood fiber–contaminated sediments from the Baltic sea. Journal of Soils and Sediments, 20(5), 2471–2483. doi:10.1007/s11368-020-02610-6
- Emery, J. A., Smullen, R. P. & Turchini, G. M. (2014). Tallow in Atlantic salmon feed. Aquaculture, 98–108. doi:10.1016/j.aquaculture.2013.12.004
- Escobar, J. C., Lora, E. S., Venturini, O. J., Yáñez, E. E., Castillo, E. F. & Almazan, O. (2009). Biofuels: environment, technology and food security. Renewable and Sustainable Energy Reviews, 13(6–7), 1275–1287. doi:10.1016/j.rser.2008.08.014
- European Food Safety Authority, EFSA. (2008). Safety of aluminium from dietary intake – Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA Journal, 6, 1–34. doi:10.2903/j.efsa.2008.754
- FAO. (2020). The State of World Fisheries and Aquaculture 2020. Sustainability in Action (Rome: Food and Agriculture Organization of the United Nations (FAO)). doi:10.4060/ca9229en
- FAO & WHO. (1995). General Standard for Contaminants and Toxins in Food and Feed. Codex Alimentarius International Food Standards, CXS 193-1995 (Food and Agriculture Organization of the United Nations (FAO) & World Health Organization (WHO)). https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf).
- Glencross, B. D., Huyben, D. & Schrama, J. W. (2020). The application of single-cell ingredients in aquaculture feeds—A review. Fishes, 5(3), 22–39. doi:10.3390/fishes5030022
- Hara, A. & Radin, N. S. (1978). Lipid extraction of tissues with a low-toxicity solvent. Analytical Biochemistry, 90(1), 420–426. doi:10.1016/0003-2697(78)90046-5
- Hatlen, B., Jakobsen, J.-V., Crampton, V., Alm, M., Langmyhr, E., Espe, M., Hevrøy, E. M., Torstensen, B. E., Liland, N. & Waagbø, R. (2015). Growth, feed utilization and endocrine responses in Atlantic salmon (Salmo salar) fed diets added poultry by-product meal and blood meal in combination with poultry oil. Aquaculture Nutrition, 21(5), 714–725. doi:10.1111/anu.12194
- Jacobsson, S. (2022). Fatty acid composition in Arctic char (Salvelinus alpinus) fed with red yeast biomass: a comparison between large and small specimens. Second cycle, A2E (Uppsala: SLU, Department of Molecular Sciences).
- Jaime-Lara, R. B., Brooks, B. E., Vizioli, C., Chiles, M., Nawal, N., Ortiz-Figueroa, R. S. E., Livinski, A. A., Agarwal, K., Colina-Prisco, C., Iannarino, N., Hilmi, A., Tejeda, H. A. & Joseph, P. V. (2023). A systematic review of the biological mediators of fat taste and smell. Physiological Reviews, 103, 855–918. doi:10.1152/physrev.00061.2021
- Karlsson, H., Ahlgren, S., Sandgren, M., Passoth, V., Wallberg, O. & Hansson, P. A. (2017). Greenhouse gas performance of biochemical biodiesel production from straw: Soil organic carbon changes and time-dependent climate impact. Biotechnology for Biofuels, 10(1), 1–15. doi:10.1186/s13068-017-0907-9
- Lundstedt, S., Bandowe, B. A. M., Wilcke, W., Boll, E., Christensen, J. H., Vila, J., Grifoll, M., Faure, P., Biache, C., Lorgeoux, C., Larsson, M., Frech Irgum, K., Ivarsson, P. & Ricci, M. (2014). First intercomparison study on the analysis of oxygenated polycyclic aromatic hydrocarbons (oxy-PAHs) and nitrogen heterocyclic polycyclic aromatic compounds (N-PACs) in contaminated soil. TrAC Trends in Analytical Chemistry, 57, 83–92. doi:10.1016/j.trac.2014.01.007
- Nagaraj, Y. N., Burkina, V., Okmane, L., Blomqvist, J., Rapoport, A., Sandgren, M., Pickova, J., Sampels, S. & Passoth, V. (2022). Identification, quantification and kinetic study of carotenoids and lipids in Rhodotorula toruloides CBS 14 cultivated on wheat straw hydrolysate. Fermentation, 8(7), 300–316. doi:10.3390/fermentation8070300
- Navarrete, P. & Tovar-Ramírez, D. (2014). Use of yeasts as probiotics in fish aquaculture. In M. P. Hernandez-Vergara & C. I. Perez-Rostro (eds.) Sustainable Aquaculture Techniques (Intech Open). doi:10.5772/57196
- Nielsen, S. (1994). Introduction to the Chemical Analysis of Foods (Boston: Jones & Bartlett Publ.), pp. 113–121. doi:10.1007/978-1-4419-1478-1
- Ochsenreither, K., Glück, C., Stressler, T., Fischer, L. & Syldatk, C. (2016). Production strategies and applications of microbial single cell oils. Frontiers in Microbiology, 7, 1539. doi:10.3389/fmicb.2016.01539
- Øverland, M., Karlsson, A., Mydland, L. T., Romarheim, O. H. & Skrede, A. (2013). Evaluation of Candida utilis, Kluyveromyces marxianus and Saccharomyces cerevisiae yeasts as protein sources in diets for Atlantic salmon (Salmo salar). Aquaculture, 1–7. doi:10.1016/j.aquaculture.2013.03.016
- Passoth, V. (2017). Lipids of yeasts and filamentous fungi and their importance for biotechnology. In A. A. Sibirny (ed.) Biotechnology of Yeasts and Filamentous Fungi (Cham: Springer), pp. 149–204. doi:10.1007/978-3-319-58829-2_6
- Passoth, V., Brandenburg, J., Chmielarz, M., Martín-Hernández, G. C., Nagaraj, Y., Müller, B. & Blomqvist, J. (2023). Oleaginous yeasts for biochemical, biofuels and food from lignocellulose-hydrolysate and crude glycerol. Yeast, 290–302. doi:10.1002/yea.3838
- Pettersson, A., Johnsson, L., Brännäs, E. & Pickova, J. (2009). Effects of rapeseed oil replacement in fish feed on lipid composition and self-selection by rainbow trout (Oncorhynchus mykiss). Aquaculture Nutrition, 15(6), 577–586. doi:10.1111/j.1365-2095.2008.00625.x
- Qin, L., Liu, L., Zeng, A. P. & Wei, D. (2017). From low-cost substrates to single cell oils synthesized by oleaginous yeasts. Bioresource Technology, 245, 1507–1519. doi:10.1016/j.biortech.2017.05.163
- Rasmussen, M. K., Ekstrand, B. & Zamaratskaia, G. (2011). Comparison of cytochrome P450 concentrations and metabolic activities in porcine hepatic microsomes prepared with two different methods. Toxicology In Vitro, 25(1), 343–346. doi:10.1016/j.tiv.2010.10.007
- Refsgaard, H. H. F., Brockhoff, P. M. B. & Jensen, B. (2000). Free polyunsaturated fatty acids cause taste deterioration of salmon during frozen storage. Journal of Agricultural and Food Chemistry, 48(8), 3280–3285. doi:10.1021/jf000021c
- Rincón, L., Castro, P. L., Álvarez, B., Hernández, M. D., Álvarez, A., Claret, A., Guerrero, L. & Ginés, R. (2016). Differences in proximal and fatty acid profiles, sensory characteristics, texture, colour and muscle cellularity between wild and farmed blackspot seabream (Pagellus bogaraveo). Aquaculture, 451, 195–204. doi:10.1016/j.aquaculture.2015.09.016
- Ruiz-Capillas, C., Herrero, A. M., Pintado, T. & Delgado-Pando, G. (2021). Sensory analysis and consumer research in new meat products development. Foods (basel, Switzerland), 10, 429–415. doi:10.3390/foods10020429
- Salini, M., Irvin, S., Bourne, N., Blyth, D., Cheers, S., Habilay, N. & Glencross, B. (2015). Marginal efficiencies of long chain-polyunsaturated fatty acid use by barramundi (Lates calcarifer) when fed diets with varying blends of fish oil and poultry fat. Aquaculture, 449, 48–57. doi:10.1016/j.aquaculture.2015.02.027
- Sánchez, ÓJ & Cardona, C. A. (2008). Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresource Technology, 99, 5270–5295. doi:10.1016/j.biortech.2007.11.013
- Sánchez-Vázquez, F. J., Yamamoto, T., Akiyama, T., Madrid, J. A. & Tabata, M. (1999). Macronutrient self-selection through demand-feeders in rainbow trout. Physiology & Behavior, 66(1), 45–51. doi:10.1016/S0031-9384(98)00313-8
- Scientific Advisory Committee on Nutrition (SACN), UK. (2004). Advice on Fish Consumption: Benefits & Risks. Committee on Toxicity (London: TSO). https://www.gov.uk/government/publications/sacn-advice-on-fish-consumption).
- Sess-Tchotch, D. A., Kedjebo, K. B. D., Faulet, B. M., Fontana-Tachon, A., Alter, P., Durand, N., Grabulos, J., Montet, D. & Guehi, T. S. (2018). Analytical method validation and rapid determination of polycyclic aromatic hydrocarbons (PAHs) in cocoa butter using HPLC-FLD. Food Analytical Methods, 11(11), 3138–3146. doi:10.1007/s12161-018-1282-2
- Shen, H., Zhang, X., Gong, Z., Wang, Y., Yu, X., Yang, X. & Zhao, Z. K. (2017). Compositional profiles of Rhodosporidium toruloides cells under nutrient limitation. Applied Microbiology and Biotechnology, 101(9), 3801–3809. doi:10.1007/s00253-017-8157-0
- Sigtryggsson, C., Karlsson Potter, H., Passoth, V. & Hansson, P. A. (2023). From straw to salmon: A technical design and energy balance for production of yeast oil for fish feed from wheat straw. Biotechnology for Biofuels and Bioproducts, 16(1), 1–12. doi:10.1186/s13068-023-02392-2
- Sinkinson, C. (2017). Discrimination testing in sensory science. Practical Handbook, 153–170. doi:10.1016/B978-0-08-101009-9.00007-1
- Swanson, D., Block, R. & Mousa, S. A. (2012). Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Advances in Nutrition, 3(1), 1–7. doi:10.3945/an.111.000893
- Swedish Food Agency. (2023). Livsmedelsverket. Fish and shellfish- advice. https://www.livsmedelsverket.se/en/food-habits-health-and-environment/dietary-guidelines/adults/fisk-och-skaldjur—rad
- Tacon, A. G. J., Hasan, M. R., & Metian, M. (2011). Demand and supply of feed ingredients for farmed fish and crustaceans: trends and prospects. FAO Fisheries and Aquaculture Technical Paper, 564, 1–87.
- Tocher, D. R. (2015). Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture, 449, 94–107. doi:10.1016/j.aquaculture.2015.01.010
- Vethaak, A. D., Baggelaar, P. K., van Lieverloo, J. H. M. & Ariese, F. (2016). Decadal trends in polycyclic aromatic hydrocarbon (PAH) contamination assessed by 1-hydroxypyrene in fish bile fluid in The Netherlands: declining in marine waters but still a concern in estuaries. Frontiers in Marine Science, 3, 215. doi:10.3389/fmars.2016.00215
- Vidakovic, A., Huyben, D., Sundh, H., Nyman, A., Vielma, J., Passoth, V., Kiessling, A. & Lundh, T. (2020). Growth performance, nutrient digestibility and intestinal morphology of rainbow trout (Oncorhynchus mykiss) fed graded levels of the yeasts Saccharomyces cerevisiae and Wickerhamomyces anomalus. Aquaculture Nutrition, 26(2), 275–286. doi:10.1111/anu.12988
- Whyte, J. J., Jung, R. E., Schmitt, C. J. & Tillitt, D. E. (2000). Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Critical Reviews in Toxicology, 30(4), 347–570. doi:10.1080/10408440091159239
- Yan, J., Karlsson, A., Zou, Z., Dai, D. & Edlund, U. (2020). Contamination of heavy metals and metalloids in biomass and waste fuels: comparative characterisation and trend estimation. Science of the Total Environment, 700, 134382. doi:10.1016/j.scitotenv.2019.134382
- Zamaratskaia, G. & Zlabek, V. (2009). EROD and MROD as markers of cytochrome P450 1A activities in hepatic microsomes from entire and castrated male pigs. Sensors, 9(3), 2134–2147. doi:10.3390/s90302134
- Zhang, T. T., Xu, J., Wang, Y. M. & Xue, C. H. (2019). Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Progress in Lipid Research, 75, 100997. doi:10.1016/j.plipres.2019.100997