ABSTRACT
Hyperactivity of coagulation is common in exertional heatstroke (EHS). Disseminated intravascular coagulation (DIC) is the most severe form of coagulation dysfunction and associated with poor outcome. DIC, temperature and Glasgow coma scale score were identified as independent risk factors for in-hospital mortality by multivariate logistic regression analysis, and we developed a nomogram for predicting in-hospital mortality in a 13-year EHS patient cohort. The nomogram was assessed by calibration curves and bootstrap with 1,000 resamples. The receiver operating characteristic curve was constructed, and the area under the curve (AUC) was compared. Two hundred and ten patients were included. The in-hospital mortality was 9.0%, and the incidence of DIC was 17.6%. The AUC of the nomogram was 0.897 (95% CI 0.848–0.935, p < .0001) and was non-inferior to SOFA and APACHE II scores but superior to SIRS score, which were widely-used score systems of disease severity. The nomogram contributed to the adverse outcome prediction of EHS.
Background
Heatstroke (HS), an acute life-threatening heat-related illness, is clinically manifested as core temperature >40°C, central nervous system abnormalities and multiple organ dysfunction syndrome (MODS). HS is categorized as either classic HS (CHS) or exertional HS (EHS) according to exogenous or endogenous source of heat (Epstein et al. Citation2019; Bouchama et al. Citation2022). With the deterioration of global warming, the heat-related mortality was estimated to increase by 257% and 535% in the 2050s and 2080s, respectively, from a baseline of around 2000 deaths (Hajat et al. Citation2014; Matthews et al. Citation2017). Since the pathogenic mechanism of HS complicated with MODS remains unclear, the mortality of CHS and EHS reaches 63.2% and 26.5% even under critical care, respectively (Bouchama et al. Citation2022). Systemic inflammatory response and overactivation of coagulation system induced by heat cytotoxicity were regarded as two vital mechanisms of MODS. Disseminated intravascular coagulation (DIC) is the most severe form of coagulation disorder and associated with organ injury in mouse model (Proctor et al. Citation2020). Thrombocytopenia, elevated fibrin degradation products and prolonged clotting times were reported in both HS canine model and patients with DIC (Diehl et al. Citation2000; Zeng et al. Citation2023). The HS patients with DIC more likely developed MODS and had a lower 30-day survival rate than those without DIC (6.3% vs 47.8%) (Zeng et al. Citation2023). The incidence of DIC in HS was 21.7–32.1% (Sithinamsuwan et al. Citation2009). Although the presence of DIC is an independent risk factor of hospital mortality in the elderly with HS and treatment with recombinant human thrombomodulin, an endothelial anticoagulant cofactor, showed potential improvement of in-hospital mortality (Kawasaki et al. Citation2014; Hifumi et al. Citation2018; Ohbe et al. Citation2019), there were few predictive models based on DIC.
The accurate prediction of onset of HS benefits from continuous attempts, such as multi-segment multi-node human thermoregulatory model developed by Deng et al. (Citation2018). Besides, the complicated condition of HS patients called for a score system that satisfies the evaluation of progression and prognosis. Sequential organ failure assessment (SOFA) score, acute physiology and chronic health evaluation II (APACHE II) score and systemic inflammatory response syndrome (SIRS) score are three of those widely used score systems in the ICU. The SOFA score contains parameters from six organ systems and is used to quantify the severity of organ damage (Vincent et al. Citation1996; Singer et al. Citation2016). The APACHE II score is a classification system including 12 physiological parameters, age and chronic health points. A higher APACHE II score suggests more severe condition and higher mortality risk (Knaus et al. Citation1985; Ginter et al. Citation2023). The SIRS score is a fundamental risk assessment for disease severity and is a predictor of mortality in various diseases such as traumatic brain injury and sepsis (Jacome and Tatum Citation2018; Qiu et al. Citation2023). Furthermore, some researches pointed out that SOFA and APACHE II score show excellent value in predicting hospital and 90-day mortality in patients with EHS (Wang et al. Citation2019; Zhong et al. Citation2022). However, these scores were not HS-specific and included few hemostatic parameters or diagnostic criteria of DIC. Yang et al. developed an EHS scoring (EHSS) system including 12 parameters and found that EHSS performs better than SOFA and APACHE II in evaluating the prognosis of EHS patients, which was confirmed by another research (Yang et al. Citation2020; Li et al. Citation2021). The EHSS system was very practical in overall evaluation; however, it was too intricate for rapid, bedside and daily evaluation. In this study, we try to evaluate whether DIC is an independent risk factor of in-hospital mortality for those young healthy people and construct a predictive model based on DIC in EHS patients.
Methods
Study design and participants
In this single-center retrospective study from 1 January 2008 to 31 December 2020, EHS patients were admitted to the intensive care unit (ICU) of a tertiary hospital in Guangzhou city with subtropical monsoon climate. The diagnostic criteria of EHS were as follows: (1) the exposure to a high ambient temperature and humidity or the history of extensive physical exercise; (2) core temperature rise above 40°C and central nervous system abnormalities (including delirium, convulsion or coma) (Liu et al. Citation2020). The diagnostic criteria of rhabdomyolysis were as follows: (1) muscle weakness, pain and dark tea-like urine; (2) elevated non-cardiogenic creatine kinase (CK): serum CK > 1,000 U/L or increased more than 5 times of the normal level (Cabral et al. Citation2020). The diagnosis of DIC was based on the standard from the International Society for Thrombosis and Haemostasis (ISTH): An ISTH score ≥5 points (Taylor et al. Citation2001). The study protocol was reviewed and approved by the institutional review board (NZLLKZ2022047). Considering the retrospective design of this study, the need to obtain informed consent was waived.
Inclusion and exclusion criteria
The inclusion criteria of patients were as follows: (1) Patients who were diagnosed with EHS; (2) Patients whose age was above 18 years old. The exclusion criteria of this study were as follows: (1) Patients whose key laboratory data were missing; (2) Patients whose were not enrolled within 48 hours from the onset of EHS; (3) Patients who died in the first 24 hours after admission; (4) Patients with malignant tumor, hematological diseases, central nervous system infection or hepatic cirrhosis.
Clinical data collection
The demographics and clinical data of the EHS patients at admission were collected, including age, gender, heart rate (HR), mean artery pressure (MAP), use of vasoactive drug (VD) and mechanical ventilation (MV), respiratory rate (RR), temperature (T), white blood cell (WBC), neutrophil, monocyte, lymphocyte, hemoglobin (Hb), platelet count (PC), activated partial thromboplastin time (APTT), prothrombin time (PT), international normalized ratio (INR), D-dimer, fibrinogen (Fib), serum creatinine (Scr), CK, blood glucose (BG) and aspartate aminotransferase (AST). The existence of rhabdomyolysis and DIC was recorded. Glasgow coma scale (GCS), systemic inflammatory response syndrome (SIRS), APACHE II and SOFA scores were also measured. The hospital mortality and the length of intensive care unit stay (LoICUS) and hospital stay (LoHS) were recorded.
Statistical analysis
The continuous variables that satisfied the normal distribution were expressed as mean ± standard deviation, and the means of these variables were compared using the independent-sample T-test. The continuous variables that did not satisfy the normal distribution were expressed as median (interquartile range, IQR) and analyzed using Mann–Whitney U-tests. Count data, expressed as N (percentage, %), were analyzed using Chi-square or Fisher’s exact test. To identify risk factors associated with hospital mortality, univariate logistic regression analysis was performed, and variables with p < .1 were included in multivariable logistic regression (Forward LR) to construct predictive model. Nomogram was developed from the final predictive model and assessed by calibration curves and bootstrap with 1,000 resamples. The receiver operating characteristic (ROC) curve of each risk factor was constructed by a non-parametric method, and the area under the curve (AUC) was calculated. The DeLong test was used for the comparison of AUCs by MedCalc (version 16.8.4). The best diagnostic critical point was determined, and the sensitivity (SEN), the specificity (SPE) and Youden Index (YI) of each factor in predicting hospital mortality were calculated. Statistical analyses were performed using SPSS Windows version 26.0 (SPSS, Chicago, IL, USA), and nomogram was developed using R software version 4.2.0. A two-tailed p < .05 was considered statistically significant.
Results
Baseline characteristics of EHS patients
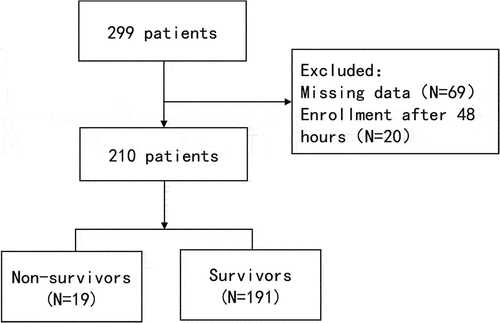
There were 299 patients with EHS that fulfilled the inclusion criteria, among which 89 patients were excluded because of the missing data or enrollment after 48 hours from the onset of EHS and 210 patients were finally analyzed (). Two hundred patients were males and 10 were females. The in-hospital mortality of patients was 9.0% (19/210). Approximately one-fifth of patients (17.6%) had ≥5 points in the ISTH score and were diagnosed as DIC. About 78 of 210 (37.1%) patients were diagnosed as rhabdomyolysis. The comparison of the clinical characteristics between survivors and non-survivors is shown in . Compared with the survivors, the non-survivors had higher T, HR, Scr, AST, CK, D-dimer, APACHE II scores, SOFA score, ISTH score and SIRS score, prolonged APTT, PT and INR (p < .05). The use of MV and VD was also higher in non-survivors. Besides, those non-survivors had decreased lymphocyte, PC, HB, Fib and GCS scores. Furthermore, LoICUS was longer in those non-survivors. The incidence of DIC and rhabdomyolysis was significantly higher in non-survivors than those survivors (57.9% vs 13.6%, p < .001; 63.2% vs 34.6%, p = .014). There was no significant difference in age, gender, month distribution, predisposing factors, underlying disease, MAP, RR, WBC, neutrophil, monocytes, BG and LoHS between two groups ().
Table 1. Baseline characteristics of patients with EHS in survivors and non-survivors group.
T, GCS score and DIC: independent risk factors of in-hospital mortality
The univariate logistic regression analysis showed that T, HR, HB, GCS scores, Scr and the existence of rhabdomyolysis and DIC were significantly associated with poor outcome of EHS patients (all p < .05) (). The multivariate logistic regression analysis showed that T, GCS score and DIC were the independent risk factors for in-hospital mortality of EHS patients (OR 1.658 95% CI 1.142–2.408, p = 0.008; OR 0.847 95% CI 0.736–0.974, p = 0.019; OR 7.616 95% CI 2.175–26.673, p = .001) ().
Table 2. Univariate logistic analysis of factors associated with in-hospital mortality in patients with EHS. T, temperature; HR, heart rate; Hb, hemoglobin; Scr, serum creatinine; AST, aspartate aminotransferase; GCS, Glasgow coma scale; DIC, disseminated intravascular coagulation; OR, odds ratio; CI confidence interval.
Table 3. Multivariate logistic analysis of factors associated with in-hospital mortality in patients with EHS.
Predictive model based on T, GCS score and DIC
Since T, GCS score and DIC were independent prognostic factors, we further combined these three indicators to the in-hospital mortality predictive model, as followed:
Y = 0.506 × T + 2.030 × DIC − 0.167 × GCS − 20.751 (model-1).
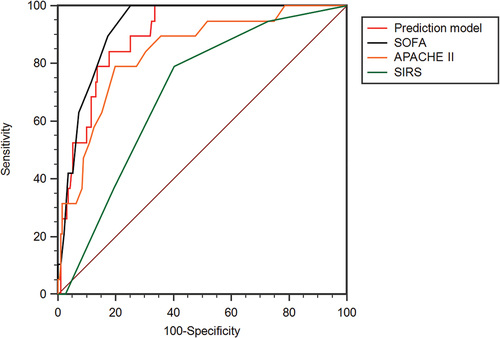
The result of the Hosmer–Lemeshow goodness-of-fit was p = 0.771 (χ2 = 4.074, df = 7) and that of the Omnibus test was p < .001 (χ2 = 40.005, df = 3). The ROC curves and AUC of three factors and predictive model are seen in and . The AUC of predictive model was 0.897 (95% CI 0.848–0.935), and the SEN and the SPE were 100% and 66.49%, respectively. The AUC of T, DIC and GCS score was 0.739 (95% CI 0.674–0.797), 0.721 (95% CI 0.656–0.781) and 0.822 (95% CI 0.764–0.872), respectively. The comparison of AUCs showed that there was no difference among these three independent prognostic factors (DeLong test: T vs GCS score, p = .1526; T vs DIC, p = .8557; GCS score vs DIC, p = .2521). The AUC of the predictive model was higher than that of T and DIC (p = .0009; p = .0021) and no significant difference existed between predictive model and GCS score (p = .0796).
Figure 2. The ROC curve analyses of predictive model, T, DIC and GCS score. T, temperature; DIC, disseminated intravascular coagulation; GCS, Glasgow coma scale.
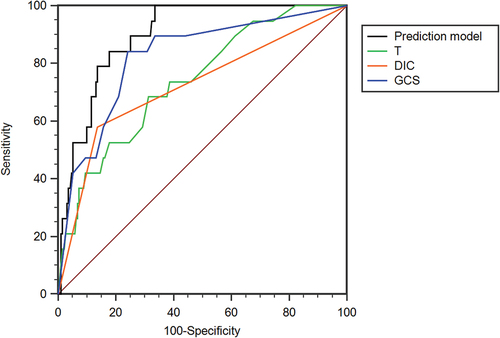
Table 4. The ROC curve analysis of predictive model, T, DIC, GCS, SOFA, APACHE II and SIRS score to predict in-hospital mortality.
The predictive efficacy of predictive model non-inferior to SOFA and APACHE II score but superior to SIRS score
The ROC curves and AUC of predictive model, SOFA, APACHE II and SIRS scores are shown in and . The AUC of SOFA score was 0.924 (95% CI 0.880–0.956), and the cutoff value was 5 (YI 0.7487, SEN 100%, SPE 74.87%). The AUC of APACHE II score was 0.838 (95% CI 0.781–0.885), and the cutoff value was 16 (YI 0.5905, SEN 78.95%, SPE 80.10%). The AUC of SIRS score was 0.699 (95% CI 0.632–0.760), and the cutoff value was 1 (YI 0.3863, SEN 78.95%, SPE 80.10%). There was no statistical difference in AUC among predictive model, SOFA and APACHE II score (DeLong test: predictive model vs SOFA, p = .3828; predictive model vs APACHE II, p = 0.1006; SOFA vs APACHE II, p = .0794). The AUC of SIRS score was lower than that of predictive model, SOFA and APACHE II score (p < .0001; p < .0001; p = .0028, respectively).
Development of the nomogram
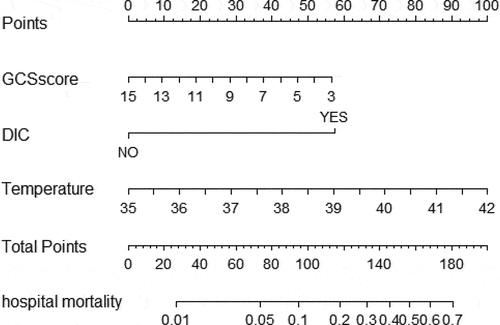
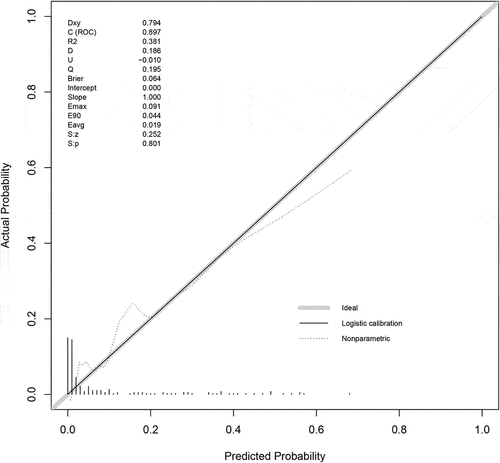
To visualize the predictive model, we developed the nomogram by R software (). The nomogram showed good performance for hospital mortality prediction, with a C-statistic of 0.897 ().
Discussion
In this study, we analyzed the independent risk factors of in-hospital mortality in EHS patients. We mainly found that T, DIC and GCS score were the independent risk factors of hospital mortality. Furthermore, the predictive model and the nomogram based on T, DIC and GCS score demonstrated the similar prognostic value with those widely used score systems like SOFA, APACHE II and SIRS scores.
We found that the non-survivors have a higher T than those survivors. The result of multivariate revealed that T was one of the independent risk factors (OR = 1.658,95%CI 1.142–2.408, p = 0.008), which meant EHS patients with higher T had a higher risk of adverse outcome. Our results suggested that the T has a modest value in predicting in-hospital mortality (AUC = 0.739, 95% CI 0.674–0.797, p = .0001) and related cutoff value was 37.6°C. The thermoregulation model constructed by Zhao et al. indicated that the time course of temperature during HS recovery contains three distinct but successive stages: a rapid cooling stage, a slow cooling stage and a rewarming stage (Zhao et al. Citation2020). The T of most patients in our study returned to a nearly normal level after continuous cooling treatment. The measure of T mainly located in the first two stages, which was supported by the manifestation of cardiovascular compensation (increasing HR and decreasing MAP). Our result indicated that measuring T in these two stages was associated with poor outcome. T was considered as an available parameter that directly represented the heat stress and heat injury (Laitano et al. Citation2019). Besides, the higher temperature also reflected an unsatisfied cooling in the early phase of EHS onset. On the contrary, some previous studies pointed out that not the degree but the longer duration of hyperthermia was associated with the poor prognosis (Shimazaki et al. Citation2020; Liu et al. Citation2021; Chen et al. Citation2023). According to the expert consensus on standardized diagnosis and treatment for HS from China in 2020, to rapidly lower core temperature below 39°C in 40 minutes or below 38.5°C in 2 hours was suggested as the most important point to rescue patients with EHS (Liu et al. Citation2020). Active cooling may improve outcome through alleviating both the primary heat toxicity and secondary systematic inflammation response and coagulation dysfunction (Bouchama et al. Citation2007). But organ injury persistently aggravated even after timely and effective cooling in some patients, implying the primary heat stress was not the only prognostic factor.
Comparing to the survivors, severe hematological dysfunction and even DIC happened more frequently in non-survivors, manifested by prolonged traditional coagulation indices and elevated levels of secondary fibrin degradation products. We found that DIC was an independent risk factor of patients with EHS (OR = 7.616, 95% CI 2.175–26.673, p = 0.001). Hifumi et al. reported the same conclusion with a little bit different result (OR = 2.16, 95% CI 1.09–4.27, p = .028) (Hifumi et al. Citation2018). The difference between two studies may originate from the diagnostic criteria of DIC, sample size or age range of patients. There were no specific diagnostic criteria of HS-related DIC so far, and most of those previous studies focused on HS tended to use the JAAM-DIC or ISTH score system, which was widely used in the diagnosis of sepsis or infection-associated DIC, to differentiate whether DIC or not. The JAAM-DIC score system was reported to be more sensitive than ISTH ones in earlier diagnosing of sepsis-induced DIC (Iba et al. Citation2019). The incidence of DIC in this present study was 17.6% (37/210), which was lower than that from Hifumi et al. (21.7%, 153/705), but higher than that from Shimazaki et al. (11.6%, 73/632) and both of them used JAAM-DIC score system (Shimazaki et al. Citation2020). Helms et al. found that there was a moderate concordance between JAAM-DIC and ISTH and both of them were usable in patients with septic shock (Helms et al. Citation2020). The pathological changes in HS animal models, including microthrombosis, endothelial injury and inflammatory cell infiltration, were similar to those of sepsis (Roberts et al. Citation2008; Bouchama et al. Citation2012). The progressive cross-talk between inflammation and coagulation takes a vital role in organ dysfunction and poor outcome.
Central nervous system dysfunction was another clinical presentation of HS; however, cranial computerized tomography (CT) or magnetic resonance imaging (MRI) examination showed poor value in severity during the early stage of HS onset. Although S100 calcium-binding protein β (S100β) and neuron-specific enolase (NSE) were proposed as promising biomarkers of HS brain injury (Chun et al. Citation2019; Schlader et al. Citation2022), clinical usage is still limited. GCS score was a useful bedside tool to prognose the outcome in severe head injuries (Jennett et al. Citation1979). The present findings showed that GCS score of non-survivors was significantly lower than that of the survivors. Besides, GCS score was an independent risk factor in patients with EHS (OR = 0.847, 95% CI 0.736–0.974, p = 0.019). Improving the GCS score by brain cooling and protection may be a vital therapeutic goal after the onset of HS, which was supported by the previous animal study from Hsu et al. (Citation2006). They demonstrated that rat brain cooling via hypothermic retrograde jugular vein flush significantly attenuated systemic inflammation response and coagulation disorder, which contribute to multiple organ dysfunction.
We constructed a predictive model based on three independent risk factors and it showed excellent efficacy in prognosing in-hospital mortality (AUC = 0.897, 95% CI 0.848–0.935, p < .0001). We also compared the predicted values of conventional score system and the predictive model and found that there was no statistical difference in AUC among predictive model, SOFA and APACHE II scores according to DeLong test results. Both the SOFA and APACHE II scores are widely used in the severity evaluation for critical illness and their advantages originate from comprehensive assessment of different vital organ functions. But one coin has two sides, the initial treatment based on SOFA and APACHE II scores may be delayed as these two score systems need more parameters. Besides, these score systems are not HS-specific and include few hemostatic parameters. The predictive model can be a routine monitoring as it included less variables which were easily available. Furthermore, the model also consisted of diagnostic criteria of DIC, which reflected the important pathogenic changes of HS.
To be honestly, our work was not the first try to use a nomogram in predicting the prognosis of HS patients. Shao and colleagues developed an impressive nomogram with C-index of 0.880 (95% CI 0.831–0.930) for predicting 7-day and 14-day survival in patients with HS. The nomogram was based on white blood cell count, creatine, alanine aminotransferase (ALT), maximum heart rate, invasive ventilation, initial mean arterial pressure and GCS score (Shao et al. Citation2022). Wei et al. constructed a comprehensive nomogram on HS patients including neutrophil/lymphocyte ratio, platelet, troponin I, creatine kinase myocardial band, lactate dehydrogenase, human serum albumin, D-dimer and APACHE-II scores. The AUC of the predictive model was 0.905 and 0.918 for 10-day and 30-day survival, respectively (Wei et al. Citation2022). Most of the participants in the previous two researches were the elderly, and they tended to suffer from CHS owing to diminished thermoregulatory capacity, such as increased vasodilatation and sweating thresholds, reduced thermal sensitivity, reduced maximal sweating capacity and lowered metabolic rate (Ou et al. Citation2023). Our nomogram had its own advantages when compared with the prior studies. The risk factors in our study, such as GCS score, coincided with those components of previous nomograms and their value was highlighted again. The difference between prior researches and our work may originate from the involved population and type of HS. Specifically, most of the patients in our study were young adults, and they suffered from EHS due to the extensive and strenuous physical activity. Our work grabbed the core parameters (GCS score, T and DIC) reflecting early changes of EHS so that contained less variables with similar efficacy, which mean more practical and available. In short, the nomogram showed a good balance between the rapid and comprehensive evaluation.
There were several limitations in our research. Firstly, it was a single-center retrospective study with limited sample size. Small size restricted the number of variables in logistic regression. Secondly, few female patients were included, so we should keep prudent when we sought to expand the statistical results to the whole population of EHS. Furthermore, we did not divide this database into training and validation cohort.
Conclusion
HS increasingly threatens people in the background of global warming. To construct an easily available predictive model based on pathogenic mechanism become an urgent need. Our research developed a nomogram, which was based on T, GCS score and the presence of DIC, as a promising tool for clinicians.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bouchama A, Abuyassin B, Lehe C, Laitano O, Jay O, O’Connor FG, Leon LR. 2022. Classic and exertional heatstroke. Nat Rev Dis Primers. 8(1):8. eng. doi: 10.1038/s41572-021-00334-6.
- Bouchama A, Al-Mohanna F, Assad L, Baturcam E, Eldali A, Owaidah T, Dehbi M. 2012. Tissue factor/factor VIIa pathway mediates coagulation activation in induced-heat stroke in the baboon. Crit Care Med. 40(4):1229–1236. eng. doi: 10.1097/CCM.0b013e3182387bef.
- Bouchama A, Dehbi M, Chaves-Carballo E. 2007. Cooling and hemodynamic management in heatstroke: practical recommendations. Crit Care. 11(3):R54. eng. doi: 10.1186/cc5910.
- Cabral BMI, Edding SN, Portocarrero JP, Lerma EV. 2020. Rhabdomyolysis. Dis Mon. 66(8):101015. eng. doi: 10.1016/j.disamonth.2020.101015.
- Chen L, Xu S, Yang X, Zhao J, Zhang Y, Feng X. 2023. Association between cooling temperature and outcomes of patients with heat stroke. Intern Emerg Med Eng. doi: 10.1007/s11739-023-03291-y.
- Chun JK, Choi S, Kim HH, Yang HW, Kim CS. 2019. Predictors of poor prognosis in patients with heat stroke. Clin Exp Emerg Med. 6(4):345–350. eng. doi: 10.15441/ceem.18.081.
- Deng Q, Zhao J, Liu W, Li Y. 2018. Heatstroke at home: prediction by thermoregulation modeling. Build Environ. 137:147–156. doi: 10.1016/j.buildenv.2018.04.017.
- Diehl KA, Crawford E, Shinko PD, Tallman RD Jr., Oglesbee MJ. 2000. Alterations in hemostasis associated with hyperthermia in a canine model. Am J Hematol. 64(4):262–270. eng. doi: 10.1002/1096-8652(200008)64:4<262:AID-AJH5>3.0.CO;2-D.
- Epstein Y, Yanovich R, Longo DL. 2019. Heatstroke. N Engl J Med. 380(25):2449–2459. eng. doi: 10.1056/NEJMra1810762.
- Ginter K, Schwab F, Behnke M, Wolkewitz M, Gastmeier P, Geffers C, Maechler F. 2023. SAPS2, APACHE2, SOFA, and core-10-TISS upon admission as risk indicators for ICU-acquired infections: a retrospective cohort study. Infection. 51(4):993–1001. eng. doi: 10.1007/s15010-022-01972-y.
- Hajat S, Vardoulakis S, Heaviside C, Eggen B. 2014. Climate change effects on human health: projections of temperature-related mortality for the UK during the 2020s, 2050s and 2080s. J Epidemiol Comm Health. 68(7):641–648. eng. doi: 10.1136/jech-2013-202449.
- Helms J, Severac F, Merdji H, Clere-Jehl R, François B, Mercier E, Quenot JP, Meziani F. 2020. Performances of disseminated intravascular coagulation scoring systems in septic shock patients. Ann Intensive Care. 10(1):92. eng. doi: 10.1186/s13613-020-00704-5.
- Hifumi T, Kondo Y, Shimazaki J, Oda Y, Shiraishi S, Wakasugi M, Kanda J, Moriya T, Yagi M, Ono M. et al. 2018. Prognostic significance of disseminated intravascular coagulation in patients with heat stroke in a nationwide registry. J Crit Care. 44:306–311. eng. doi: 10.1016/j.jcrc.2017.12.003.
- Hsu SF, Niu KC, Lin CL, Lin MT. 2006. Brain cooling causes attenuation of cerebral oxidative stress, systemic inflammation, activated coagulation, and tissue ischemia/injury during heatstroke. Shock. 26(2):210–220. eng. doi: 10.1097/01.shk.0000223124.49265.10.
- Iba T, Levy JH, Warkentin TE, Thachil J, van der Poll T, Levi M. 2019. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 17(11):1989–1994. eng. doi: 10.1111/jth.14578.
- Jacome T, Tatum D. 2018. Systemic inflammatory response syndrome (SIRS) score independently predicts poor outcome in isolated traumatic brain injury. Neurocrit Care. 28(1):110–116. eng. doi: 10.1007/s12028-017-0410-y.
- Jennett B, Teasdale G, Braakman R, Minderhoud J, Heiden J, Kurze T. 1979. Prognosis of patients with severe head injury. Neurosurgery. 4(4):283–289. eng. doi: 10.1227/00006123-197904000-00001.
- Kawasaki T, Okamoto K, Kawasaki C, Sata T. 2014. Thrombomodulin improved liver injury, coagulopathy, and mortality in an experimental heatstroke model in mice. Anesth Analg. 118(5):956–963. eng. doi: 10.1213/ANE.0000000000000170.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med. 13(10):818–829. eng. doi: 10.1097/00003246-198510000-00009.
- Laitano O, Leon LR, Roberts WO, Sawka MN. 2019. Controversies in exertional heat stroke diagnosis, prevention, and treatment. J Appl Physiol (1985). 127(5):1338–1348. eng. doi: 10.1152/japplphysiol.00452.2019.
- Liu SY, Song JC, Mao HD, Zhao JB, Song Q. 2020. Expert consensus on the diagnosis and treatment of heat stroke in China. Mil Med Res. 7(1):1. eng. doi: 10.1186/s40779-019-0229-2.
- Liu S, Xing L, Wang Q, Xin T, Mao H, Tao Y, Zhao J, Li X, Li C, Li Q. et al. 2021. Association between early stage-related factors and mortality in patients with exertional heat stroke: a retrospective study of 214 cases. Int J Gen Med. 14:4629–4638. eng. doi: 10.2147/IJGM.S322910.
- Li P, Yang L, Liu R, Chen RL. 2021. The value of the exertional heat stroke score for the prognosis of patients with exertional heat stroke. Am J Emerg Med. 50:352–355. eng. doi: 10.1016/j.ajem.2021.08.036.
- Matthews TK, Wilby RL, Murphy C. 2017. Communicating the deadly consequences of global warming for human heat stress. Proc Natl Acad Sci U S A. 114(15):3861–3866. eng. doi: 10.1073/pnas.1617526114.
- Ohbe H, Isogai S, Jo T, Matsui H, Fushimi K, Yasunaga H. 2019. Treatment with antithrombin or thrombomodulin and mortality from heatstroke-induced disseminated intravascular coagulation: a nationwide observational study. Semin Thromb Hemost. 45(8):760–766. eng. doi: 10.1055/s-0039-1700520.
- Ou Y, Wang F, Zhao J, Deng Q. 2023. Risk of heatstroke in healthy elderly during heatwaves: a thermoregulatory modeling study. Build Environ. 237:110324. doi:10.1016/j.buildenv.2023.110324.
- Proctor EA, Dineen SM, Van Nostrand SC, Kuhn MK, Barrett CD, Brubaker DK, Yaffe MB, Lauffenburger DA, Leon LR. 2020. Coagulopathy signature precedes and predicts severity of end-organ heat stroke pathology in a mouse model. J Thromb Haemost. 18(8):1900–1910. eng. doi: 10.1111/jth.14875.
- Qiu X, Lei YP, Zhou RX. 2023. SIRS, SOFA, qSOFA, and NEWS in the diagnosis of sepsis and prediction of adverse outcomes: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 21(8):891–900. eng. doi: 10.1080/14787210.2023.2237192.
- Roberts GT, Ghebeh H, Chishti MA, Al-Mohanna F, El-Sayed R, Al-Mohanna F, Bouchama A. 2008. Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arterioscler Thromb Vasc Biol. 28(6):1130–1136. eng. doi: 10.1161/ATVBAHA.107.158709.
- Schlader ZJ, Davis MS, Bouchama A. 2022. Biomarkers of heatstroke-induced organ injury and repair. Exp Physiol. 107(10):1159–1171. eng. doi: 10.1113/EP090142.
- Shao F, Shi X, Huo SH, Liu QY, Shi JX, Kang J, Gong P, Yan ST, Wang GX, Qin LJ, et al. 2022. Development and evaluation of a predictive nomogram for survival in heat stroke patients: a retrospective cohort study. World J Emerg Med. 13(5):355–360. eng. doi: 10.5847/wjem.j.1920-8642.2022.092.
- Shimazaki J, Hifumi T, Shimizu K, Oda Y, Kanda J, Kondo Y, Shiraishi S, Takauji S, Hayashida K, Moriya T, et al. 2020. Clinical characteristics, prognostic factors, and outcomes of heat-related illness (heatstroke study 2017-2018). Acute Med Surg. 7(1):e516. eng. doi: 10.1002/ams2.516.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). Jama. 315(8):801–810. eng. doi: 10.1001/jama.2016.0287.
- Sithinamsuwan P, Piyavechviratana K, Kitthaweesin T, Chusri W, Orrawanhanothai P, Wongsa A, Wattanathum A, Chinvarun Y, Nidhinandana S, Satirapoj B, et al. 2009. Exertional heatstroke: early recognition and outcome with aggressive combined cooling–a 12-year experience. Mil Med. 174(5):496–502. eng. doi: 10.7205/MILMED-D-02-5908.
- Taylor FB Jr., Toh CH, Hoots WK, Wada H, Levi M. 2001. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 86(5):1327–1330. eng. doi: 10.1055/s-0037-1616068.
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. 1996. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive Care Medicine. Intensive Care Med. 22(7):707–710. eng. doi: 10.1007/BF01709751.
- Wang Y, Xiao QM, Qi HN, Li W, Zhu BY, Liu YJ, Wang P, Wang WZ. 2019. Value of APACHE.II score and DIC score in predicting the death of patients with heat stroke. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 37(1):43–45. chi. doi: 10.3760/cma.j.issn.1001-9391.2019.01.009.
- Wei D, Gu T, Yi C, Tang Y, Liu F. 2022. A NOMOGRAM for PREDICTING PATIENTS with SEVERE HEATSTROKE. Shock. 58(2):95–102. eng. doi: 10.1097/SHK.0000000000001962.
- Yang MM, Wang L, Zhang Y, Yuan R, Zhao Y, Hu J, Zhou FH, Kang HJ. 2020. Establishment and effectiveness evaluation of a scoring system for exertional heat stroke by retrospective analysis. Mil Med Res. 7(1):40. eng. doi: 10.1186/s40779-020-00269-1.
- Zeng Q, Zhong L, Zhang N, He L, Lin Q, Song J. 2023. Nomogram for predicting disseminated intravascular coagulation in heatstroke patients: a 10 years retrospective study. Front Med. 10:1150623. eng. doi: 10.3389/fmed.2023.1150623.
- Zhao J, Wang H, Li Y, Xiao F, Deng Q. 2020. Heatstroke recovery at home as predicted by human thermoregulation modeling. Build Environ. 173:106752. doi: 10.1016/j.buildenv.2020.106752.
- Zhong L, Wu M, Ji J, Liu Z. 2022. Usefulness of sequential organ failure assessment score on admission to predict the 90-day mortality in patients with exertional heatstroke: an over 10-year intensive care survey. Am J Emerg Med. 61:56–60. eng. doi: 10.1016/j.ajem.2022.08.042.