ABSTRACT
The association of air pollution and greenspace with respiratory pathogen acquisition and respiratory health was investigated in a community-based birth-cohort of 158 Australian children. Weekly nasal swabs and daily symptom-diaries were collected for 2-years, with annual reviews from ages 3-7-years. Annual exposure to fine-particulate-matter (PM2.5), nitrogen-dioxide (NO2), and normalised-difference-vegetation-index (NDVI) was estimated for pregnancy and the first 2-years-of-life. We examined rhinovirus, any respiratory virus, Streptococcus pneumoniae, Moraxella catarrhalis, and Haemophilus influenzae detections in the first 3-months-of-life, age at initial pathogen detection, wheezing in the first 2-years, and asthma at ages 5-7-years. Our findings suggest that higher NDVI was associated with fewer viral and M. catarrhalis detections in the first 3-months, while increased PM2.5 and NO2 were linked to earlier symptomatic rhinovirus and H. influenzae detections, respectively. However, no associations were observed with wheezing or asthma. Early-life exposure to air pollution and greenspace may influence early-life respiratory pathogen acquisition and illness.
Introduction
Air pollution exposure in early life can have detrimental effects upon respiratory health by increasing the risk and severity of respiratory infections, which in young children may also impair future lung growth and development (Bowatte et al. Citation2015; Lambert et al. Citation2017; Sly et al. Citation2019; Johnson et al. Citation2021). Higher air pollution levels are associated with more frequent respiratory virus infections, including those caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (Conticini et al. Citation2020), respiratory syncytial virus (RSV) (Wrotek et al. Citation2021), and rhinovirus (Rodrigues et al. Citation2019). A meta-analysis of 10 European birth cohorts found an association between air pollution and physician-diagnosed pneumonia as measured by nitrogen dioxide (NO2) (adjusted odds ratio 1.30; 95% confidence interval [CI]: 1.02, 1.65, per 10 μg/m3 average annual increase) and particulate matter ≤10 µm in aerodynamic diameter (PM10) (adjusted odds ratio 1.76; 95%CI: 1.00, 3.09, per 10 μg/m3 average annual increase), respectively (MacIntyre et al. Citation2014). In contrast, greenspace, defined here as distinct areas of grass, trees and other vegetation set aside from an otherwise urban environment, is an environmental exposure that in some regions is associated with improved respiratory health in childhood and fewer lower respiratory infections (Tischer et al. Citation2017).
Early life exposure to air pollution can have other detrimental effects upon respiratory health, although findings from previous studies have been inconsistent. A systematic review of 21 birth cohort studies examined the impact of traffic-related air pollution during the first 1000-days-of-life upon the development of wheezing and asthma in childhood (Bettiol et al. Citation2021). Exposure to particulate matter (PM) and nitric oxides (NOx) during pregnancy was consistently associated with an increased risk of developing asthma, while the association with wheezing was unclear. Similarly, for exposures during the first 2-years-of-life, most studies found a positive association between PM and NOx and the risk of asthma, but not for wheezing episodes. However, in a meta-analysis of 18 studies in children aged from birth to 14-years-of-age, antenatal exposure to NO2 and PM2.5 were both associated with childhood wheeze, and NO2 was associated with asthma (Hehua et al. Citation2017). Two cohort studies have examined associations between greenspace exposure and respiratory health. A Portuguese study found that 7-year-olds living near greenspace had a lower prevalence of asthma (Cavaleiro Rufo et al. Citation2021) than other children of the same age. In contrast, a meta-analysis of nine European cohort studies reported exposure to greenspace resulted in an increased risk of wheezing, asthma and allergic rhinitis (Parmes et al. Citation2020).
Although air pollution and its association with respiratory health have been studied widely, less is known about the association between antenatal exposure to air pollution and respiratory infections in early life (Madsen et al. Citation2017). In a large prospective cohort of 17,533 Norwegian participants, there was no association between exposure to moderate NO2 levels (13.6 µg/m3, range 0.01–60.4 µg/m3) in pregnancy and lower respiratory infections to age 6-months (adjusted relative risk (aRR) 0.99; 95%CI: 0.84, 1.17 per 10 µg/m3), or at 6–18-months (aRR 1.05; 95%CI: 0.94, 1.16 per 10 µg/m3) (Madsen et al. Citation2017). However, other air pollutants, including PM2.5 were not examined.
The composition of commensal bacterial communities in the nasal airways of children in the first weeks of life is altered by transient incursions of potential bacterial pathogens within the Moraxella, Streptococcus and Haemophilus genera (Teo et al. Citation2015). These events are associated with respiratory viral infections, and an increased risk of lower respiratory illnesses and subsequent asthma. The link between early life respiratory viral infections and bacterial pathogen acquisitions with lung health later in childhood is complex and multifactorial, but exposure to environmental factors may play a key role by compromising both adaptive immunity and the local innate immune responses in the respiratory epithelium resulting in more severe respiratory illnesses (Johnson et al. Citation2021).
The aim of this study was to use data from an Australian community-based birth cohort to evaluate the association between exposure to the air pollutants, NO2 and PM2.5, and to greenspace during the full antenatal period and the first 2-years-of-life and (i) respiratory pathogens detected in the first 3-months-of-life, and (ii) the timing of first detections of respiratory pathogens from birth until the child’s second birthday. In addition, we explored the associations between air pollutants and greenspace with wheeze in the first 2-years-of-life and asthma at age 5–7-years.
Material and methods
Study population and data collection
The Observational Research in Childhood Infectious Diseases (ORChID) birth cohort progressively enrolled healthy, term-born infants in Brisbane, Australia, between September 2010 and October 2012 (Lambert et al. Citation2012). Children were followed from birth until their second birthday. At the end of their involvement with the ORChID project, children and their parents/caregivers were invited to participate in an extension study (Early Life Lung Function; ELLF), which required an annual review by research staff between ages 3–7-years and included the completion of a standardised respiratory health questionnaire and clinical examination (Sly et al. Citation2019). Further details are provided in the Supplementary Methods. The Royal Brisbane and Women’s Hospital (HREC/10/QRBW125) Human Research Ethics Committee (HREC), the Children’s Health Queensland (HREC/10/QRCH/16 and HREC/13/QRCH/156) and The University of Queensland (2010/HE00820 and 2013/HE001291) HRECs approved the studies.
Demographic, social and clinical characteristics
Parents provided socio-demographic and health information at enrolment, including pregnancy and birth details. Parents thereafter completed a daily tick-box diary from birth until the child’s second birthday. This required recording pre-defined respiratory symptoms, including wheeze, which research staff had trained them to recognise (Lambert et al. Citation2012). When symptoms developed, parents also completed an illness-burden diary, which included any healthcare attendance. Thereafter, parents were interviewed by telephone every 3-months until the child’s second birthday to update feeding and childcare information. Exclusive breastfeeding was defined as receipt of breastmilk without milk formula or solids (Lambert et al. Citation2012). Childcare was categorised as formal (regulated care outside the child’s home) and informal (non-regulated care by family or friends). Vaccination status was confirmed using the Australian Immunisation Register. A symptomatic episode captured by the daily diary was defined when any of nasal congestion/discharge, dry cough, doctor-diagnosed acute otitis media, moist cough, rattle-like breathing, shortness of breath, wheeze, or doctor-diagnosed pneumonia were present.
Exposure: air pollution
Air pollution levels were represented by NO2 and PM2.5. Annual average NO2 and fine particulate matter ≤2.5 µm in aerodynamic diameter (PM2.5) levels were estimated from a validated, national land-use regression model for Australia (Knibbs et al. Citation2018). Residential addresses were geocoded and used to assign exposure. Exposures were estimated for the full antenatal period (as time-fixed) or first 2-years-of-life (as time-varying), depending upon the analysis.
Exposure: greenspace
Greenspace within the subtropical urban environment of Brisbane was measured by the annual mean normalised difference vegetation index (NDVI), a proxy for photosynthesising green vegetation. NDVI annual averages were estimated based upon 16-day Landsat images, with spatial resolution of 30-metres (Knibbs et al. Citation2018), and the NDVI values were calculated for a 500-metre buffer around the geocoded residential address. Exposures were estimated for the full antenatal period (as time-fixed) or first 2-years-of-life (as time-varying), depending upon the analysis.
Outcome: respiratory virus and bacteria detection
Parents collected weekly anterior nasal swabs from birth until the child’s second birthday. Swabs were surface-mailed, taking a median 3-days (interquartile range [IQR] 2–4) to reach the research laboratory, where they were processed and stored at −80°C. The median interval between swab collections was 7-days [IQR:7–12].
Swabs were batch-tested for 17 respiratory viruses and the bacterial pathogens, Streptococcus pneumoniae, Moraxella catarrhalis and Haemophilus influenzae by previously validated real-time polymerase chain reaction assays (Sarna et al. Citation2018; Palmu et al. Citation2019). Respiratory virus and bacterial pathogen detections with cycle threshold values < 40 were considered positive. A virus detection was classified as symptomatic if symptoms were first detected in the week prior to the first virus detection for that episode, or if symptoms were present in the week after the first virus detection.
Outcome: wheeze and asthma
Presence of wheeze reported in the first 2-years-of-life was extracted from the symptom diary. Children who failed to return any diary data were excluded from this analysis. At ages 5–7-years, parents were asked whether their child had ever received a diagnosis of asthma from a doctor, or if they had used an inhaled beta-2 agonist or inhaled corticosteroid asthma medication in the previous 12-months.
Analysis
We considered the association between air pollution (NO2 and PM2.5) and greenspace (NDVI) and (i) frequency of detections of rhinovirus, any virus, symptomatic viral detection episodes, S. pneumoniae, M. catarrhalis, and H. influenzae in the first 3-months-of-life; (ii) the timing of when the various pathogen species were first detected between birth and the child’s second birthday; (iii) wheeze in the first 2-years-of-life; and (iv) asthma at age 5–7-years. Associations between air pollution and greenspace, and the frequency of pathogen detection were investigated using linear regression with robust standard errors to account for repeated swabs from participants. Associations between air pollution and greenspace and the time-to-first pathogen detections were investigated using Cox regression. Associations between air pollution and greenspace and the presence of wheeze in the first 2-years-of-life were investigated using mixed-effects Poisson regression, and between air pollution and greenspace and asthma at ages 5–7-years were investigated using mixed-effects logistic regression. All mixed-effects models included child as a random effect to account for repeated measures.
All analyses used a similar approach and are reported per each IQR increase. First, unadjusted analyses were undertaken. Three separate regression analyses were conducted, with each analysis including a single main effect of NO2, PM2.5 or NDVI. Then, the models using a single air pollution or greenspace exposure, with each exposure adjusted for potentially confounding variables, were analysed (see Supplementary material for details). Next, the adjusted model included all air pollution and greenspace variables as main effects with potentially confounding variables (season of birth and maternal education for frequency of detection in the first 3-months-of-life and timing of first detections in the first 2-years-of-life; and season of birth for the wheeze outcome and maternal education for the asthma outcome) as covariables (see Figures S1–S3 for directed acyclic graphs) (Sarna et al. Citation2018; Palmu et al. Citation2019). The covariables selected were based upon these recent studies, expert clinician opinion and directed acyclic graphs. Environmental factors were treated as time-fixed (full antenatal period) or time-varying (first 2-years-of-life) depending on the analysis in regression models and were standardised by subtracting the median value from each environmental factor value and dividing it by the IQR. Interaction effects were examined by assessing the statistical significance (with alpha set at 0.05) of interaction terms, and comparing the magnitude of the main exposure effect sizes with, and without, the interaction terms. Interactions were examined between NO2, PM2.5 and NDVI, and interactions between each exposure (NO2, PM2.5 and NDVI) and season of birth. Sensitivity and additional analyses are detailed in the Supplementary Methods. All analyses were conducted using Stata statistical software v13 (StataCorp, College Station, TX, USA).
Results
One-hundred and fifty-eight children were included in the ORChID study, with 154 children supplying diary data for the wheeze outcome and 84 children supplying asthma data at age 5–7-years in the ELLF extension study. A total of 1,777 and 11,126 swabs were returned during the first 3- and 24-months-of-life, respectively (). Overall 87,547 days (78% of the maximum expected) of diary data were returned in the first 2-years-of-life. Socio-demographic and clinical characteristics are described in and Table S1. Most children were born between 39–41-weeks gestation into a single-child household. The median value of average NO2 levels in the year at the time of birth was 6.84 parts per billion (IQR: 5.87–8.00), for PM2.5 it was 6.26 μm/m3 (IQR: 5.69–6.84), and for the NDVI it was 0.43 (IQR: 0.38–0.49) (Table S2, Figure S4).
Figure 1. Flow chart of nasal swabs and symptom diaries from children in the Observational Research in childhood infectious diseases study.
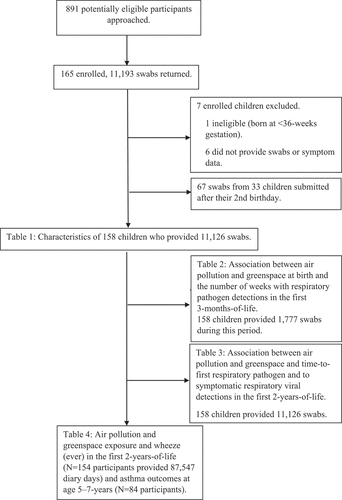
Table 1. Characteristics of the observational research in the childhood infectious diseases (ORChID) cohort (N = 158).
Association between air pollution and greenspace, and frequency of respiratory virus and bacteria detections in the first 3-months-of-life
In the first 3-months-of-life there were 153 rhinovirus and 182 “any virus” (including rhinovirus) detections (Table S3). During this 3-month period rhinoviruses were detected for a mean (standard deviation; SD) 0.94 (1.43) weeks, while “any viruses” were detected for a mean 1.50 (2.26) weeks. In adjusted models, there was no statistically significant association between air pollution and frequency of rhinovirus detections. However, each IQR unit increase in the NDVI significantly decreased the frequency of any virus detections by 0.45-weeks (95%CI:-0.88, −0.02). ().
Table 2. Association between air pollution and greenspace at birth, and the number of weeks with respiratory pathogen detections in the first 3-months-of-life (158 children; 1,717 swabs).
Similarly, in the first 3-months-of-life, S. pneumoniae, M. catarrhalis, and H. influenzae were detected on 265, 206 and 44 occasions, respectively. The mean (SD) number of weeks detected for S. pneumoniae was 1.30 (2.02), for M. catarrhalis 1.20 (2.61), and for H. influenzae 0.25 (0.92). There were no statistically significant associations between air pollution and bacterial detections, however, for each increase of one IQR unit in NDVI values, M. catarrhalis detections decreased by 0.50-weeks (95%CI: −0.93, −0.08) (). Results were similar for sensitivity analyses (Table S4).
Stratified analysis by exclusive breastfeeding in the first 3-months-of-life showed an enhanced protective effect of NDVI, with each IQR increase in the NDVI significantly decreasing the frequency of any virus detections by 0.68 weeks (95%CI:-1.31, -0.04) and M.catarrhalis detections by 0.77 weeks (95% CI: -1.39, -0.14, Supplementary Table 5).
Association between air pollution and greenspace, and time-to-first respiratory pathogen and to first symptomatic respiratory virus detections in the first 2-years-of-life
A one IQR unit increase in PM2.5 was associated with earlier first symptomatic rhinovirus detections after adjusting for season of birth and maternal education (; hazard ratio 1.75; 95%CI: 1.04, 2.97). In addition, a one IQR unit increase of NO2 was associated with an earlier first detection of H. influenzae (hazard ratio 1.96; 95%CI: 1.22, 3.16) in adjusted analyses. In sensitivity analyses, only NO2 was associated with an earlier first H. influenzae detection (Tables S6 and S7).
Table 3. Association between air pollution and greenspace, and time-to-first respiratory pathogen and to first symptomatic respiratory virus detections in the first 2-years-of-life (158 children; 11126 swabs).
Association between air pollution and greenspace, and wheeze in the first 2-years-of-life
There were 55/154 (35.7%) children whose parents reported they wheezed in the first 2-years-of-life ( and Table S8). There was no association between air pollution exposure in the first 2-years-of-life and wheeze during this period. However, each IQR increase in NDVI reduced wheeze incidence (incidence rate ratio 0.75; 95%CI: 0.56, 1.00).
Table 4. Air pollution and greenspace exposure and risk of wheezing (ever) in the first 2-years-of-life (N = 154 participants and 87,547 diary-days) and asthma outcomes at ages 5–7-years (N = 84 participants).
Association between air pollution and greenspace, and asthma at 5–7 years-of-age
There were 29/84 (34.5%) children identified as having asthma at age 5–7-years ( and Table S8). There was no statistically significant association between air pollution and NDVI exposure in the first 2-years-of-life and asthma at age 5–7-years.
Discussion
In this community-based birth cohort of healthy Australian children, exposure to increased residential greenspace was significantly associated with fewer respiratory virus and M. catarrhalis detections in the first 3-months-of-life. Increased NO2 was associated with earlier first detections of H. influenzae, and increased PM2.5 was associated with earlier first symptomatic rhinovirus detection episodes. No associations between either air pollution or greenspace and wheeze or asthma were observed. Together, these data suggest improved air quality and greenspace exposures may reduce early respiratory virus infections and acquisition of respiratory bacterial pathogens.
Studies on neighbourhood greenness and health outcomes have focused on non-communicable diseases such as obesity, mental health, birth and developmental outcomes, cardiovascular disease, and mortality (James et al. Citation2015). Few studies have examined the relationship with respiratory health outcomes or respiratory pathogen detection (Lambert et al. Citation2017; Tischer et al. Citation2017). A recent longitudinal study of 47 infants in Switzerland examining air pollutants and greenspace using 16S rRNA pyrosequencing of nasal microbiota as an outcome reported that low-to-moderate levels of air pollution were negatively associated with Corynebacteriaceae abundance and positively associated with bacterial community stability (Gisler et al. Citation2021). As the authors noted, these findings are counterintuitive, since in young infants Corynebacteriaceae abundance in the upper airways is associated with good respiratory health, while bacterial community instability is associated with increased susceptibility to respiratory infections. Although, air pollution does seem to impact nasal microbiota, this was not observed with exposure to greenspace, and the clinical significance of these results was not explored (Gisler et al. Citation2021).
Meanwhile, a study evaluating greenspace and SARS-CoV-2 incidence in the United States reported that each 0.1 unit increase in the NDVI was associated with a 6% (95%CI: 3, 10%) decrease in SARS-CoV-2 incidence after adjustment for area-level confounders (Klompmaker et al. Citation2021). Associations were strongest in counties with the highest population densities or with “stay at home” orders. In contrast, a study in London found that highly connected greenspaces increased the risk of SARS-CoV-2 infections, which was attributed to more social contact (Pan et al. Citation2021). These conflicting findings may be explained by differences in greenspace types. A study exploring greenspace and SARSCoV-2 infections, showed forests and pastures were associated with lower infection rates, but parks were associated with higher infection rates (Jiang et al. Citation2022). Together, these papers suggest that greenspaces may have value in reducing infections, but the local context and park type may play a role.
Our understanding of the mechanisms by which greenspaces exert positive benefits is poorly understood. Greenspaces, especially those spaces with a higher biodiversity index, contain environmental microbiota, which may assist in immune priming (Aerts et al. Citation2018). Moreover, exclusive breastfeeding enhanced the protective effect of greenspace exposure in the first 3-months-of-life, possibly by influencing the gut-lung axis (Granger et al. Citation2021). Bacterial communities in outdoor air also penetrate into indoor spaces, which then have their own human-associated bacterial communities (Meadow et al. Citation2014). It is possible that there is a complex interplay between these two communities that influences human immune health with synergistic effects; a topic requiring further research. However, simpler explanations also exist, and depending upon the type, increased greenspace may indicate decreased air pollution and/or human population density levels. Further research is warranted to unravel how increased greenspace improves respiratory outcomes.
The effects of exposure to air pollutants and childhood respiratory infections are uncertain, with conflicting results (Lu et al. Citation2021). One study reported that closer distance to a busy road during pregnancy was associated with an increased risk of respiratory infections in children (Rice et al. Citation2015). However, most available studies have found no statistically significant associations (Aguilera et al. Citation2013; Madsen et al. Citation2017). Nevertheless, a positive interaction between PM2.5 and symptomatic rhinovirus in children with asthma has been reported (Vempilly et al. Citation2013). In addition to the current report, a South African birth cohort study found that indoor air levels of NO2 and PM10 were positively associated with nasopharyngeal carriage of M. catarrhalis and H. influenzae at 6-months-of-age, and of M. catarrhalis carriage at age 12-months (Vanker et al. Citation2019). A possible mechanistic explanation is provided by an in-vitro study employing rat lung epithelial cells that observed exposure to diesel exhaust particles resulted in oxidative stress and upregulated mRNA expression of the cellular surface proteins, intercellular adhesion molecule-1, low-density lipoprotein and platelet-activating factor, each of which serve as receptors for both viruses and bacteria (Ito et al. Citation2006).
Although we did not find an association between air pollution and either wheeze or asthma in children, a meta-analysis of 11 birth cohort studies found increased childhood exposure to PM2.5 was associated with a slightly increased risk of subsequent asthma in childhood (PM2.5: odds ratio 1.14, 95%CI: 1.00, 1.30 per 2 μg/m3) (Bowatte et al. Citation2015). A large cohort study in the United States also found exposure to air pollution during early childhood increased the risk of developing physician-diagnosed asthma by age 5-years (odds ratio 1.74; 95%CI:1.02, 2.96) (Salam Muhammad et al. Citation2004). In contrast, a recent literature review of greenspace and health was inconclusive (Johannessen et al. Citation2023). Of the three included papers, two (Servadio et al. Citation2019; Nordeide Kuiper et al. Citation2021) found no association between greenness exposure and asthma, while the third reported greenness exposure could both increase and decrease the risk of asthma in adults. The inconsistency is probably from using loose definitions for both exposure and outcome. Overall, the relationship between greenness and asthma outcomes remains complex and further research is needed to clarify these findings (Donovan et al. Citation2021).
The ORChID study’s strengths include its longitudinal design and weekly sampling of nasal swabs in an unselected healthy community-based cohort. It does, however, have several limitations. First, environmental data were annually-averaged, and consequently do not reflect the seasonality and temporal changes of these values, or peaks at certain times, such as during wildfire season. Second, suboptimal swabbing techniques may have missed pathogen detections, but we have shown previously that parent-collected nasal swabs have similar virus and pathogenic bacteria detection rates to those obtained by health personnel (Zoch-Lesniak et al. Citation2020). Thirdly, we did not undertake unbiased next-generation sequencing to examine alterations in microbial community profiles during the first 3-months-of-life. However, low bacterial DNA loads in nasal swabs limit these studies to 16S rRNA gene sequencing where organisation taxonomic unit discrimination does not often go beyond the genus level. Fourthly, wheeze and asthma outcomes were also aggregated measures, which may lead to a lack of power, not fully capturing temporal differences caused by air pollution fluctuations and seasonality changes. Another limitation is the reliance upon mean annual exposures as proxies for air pollution and greenspace since we did not directly account for seasonal variations in these factors. This limitation may introduce uncertainty in the estimates and could impact the robustness of the findings. Furthermore, the high degree of correlation between antenatal exposures and those in the first 2-years-of-life makes it difficult to disentangle the effects of these exposures upon postnatal outcomes. Finally, as is often the case with intense studies of this nature, the results may not generalise to children from other backgrounds and environmental settings, especially for greenspace.
Conclusion
Improved air quality (decreased NO2 and PM2.5 levels) and greenspace exposures may reduce early acquisition of respiratory pathogens in the upper airways, and decrease rates of early life symptomatic respiratory virus infections. The present study provides insight into the complex interplay between environmental factors and respiratory health in early childhood. Further research is needed to better understand the mechanisms by which air quality and greenspace influence microbial acquisition in early life and whether this impacts the lifelong risk of respiratory disease and poor respiratory health outcomes.
Supplemental Material
Download MS Word (450.9 KB)Supplemental Material
Download MS Word (451.1 KB)Acknowledgements
The authors acknowledge the generosity of the study families who participated in the study and the efforts of the recruitment nurses and volunteer staff members for administrative assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
De-identified participant data, the data dictionary, and related documents (e.g. case report forms) will be made available on written request to the senior author. Requests must be accompanied by a formal protocol for the use of the data and approval from the relevant Human Research Ethics Committees. A written and signed data access agreement will be required.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09603123.2023.2299225.
Additional information
Funding
References
- Aerts R, Honnay O, Van Nieuwenhuyse A. 2018. Biodiversity and human health: mechanisms and evidence of the positive health effects of diversity in nature and green spaces. Br Med Bull. 127(1):5–22. doi: 10.1093/bmb/ldy021.
- Aguilera I, Pedersen M, Garcia-Esteban R, Ballester F, Basterrechea M, Esplugues A, Fernández-Somoano A, Lertxundi A, Tardón A, Sunyer J. 2013. Early-life exposure to outdoor air pollution and respiratory health, ear infections, and eczema in infants from the INMA study. Environ Health Perspect. 121(3):387–392. eng. doi:10.1289/ehp.1205281.
- Bettiol A, Gelain E, Milanesio E, Asta F, Rusconi F. 2021. The first 1000 days of life: traffic-related air pollution and development of wheezing and asthma in childhood. A systematic review of birth cohort studies. Environ Health. 20(1):46. doi: 10.1186/s12940-021-00728-9.
- Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, Matheson M, Dharmage SC. 2015. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies [https: //doi.Org/10.1111/all.12561]. Allergy. 70(3):245–256. doi: 10.1111/all.12561.
- Cavaleiro Rufo J, Paciência I, Hoffimann E, Moreira A, Barros H, Ribeiro AI. 2021. The neighbourhood natural environment is associated with asthma in children: a birth cohort study. Allergy. 76(1):348–358. eng. doi:10.1111/all.14493.
- Conticini E, Frediani B, Caro D. 2020. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ Pollut. 261:114465. eng. doi: 10.1016/j.envpol.2020.114465.
- Donovan GH, Landry SM, Gatziolis D. 2021. The natural environment, plant diversity, and adult asthma: a retrospective observational study using the CDC’s 500 cities project data. Health & Place. 67:102494. eng. doi: 10.1016/j.healthplace.2020.102494.
- Gisler A, Korten I, de Hoogh K, Vienneau D, Frey U, Decrue F, Gorlanova O, Soti A, Hilty M, Latzin P. et al. 2021. Associations of air pollution and greenness with the nasal microbiota of healthy infants: a longitudinal study. Environ Res. eng. 202:111633. doi:10.1016/j.envres.2021.111633.
- Granger CL, Embleton ND, Palmer JM, Lamb CA, Berrington JE, Stewart CJ. 2021. Maternal breastmilk, infant gut microbiome and the impact on preterm infant health. Acta Paediatr. 110(2):450–457. doi: 10.1111/apa.15534.
- Hehua Z, Qing C, Shanyan G, Qijun W, Yuhong Z. 2017. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: a systematic review. Environ Res. 159:519–530. eng. doi: 10.1016/j.envres.2017.08.038.
- Ito T, Okumura H, Tsukue N, Kobayashi T, Honda K, Sekizawa K. 2006. Effect of diesel exhaust particles on mRNA expression of viral and bacterial receptors in rat lung epithelial L2 cells. Toxicol Lett. 165(1):66–70. doi: 10.1016/j.toxlet.2006.01.015.
- James P, Banay RF, Hart JE, Laden F. 2015. A review of the health benefits of greenness. Curr Epidemiol Rep. 2(2):131–142. doi: 10.1007/s40471-015-0043-7.
- Jiang B, Yang Y, Chen L, Liu X, Wu X, Chen B, Webster C, Sullivan WC, Larsen L, Wang J, et al. 2022. Green spaces, especially nearby forest, may reduce the SARS-CoV-2 infection rate: a nationwide study in the United States. Landsc Urban Plan. 228:104583. doi: 10.1016/j.landurbplan.2022.104583.
- Johannessen A, Xu S, Abbah AP, Janson C. 2023. Greenness exposure: beneficial but multidimensional. Breathe (Sheff). 19(2):220221. eng. doi:10.1183/20734735.0221-2022.
- Johnson NM, Hoffmann AR, Behlen JC, Lau C, Pendleton D, Harvey N, Shore R, Li Y, Chen J, Tian Y, et al. 2021. Air pollution and children’s health—a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ Health Prev Med. 26(1):72. doi:10.1186/s12199-021-00995-5.
- Klompmaker JO, Hart JE, Holland I, Sabath MB, Wu X, Laden F, Dominici F, James P. 2021. County-level exposures to greenness and associations with COVID-19 incidence and mortality in the United States. Environ Res. 199(111331):111331. eng. doi:10.1016/j.envres.2021.111331.
- Knibbs LD, van Donkelaar A, Martin RV, Bechle MJ, Brauer M, Cohen DD, Cowie CT, Dirgawati M, Guo Y, Hanigan IC, et al. 2018. Satellite-based land-use regression for continental-scale long-term ambient PM(2.5) exposure assessment in Australia. Environ Sci Technol. 52(21):12445–12455. eng. doi: 10.1021/acs.est.8b02328.
- Lambert KA, Bowatte G, Tham R, Lodge C, Prendergast L, Heinrich J, Abramson MJ, Dharmage SC, Erbas B. 2017. Residential greenness and allergic respiratory diseases in children and adolescents – a systematic review and meta-analysis. Environ Res. 159:212–221. doi: 10.1016/j.envres.2017.08.002.
- Lambert SB, Ware RS, Cook AL, Maguire FA, Whiley DM, Bialasiewicz S, Mackay IM, Wang D, Sloots TP, Nissen MD, et al. 2012. Observational Research in childhood infectious diseases (ORChID): a dynamic birth cohort study: table 1. BMJ Open. 2(6):e002134. doi:10.1136/bmjopen-2012-002134.
- Lu C, Peng W, Kuang J, Wu M, Wu H, Murithi RG, Johnson MB, Zheng X. 2021. Preconceptional and prenatal exposure to air pollution increases incidence of childhood pneumonia: a hypothesis of the (pre-)fetal origin of childhood pneumonia. Ecotoxicol Environ Saf. 210:111860. doi: 10.1016/j.ecoenv.2020.111860.
- MacIntyre EA, Gehring U, Mölter A, Fuertes E, Klümper C, Krämer U, Quass U, Hoffmann B, Gascon M, Brunekreef B, et al. 2014. Air pollution and respiratory infections during early childhood: an analysis of 10 European birth cohorts within the ESCAPE project. Environ Health Perspect. 122(1):107–113. doi:10.1289/ehp.1306755.
- Madsen C, Haberg SE, Magnus MC, Aamodt G, Stigum H, London SJ, Nystad W, Nafstad P. 2017. Pregnancy exposure to air pollution and early childhood respiratory health in the Norwegian mother and child cohort study (MoBa). BMJ Open. 7(12):e015796. doi: 10.1136/bmjopen-2016-015796.
- Meadow JF, Altrichter AE, Kembel SW, Kline J, Mhuireach G, Moriyama M, Northcutt D, O’Connor TK, Womack AM, Brown GZ, et al. 2014. Indoor airborne bacterial communities are influenced by ventilation, occupancy, and outdoor air source [https: //doi.Org/10.1111/ina.12047]. Indoor Air. 24(1):41–48. doi:10.1111/ina.12047.
- Nordeide Kuiper I, Svanes C, Markevych I, Accordini S, Bertelsen RJ, Bråbäck L, Heile Christensen J, Forsberg B, Halvorsen T, Heinrich J. et al. 2021. Lifelong exposure to air pollution and greenness in relation to asthma, rhinitis and lung function in adulthood. Environ Int. eng. 146:106219. doi:10.1016/j.envint.2020.106219.
- Palmu AA, Ware RS, Lambert SB, Sarna M, Bialasiewicz S, Seib KL, Atack JM, Nissen MD, Grimwood K. 2019. Nasal swab bacteriology by PCR during the first 24-months of life: a prospective birth cohort study. Pediatr Pulmonol. 54(3):289–296. doi: 10.1002/ppul.24231.
- Pan J, Bardhan R, Jin Y. 2021. Spatial distributive effects of public green space and COVID-19 infection in London. Urban For Urban Green. 62:127182. doi: 10.1016/j.ufug.2021.127182.
- Parmes E, Pesce G, Sabel CE, Baldacci S, Bono R, Brescianini S, D’Ippolito C, Hanke W, Horvat M, Liedes H, et al. 2020. Influence of residential land cover on childhood allergic and respiratory symptoms and diseases: evidence from 9 European cohorts. Environ Res. 183:108953. doi: 10.1016/j.envres.2019.108953.
- Rice MB, Rifas-Shiman SL, Oken E, Gillman MW, Ljungman PL, Litonjua AA, Schwartz J, Coull BA, Zanobetti A, Koutrakis P, et al. 2015. Exposure to traffic and early life respiratory infection: a cohort study [https: //doi.Org/10.1002/ppul.23029]. Pediatr Pulmonol. 50(3):252–259. doi:10.1002/ppul.23029.
- Rodrigues AF, Santos AM, Ferreira AM, Marino R, Barreira ME, Cabeda JM. 2019. Year-long rhinovirus infection is influenced by atmospheric conditions, outdoor air virus presence, and immune system-related genetic polymorphisms. Food Environ Virol. 11(4):340–349. eng. doi:10.1007/s12560-019-09397-x.
- Salam Muhammad T, Li Y-F, Langholz B, Gilliland Frank D. 2004. Early-life environmental risk factors for asthma: findings from the Children’s health study. Environ Health Perspect. 112(6):760–765. doi: 10.1289/ehp.6662.
- Sarna M, Ware RS, Lambert SB, Sloots TP, Nissen MD, Grimwood K. 2018. Timing of first respiratory virus detections in infants: a community-based birth cohort study. J Infect Dis. 217(3):418–427. doi: 10.1093/infdis/jix599.
- Servadio JL, Lawal AS, Davis T, Bates J, Russell AG, Ramaswami A, Convertino M, Botchwey N. 2019. Demographic Inequities in Health Outcomes and air pollution exposure in the Atlanta Area and its relationship to urban infrastructure. J Urban Health. 96(2):219–234. eng. doi:10.1007/s11524-018-0318-7.
- Sly PD, Cormier SA, Lomnicki S, Harding JN, Grimwood K. 2019. Environmentally persistent free radicals: linking air pollution and poor respiratory health? Am J Respir Crit Care Med. 200(8):1062–1063. doi: 10.1164/rccm.201903-0675LE.
- Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, Holt BJ, Hales BJ, Walker ML, Hollams E, et al. 2015. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host & Microbe. 17(5):704–715. doi:10.1016/j.chom.2015.03.008.
- Tischer C, Gascon M, Fernández-Somoano A, Tardón A, Lertxundi Materola A, Ibarluzea J, Ferrero A, Estarlich M, Cirach M, Vrijheid M, et al. 2017. Urban green and grey space in relation to respiratory health in children. Eur Respir J. 49(6):1502112. doi:10.1183/13993003.02112-2015.
- Vanker A, Nduru PM, Barnett W, Dube FS, Sly PD, Gie RP, Nicol MP, Zar HJ. 2019. Indoor air pollution and tobacco smoke exposure: impact on nasopharyngeal bacterial carriage in mothers and infants in an African birth cohort study. ERJ Open Research. 5(1):00052–02018. doi: 10.1183/23120541.00052-2018.
- Vempilly J, Abejie B, Diep V, Gushiken M, Rawat M, Tyner TR. 2013. The synergetic effect of ambient PM2.5 exposure and rhinovirus infection in airway dysfunction in asthma: a pilot observational study from the central valley of california. Exp Lung Res. 39(10):434–440. doi: 10.3109/01902148.2013.840693.
- Wrotek A, Badyda A, Czechowski PO, Owczarek T, Dąbrowiecki P, Jackowska T. 2021. Air pollutants’ concentrations are associated with increased number of RSV hospitalizations in polish children. J Clin Med. 10(15):3224. eng. doi:10.3390/jcm10153224.
- Zoch-Lesniak B, Ware RS, Grimwood K, Lambert SB. 2020. The respiratory specimen collection trial (ReSpect): a randomized controlled trial to compare quality and timeliness of respiratory sample collection in the home by parents and healthcare workers from children aged <2 years. J Pediatric Infect Dis Soc. 9(2):134–141. doi: 10.1093/jpids/piy136.

