ABSTRACT
The demand for food is increasing and the use of soil organic amendments in agricultural management practices has been instructed to increase crop yield and reduce dependence on synthetic inorganic fertilizers at low cost to limited resource farmers. However, the effect of organic amendments on the quality and nutritional composition of edible plants has received little attention. Locally available organic amendments (sewage sludge SS, chicken manure CM, cow manure Cow, vermicompost Vermi, and biochar Bio) were chosen to test their impact on field-grown sweet potato, Ipomoea batatas L. yield, root quality, and root nutritional composition. The results indicated that utilizing Cow manure in growing sweet potatoes significantly promoted root yield and root nutritional composition. Cow treatment produced the greatest number of roots compared to Bio, CM, SS, and the control treatments. The results also revealed that the concentrations of vitamin C (260. 3 µg g−1), β-carotene (45.4 µg g−1), soluble sugars (16.7 mg g−1), and total phenols (196.3 3 µg g−1 fresh roots) were greater in the roots of plants grown in Cow compared to the roots of the control treatment. The results indicated the low impact of biochar whereas Cow is recommended for enhancing sweet potato yield and nutritional composition.
Introduction
Sweet potato, Ipomoea batatas L. roots is an important worldwide economic food crop due to its naturally occurring vitamins and antioxidant composition that have been associated with suppressing various health-related disorders, such as melanogenesis and hematoma invasion (Yagasaki et al. Citation2000) in conjunction with meeting the food nutritional requirements, decreasing poverty, and the growing worldwide food security (El-Sheikha and Ray Citation2017). In the U.S., the commercially popular variety is the orange-fleshed sweet potato called “yam” with great β-carotene and sugar content used for combating vitamin A deficiency in children, lactating mothers, and pregnant women (Hotz et al. Citation2012). In addition, sweet potato contains polyphenols that have anti-inflammatory, anticancer, antidiabetic, and hepatoprotective activity effects (Lim et al. Citation2013; Hu et al. Citation2016). Recently, sweet potato utilization has been considerably increased by the common commercial availability of “French-fried” sweet potato roots. According to Firon et al. (Citation2009), the sweet potato root appearance quality is determined by the root shape and the differential rates of longitudinal and lateral growth which could be highly variable making it difficult to optimize the size and shape uniformity. However, the United States Department of Agriculture (USDA) has established standards for sweet potato root grades for the marketplace (USDA Citation2005).
On the other hand, the global challenge of waste disposal and limited landfill space emphasizes the crucial need for alternative waste management practices. In agricultural production systems, animal manure (chicken manure, horse manure, vermicompost, municipal sewage sludge), and other organic amendments such as biochar have been recommended as alternatives to elemental inorganic fertilizers (Antonious Citation2023; Antonious et al. Citation2022 a; Citationb). Biochar, the product of biomass pyrolysis (thermal decomposition), has been recommended to combat global warming and promote soil health. The potential benefits of biochar in improving agricultural soil quality have been found to enhance the soil’s capacity to retain nutrients and water, potentially leading to accelerated plant growth (Renner Citation2007). Biochar also can serve as a habitat for soil microorganisms, aiding in the breakdown of organic matter in the soil (Shen et al. Citation2016).
Investigators (El Sheikha Citation2016) studied the release of nutrients from elemental inorganic and organic fertilizers and reported that nutrients should be available to plants at optimum quantities for optimum plant growth. Nutrients in organic amendments such as animal manure are usually released in slow-release quantities for plant uptake due to their slow rate of mineralization by soil microorganisms. On the contrary, nutrients in inorganic fertilizers are available to plants immediately following their application. This fast availability of nutrients in inorganic fertilizers allows their volatilization and mobility to runoff and seepage water. Accordingly, none of these types of soil amendments can maintain crop nutritional needs, and combining organic manure and inorganic fertilizers is needed for sustainable agricultural production systems. Recently, El Sheikha et al. (Citation2022) grew snap beans (Phaseolus vulgaris L.) under several natural biostimulants (humic acid, vermicompost tea, moringa leaf extract, and yeast extract to investigate their impact on the plant yield and nutritional value (vitamin C and amino acids). They concluded that moringa leaf extracts and vermicompost tea positively increased bean yield and amino acid composition. In addition, several investigators (Castellanos et al. Citation2023) studied the impact of replacing inorganic fertilizer with manure as organic fertilizer on the yield of almonds, olives, and barely and concluded that manure compost significantly enhanced crop yield and reduced the production costs of the three crops.
Accordingly, the objectives of this investigation were to 1) study the impact of mixing native agricultural soil with animal manure (cow manure, chicken manure, sewage sludge, and vermicompost) and biochar on sweet potato (Ipomoea batatas L.) root yield and root quality; 2) determine the concentrations of ascorbic acid (vitamin C), total phenols, free sugars, and β-carotene (ancestor of vitamin A) in sweet potato roots grown under different soil management practices. Identifying soil management practices that meet soil nutrition needs, and support crop production and food quality is the focus of this investigation.
Materials and methods
Experimental design and field description
A randomized complete block design (RCBD) experiment at Kentucky State University Research Farm was established in the summer of 2021 in 18 field plots used for growing sweet potato, Ipomoea batatas L. variety Mahon Yam slips obtained from Johnny’s Selected Seeds (Waterville, Maine, USA). Plots (3.7 m wide × 22 m long each), separated with stainless steel borders along each plot side were built to prevent cross-contamination between adjacent treatments. The native soil in three experimental plots was mixed with chicken manure CM, three plots mixed with sewage sludge SS, three plots mixed with cow manure Cow, three plots mixed with vermicompost Vermi, three plots mixed with biochar Bio, and the native soil in three plots was used as control treatment. Each of the five soil amendments used in this investigation was mixed with the native soil at 5% nitrogen (N) on a dry weight basis to eliminate variations among the soil amendments composition due to their natural variability in N content since N fertilization has been identified as the major factor that influences NO3- content of vegetables (Gruda Citation2005; Santamaria Citation2006). SS which is used as is by growers in Kentucky, naturally contains 5% N was purchased from the Metropolitan Sewer District in Louisville (KY, USA) and applied to native soil at 2245.9 kg hectare −1, CM that contains 1.1% N was obtained from the Department of Animal and Food Sciences, University of Kentucky (Lexington, KY, USA) and applied at 10,179.8 kg hectare−1. Cow manure (0.5%. N) was purchased from Lowe’s (Frankfort, KY) and applied to native soil at 22,417 kg hectare −1. Vermicompost was obtained from Worm Powers (Montpelier, Vermont, USA) and applied at 9340.1 kg hectare−1, whereas biochar was purchased from Wakefield Agricultural Carbon Company (Columbia, MO) and applied at 0.56 kg hectare −1. Each of the soil amendments was mixed with native soil and rototilled to a depth of 15 cm (~0.5 ft.) topsoil before sweet potato planting to ensure uniform distribution of each amendment. Sweet potato slips (vine cuttings purchased from Johnny’s Selected Seeds, PO Box 299 Waterville, Maine, USA), were purchased and planted on 30 June 2021).
Yield and root quality
At harvest, 120 d sweet potato roots were collected using a single-row potato digger. Roots were weighed and graded according to the USDA (Citation2005) standards for grades into U.S. Extra No. 1 that is well-shaped roots with no cuts, bruises, or surface blemishes, length 3–9 inches (7.6–22.9 cm), maximum weight < 18 ounces (<510.3 g), maximum diameter not > 3–1/4 inches (<8.3 cm), and minimum diameter not < 1–3/4 inches (<4.45 cm). U.S. No. 1 grade is well-shaped with no cuts, bruises, or surface blemishes maximum diameter not > 3–1/2 inches (8.9 cm), maximum weight should not be > 20 ounces (567 g), length should be not <3 inches (7.6 cm) or more than 9 inches (22.9 cm), and minimum diameter not < 1–3/4 inches (<4.45 cm). U.S. No. 1 grade Petite is well-shaped with no cuts, bruises, or surface blemishes, diameter should be not < 1–1/2 inches (<3.8 cm) or more than 2–1/4 inches (5.7 cm) and should be not <3 inches (7.2 cm) or >7 inches (17.9 cm) in length. U.S. Commercial grade consists of sweet potatoes which meet all the requirements of the U.S. No. 1 grade except that an increased tolerance for defects is allowed. U.S. No. 2 diameter should be not < 1–1/2 inches (3.8 cm) and the maximum weight not > 36 ounces (1020.6 g). Whereas cull is the sweet potato root that is not classified according to USDA grades, and not marketable.
Chemical analysis
Replicate samples (n = 6) of sweet potato roots (underground stem) were collected at random from the soil treatments. The entire roots were cut into small pieces (1 cm cubes) and representative subsamples of 20 g were blended with 150 mL of ethanol to extract total phenols. Homogenates were filtered through Whatman No. 1 filter paper (Fisher Scientific, Pittsburgh, PA), and one-mL aliquots of filtrate were used for the determination of total phenols (McGrath et al. Citation1982) using a standard calibration curve (1 to 16 μg mL−1) of chlorogenic acid. Ascorbic acid was extracted by blending 20 g of roots with 100 mL of 0.4% (w/v) oxalic acid solution and determined by the dichlorophenolindophenol method (Association of Official Analytical Chemists AOAC Citation1970). Thirty (30) g representative root subsamples were blended at a high speed with 100 mL of acetone for 2 min in dim light to extract β-carotene (Antonious and Kasperbauer Citation2002). The homogenate was filtered with suction through a Buchner funnel containing Whatman filter paper No.1 The resulting thick paste was extracted twice with acetone until the extract was colorless. The filtrates were combined and transferred to a separatory funnel containing 50 mL of 4% aqueous NaCl and 100 mL of petroleum ether (BP 40–60◦C). Absorption of the petroleum ether layer was measured at 450 nm in dim light. A calibration curve was prepared for each group of samples using 99% pure β-carotene in the range of 10–100 μg mL − 1. Soluble sugars were extracted from sweet potato roots with 80% ethanol and quantified colorimetrically using the methods modified by Antonious et al. (Citation1996). A calibration curve was established with each group of samples using 10, 20, 30, and 50 μg mL−1 glucose standards. Purified standards of chlorogenic acid, β-carotene, ascorbic acid, and glucose were obtained from Sigma-Aldrich Inc. (Saint Louis, MO 63,103, USA) and used to obtain calibration curves.
Statistical analysis
Data containing sweet potato yield, root number, root quality characteristics, and concentrations of vitamin C, β-carotene, total phenols, and soluble sugars in sweet potato roots was statistically analyzed using one-way analysis of variance (ANOVA) (SAS Institute Inc Citation2016), and the means were compared using Duncan’s multiple range test.
Results and discussion
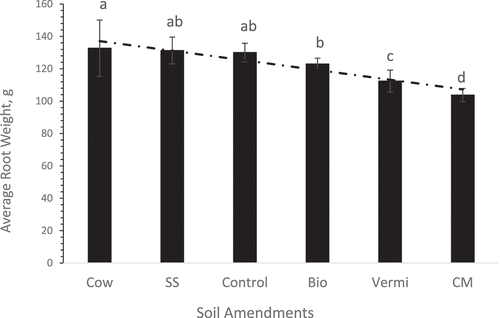
The average sweet potato root weight was greatest (133 g root −1) in plots amended with Cow manure compared to the Bio, Vermi, and CM soil amendments (). This increase might be due to improved soil fertility, nutrient retention, soil porosity, and water-holding capacity after the addition of Cow manure to native soil. Ruiz et al. (Citation2018), reported that among the total amount of animal manure produced annually, sewage sludge is on the top with 7200 t year −1 followed by pig and cow manure with 6500 t year−1. Accordingly, the huge production of Cow manure is an excellent source of renewable energy for use as an alternative source to substitute the inorganic elemental fertilizers commonly used in agricultural production systems. Cow manure is a biodegradable organic waste that contains high levels of nutrients, it can contain about 10 lbs. (4.54 kg). t−1 of potash, 5 lbs. (2.27 kg). t−1 of phosphate, and 10 lbs. (4.54 kg). t−1 of nitrogen (Neshat et al. Citation2017). In addition, the increased crop yields are often attributed to increased soil organic matter content and improvements in the physical properties of the soil after the incorporation of composted materials. These include increased soil aggregate stability (Hernando et al. Citation1989), increased moisture holding capacity, and reduced soil bulk density (Tester Citation1990).
Figure 1. Overall average of sweet potato root weight of plants grown under six soil management practices (Cow = cow manure, SS = sewage sludge, Control = no-mulch native soil, Bio = biochar, Vermi = vermicompost, and CM = chicken manure). Each bare is an average of 6 replicates ± standard error. Values in each treatment accompanied by the same letter(s) are not significantly different (p≥ 0.05) using Duncan’s multiple range test (SAS Institute Inc Citation2016).
The number of sweet potato roots varied greatly among individual soil treatments, leading to variations in total yield as well as the size and shape of the roots that impact the market value. revealed that Cow manure treatment produced the greatest number of roots compared to other treatments including biochar, CM, SS, and the control treatments. Investigators (Dong et al. Citation2023) found that organic amendments have been exploited as a source of nutrients to increase the advance and yield of several crops, including sweet potatoes. On the contrary, shows that CM-amended soil significantly (P> 0.05) reduced the number of roots compared to all the other amendments tested. Recent results (Dong et al. Citation2023) indicated that excessive soil available N introduced by poultry manure amendment could inhibit the formation of sweet potato storage roots.
Table 1. Average number of sweet potato roots categorized according to the USDA standard grades of plants grown under each soil treatment.
Regarding the impact of animal manure on the sweet potato root grades, Cow manure, SS, and Vermi were effective in increasing the US No.1 grade significantly (P< 0.05) compared to the other root grades ( respectively). In addition, CM was effective in increasing the US No.1 root grade () whereas biochar (Bio) treatment was effective in promoting US Extra No.1 root grade (the greatest sweet potato grade based on the USDA standard grades) (). Biochar is a high-carbon product produced by pyrolysis of biomass in low-oxygen surroundings. The positive effect of biochar on promoting the greatest root quality could be attributed to the improvements in soil water-holding capacity (Cheng et al. Citation2008) improved soil bulk density, and porosity (Lu et al. Citation2014), thereby promoting early root growth through the ability of plants to access more soil nutrients (Antonious and Turley Citation2020; Antonious et al. Citation2021). Regardless of soil amendment type, revealed that the US No.1 grade had the greatest sweet potato root weight compared to the US Extra, US Petite, and US No. 2 grades.
Figure 2. Overall sweet potatoes average root weight of each USDA standard grade, regardless of soil treatment. Each bare is an average of three replicates ± standard error. Values in each grade accompanied by the same letter(s) are not significantly different (p≥ 0.05) using Duncan’s multiple range test (SAS Institute Inc Citation2016).
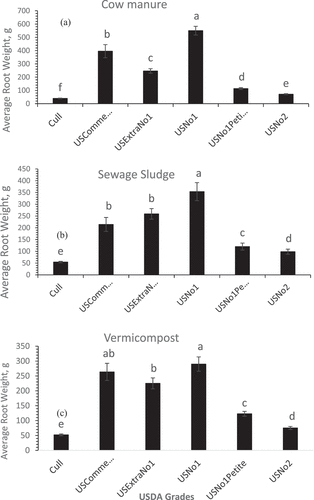
Figure 3. Overall sweet potatoes average root weight of each USDA standard grade, regardless of soil treatment. Each bare is an average of three replicates ± standard error. Values in each grade accompanied by the same letter(s) are not significantly different (p ≥ 0.05) using Duncan’s multiple range test (SAS Institute Inc Citation2016).
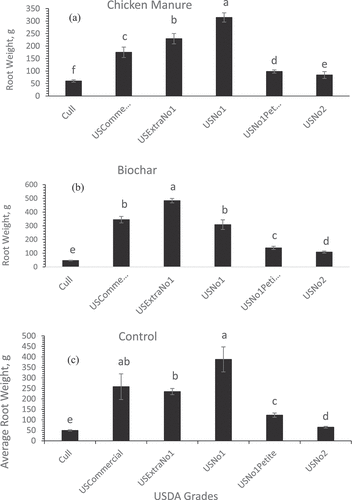
Figure 4. Overall sweet potato root standard USDA grades of plants grown under field conditions regardless of soil treatments. Each bare is an average of three replicates ± standard error. Values in each grade accompanied by the same letter(s) are not significantly different (p ≥ 0.05) using Duncan’s multiple range test (SAS Institute Inc Citation2016).
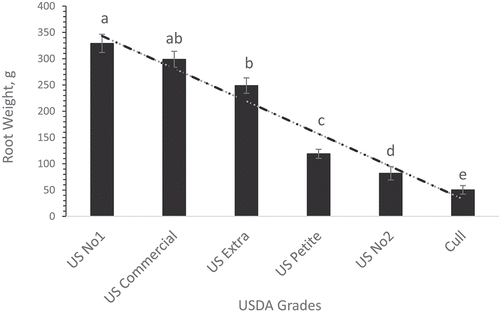
Animal manures used as organic amendments are exceptional fertilizers. The results in represent the overall impact of soil amendments on sweet potato USDA standard grades indicating that the average weight of US No1 grade (well-shaped roots with no cuts, bruises, or surface blemishes) was greater than US Extra, US Petite, and US No2 grades.
As indicated earlier, microorganisms in animal manures (Antonious et al. Citation2020, Citation2021) break down complex forms of nutrients and facilitate the slow release of N, P, and K and other nutrients from soil organic matter for plant uptake. Many vegetable species and cultivars of the same species have not been analyzed for concentrations of vitamin C, β-carotene (vitamin A), and phenolic compounds, which are important antioxidants that have several benefits for human health. Results in revealed that the concentrations of ascorbic acid (vitamin C) varied significantly among sweet potato roots of plants grown under different animal manures used as soil amendments. Ascorbic acid was greatest in the roots of plants grown in Cow manure-amended soil and lowest in the roots of plants grown in the control treatment (the no-mulch native soil). This represents a 29% increase in vitamin C compared to the control treatment. All the other organic amendments including biochar were also effective in promoting the concentration of this vitamin. β-carotene in sweet potato roots was greater by 64% in plants grown in CM-amended soil compared to the control treatment ().
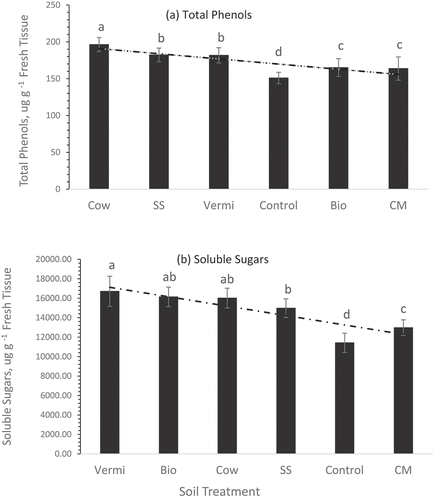
represents the variability in the concentrations of total phenols in the roots of sweet potatoes grown under the soil management practices investigated in the current study. Plants grown in Cow manure-amended soil had greater concentrations of phenols (196.3 µg g−1 fresh roots) compared to the other five treatments including the control. SS and Vermi had significantly similar concentrations of phenols (182.3 and 181.7 µg g−1, respectively) but greater than in the roots of plants grown in the control treatment. Similarly, concentrations of phenols in the roots of plants grown in Bio or CM treatments were not significantly different but greater than the control treatment.
Significant concentrations (p ≤0.05) of soluble sugars (16.7 mg g−1 fresh roots) were detected in the roots of sweet potato plants grown in Vermi amended treatments compared to the roots of plants grown in the unamended (control) treatments (10.9 mg g−1 fresh roots). Whereas sugars in plants grown in soil amended with Bio, Cow manure, or SS were not significantly different (). Studies revealed that soil fertilization is the utmost significant and manageable factor affecting the phytochemicals and nutritional value of vegetables (Antonious Citation2023). Variability of phytochemicals concentrations among plant species and among plants of the same species grown under altered soil agricultural practices such as the application of animal manures in land farming has been investigated. Most cattle and chicken are raised worldwide in large-scale concentrated animal-feeding operations, which necessitate the use of antibiotics, and inorganic and organic compounds (hormones, antibiotics, and pesticides) that when combined in the agricultural soils create a contamination problem due to their impact on the activity of soils microorganisms and their enzymatic secretion. Some of these contaminants have a negative impact on soil enzymes and microorganisms’ secretion.
Figure 5. Concentrations of ascorbic acid (a) and β-carotene (b) ± std. error in sweet potato roots of plants grown under six soil management practices (chicken manure, CM; cow manure cow, vermicompost vermi, biochar bio, sewage sludge SS, and no mulch control treatment. Statistical comparisons were performed among six treatments. Bars associated with the same letter(s) are not significantly different (p ≥ 0.05) using Duncan’s multiple range test.
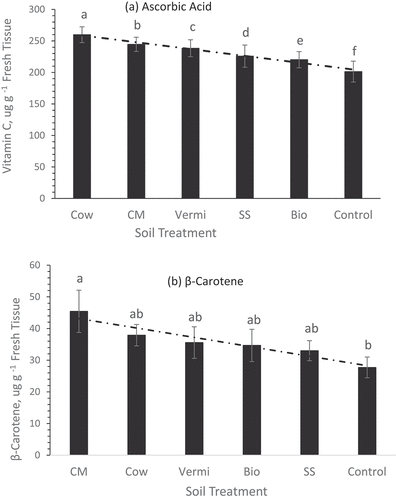
Figure 6. Concentrations of total phenols (a) and soluble sugars ± std. error (b) in sweet potato roots of plants grown under sex soil management practices (chicken manure, CM; cow manure cow, vermicompost vermi, biochar bio, sewage sludge SS, and no mulch NM control. Statistical comparisons were performed among six treatments. Bars associated with the same letter(s) are not significantly different (p ≥ 0.05) using Duncan’s multiple range test.
Soil enzymes have been investigated by several researchers and proven effective in breaking down the complex forms of organic matter in soil amendments into simple nutrients that can easily absorbed by the growing plants (Antonious Citation2023). Accordingly, the composition of the soil amendments (i.e. the presence of pesticides and heavy metals) can impact the beneficial soil microorganisms and their secreting enzymes that impact plant nutrient availability and crop yield and quality.
Variability of total phenols and soluble sugars content in sweet potato grown in the different soil amendments was observed. Cow manure increased total phenols whereas Vermi-amended soil increased soluble sugars in the roots. Variability of ascorbic acid (vitamin C), β-carotene, phenols, and soluble sugars in sweet potato roots of plants grown in the different manure-amended soil could be due to the higher synthesis of these phytochemicals by the leaves due to the nutrients added by each soil amendment. It might also be due to improved NPK nutrient absorption from the soil rhizosphere (the soil area around the plant root). This increase might also be due to increased soil organic matter and microbial activity after the addition of animal manures. Animal manure contains several substrates of many soil enzymes that activate soil hydrolyzing enzymes (i.e. urease, invertase, alkaline phosphatase, etc.). Accordingly, the pronounced increase in these studied phytochemicals could be due to increased microbial activity and their enzyme excretions after mixing native soil with animal manures.
Conclusion
There is a lack of data on the impact of animal manure on plants’ phytochemical composition and their nutritional and antioxidant properties. Researchers and growers have focused on the sweet potato yield and soil’s physical and chemical attributes following the combination of organic and inorganic amendments with very minor data on the plant’s nutritional and antioxidant contents. The current investigation revealed that the yield, quality, and nutritional composition of sweet potato roots of plants grown under various soil management practices varied significantly. The average root weight of sweet potato plants grown under Cow manure-amended soil (133 g root−1) was significantly greater compared to the roots of plants grown in the CM and Vermi amended treatments (103 and 112 g root−1, respectively). The concentrations of vitamin C in the roots of plants grown in Cow amended soil (260 3 µg g−1 fresh roots), β-carotene in the roots of plants grown in CM amended soil (45.4 3 µg g−1 fresh roots), soluble sugars in roots of plants grown in Vermi amended soil (16.7 mg g−1 fresh roots, and total phenols in roots of plants grown in Cow amended soil (196.3 3 µg g−1 fresh roots) were significantly greater than the concentrations in the roots of plants grown in the control treatments. No single amendment promoted the concentration of all the phytochemicals tested in sweet potato roots. Future objectives will include more than one mixed formulation of animal manures in a trial to promote sweet potato yield, quality, and phytochemical composition of the growing plants.
Acknowledgements
I recognize Eric Turley, Anjan Nepal, and the KSU farm team for maintaining the sweet potato field plots.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Antonious GF. 2023. The impact of organic, inorganic fertilizers, and Biochar on phytochemicals content of three Brassicaceae vegetables. J Appl Sci. 13(15):8801. doi: 10.3390/app13158801.
- Antonious GF, Dawood MH, Turley ET, Paxton RB. 2022 a. Biochar and animal manures increased the yield of three varieties of turnips. Internat J Appl Agricul Sci (IJAAS). 8(1):50–56. doi: 10.11648/j.ijaas.20220801.16.
- Antonious GF, Dawood MH, Turley ET, Trivette TG. 2022 b. Soil amendments enhanced summer squash yield, fruit composition, quality, and soil enzyme activity. Agricul Sci. 13(6):684–701. doi: 10.4236/as.2022.136045.
- Antonious GF, Kasperbauer MJ. 2002. Color of light reflected leaves modify nutrient content of carrot roots. Crop Sci. 42:1211–1216. doi: 10.2135/cropsci2002.1211.
- Antonious GF, Kasperbauer MJ, Byers ME. 1996. Light reflected from colored mulches to growing turnip leaves affects glucosinolate and sugar contents of edible roots. Photochem Photobiol. 64(3):605–610. doi: 10.1111/j.1751-1097.1996.tb03112.x.
- Antonious GF, Turley ET. 2020. Trace elements composition and enzymes activity of soil amended with municipal sewage sludge at three locations in Kentucky. Internat J Appl Agricul Sci (IJAAS). 6(5):89–95. doi: 10.11648/j.ijaas.20200605.11.
- Antonious GF, Turley ET, Dawood M. 2020. Monitoring soil enzyme activity before and after animal manure application. J. Of Agricul. 10(5):166. doi: 10.3390/agriculture10050166.
- Antonious GF, Turley ET, Shrestha DS, Dawood MH. 2021. Variability of biochar performance among soil amendments and enzyme activity. Internat J Appl Agricul Sci (IJAAS). 7(1):66–76. doi: 10.11648/j.ijaas.20210701.16.
- Association of Official Analytical Chemists (AOAC). 1970. Official methods for analysis of the Association of Analytical Chemists. 11th ed. Washington, D.C: AOAC.
- Castellanos GB, García GB, García GJ. 2023. Economic and environmental effects of replacing inorganic fertilizers with organic fertilizers in three rainfed crops in a semi-arid area. Sustainability. 15:16897. doi: 10.3390/su152416897.
- Cheng CH, Lehmann J, Engelhard MH. 2008. Natural oxidation of black carbon in soils: changes in molecular form and surface charge along a clip sequence. Geochem. Cosmochim.Acta. 72(6):1598–1610. doi: 10.1016/j.gca.2008.01.010.
- Dong HT, Li Y, Brown P, Xu CY. 2023. Sweet potato storage root formation as affected by soil organic amendment applications. Acta Physiol Plant. 45(7):88. doi: 10.1007/s11738-023-03570-3.
- El Sheikha AF. 2016. Mixing manure with chemical fertilizers, why? and what isAfter? Nutr Food Technol. 2(1):doi. doi: 10.16966/2470-6086.112.
- El Sheikha AF, Allam AY, Taha M, Varzakas T. 2022. How does the addition of biostimulants affect the growth, yield, and quality parameters of the snap bean (Phaseolus vulgaris L.)? How is this reflected in its nutritional value? Appl Sci. 12(2):776. doi: 10.3390/app12020776.
- El-Sheikha AF, Ray RC. 2017. Potential impacts of bioprocessing of sweet potato: review. Crit Rev Food Sci. & Nutr. 57(3):455–471. doi: 10.1080/10408398.2014.960909.
- Firon N, LaBonte D, Villordon A, McGregor C, Kfir Y, Pressman E. 2009. Botany and physiology: storage root formation and development. In: Loebstein G Thottappilly G, editors. The sweet potato. Dordrecht, Netherlands: Springer; pp. 13–26.
- Gruda N. 2005. Impact of environmental factors on product quality of greenhouse vegetables for fresh consumption. Crit Rev Plant Sci. 24(3):227–247. doi: 10.1080/07352680591008628.
- Hernando S, Lobo MC, Polo A. 1989. Effect of application of a municipal refuse compost on the physical and chemical properties of a soil. Sci And The Total Environ. 81(82):589–596. doi: 10.1016/0048-9697(89)90167-8.
- Hotz C, Loech C, de Brauw A, Eozenou P, Gilligan D, Moursi M, Munhaua B, van Jaarsveld P, Carriquiry A, Meenakshi JV. 2012. A large-scale intervention to introduce orange sweet potato in rural Mozambique increases vitamin A intake among children and women. Brit J Nutr. 108(1):163–176. doi: 10.1017/S0007114511005174.
- Hu Y, Deng L, Chen J, Zhou S, Liu S, Fu Y, Yang C, Liao Z, Chen M. 2016. An analytical pipeline to compare and characterize the anthocyanin antioxidant activities of purple sweet potato cultivars. Food Chem. 194:46–54. doi: 10.1016/j.foodchem.2015.07.133.
- Lim S, Xu J, Kim J, T-Y C, Su X, Standard J, Carey E, Griffin J, Herndon B, Katz B, et al. 2013. Role of anthocyanin-enriched, purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol Nutr & Food Res. 57(11):1908–1917. doi: 10.1002/mnfr.201300040.
- Lu S-G, Sun F-F, Zong Y-T. 2014. Effect of rice husk biochar and coal fly ash on some physical properties of expansive clayey soil (vertisol). Catena. 114:37–44. doi: 10.1016/j.catena.2013.10.014.
- McGrath RM, Kaluza WZ, Daiber KH, Van der Riet WR, Glennie CW. 1982. Polyphenols of sorghum grain, their changes during malting, and their inhibitory nature. J Agric Food Chem. 30:450–456. doi: 10.1021/jf00111a010.
- Neshat SA, Mohammadi M, Najafpour GD, Lahijani P. 2017. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogasproduction. Renew Sustain Energy Rev. 79:308–322. doi: 10.1016/j.rser.2017.05.137.
- Renner R. 2007. Rethinking biochar. Environ Sci Technol. 41(17):5932–5933. doi: 10.1021/es0726097.
- Ruiz D, San Miguel G, Corona B, Gaitero A, Domínguez A. 2018. Environmental and economic analysis of power generation in a thermophilic biogas plant. Sci Total Environ. 633:1418–1428. doi: 10.1016/j.scitotenv.2018.03.169.
- Santamaria P. 2006. Nitrate in vegetables: toxicity, content, intake and EC regulation (review). J Sci Food Agric. 86(1):10–17. doi: 10.1002/jsfa.2351.
- SAS Institute Inc. 2016. SAS/STAT Guide, version 9.4. Campus drive. Cary, NC, USA: SAS Institute Inc.; p. 27513.
- Shen Z, Soma AM, Wang F, Jin F, Mcmillan O, Al-Tabbaa A. 2016. Long-term impact of biochar on the immobilization of nickel (II) and zinc (II) and the revegetation of a contaminated site. Sci. Total Enviro. 542:771–776. doi: 10.1016/j.scitotenv.2015.10.057.
- Tester CF. 1990. Organic amendment effects on physical and chemical properties of a sandy soil. J Soil Sci. Society Of Amer. 54(3):827–831. doi: 10.2136/sssaj1990.03615995005400030035x.
- USDA (2005). United States Standards for Grades of Sweet Potatoes, April 1, 2005. United States Dept. of Agriculture, Washington, DC. [Online] Available at: https://www.ams.usda.gov/sites/default/files/media/Sweetpotato_Standard%5B1%5D.pdf.
- Yagasaki K, Miura Y, Okauchi R, Furuse T. 2000. Inhibitory effects of chlorogenic acid and its related compounds on the invasion of hepatoma cells in culture. Cytotechnology. 33(1/3):229–235. doi: 10.1023/A:1008141918852.

