Abstract
Neurosteroid and immunological actions of vitamin D may regulate depression-linked physiology. Meta‐analyses investigating the effect of vitamin D on depression have been inconsistent. This meta-analysis investigated the efficacy of vitamin D in reducing depressive symptoms among adults in randomized placebo-controlled trials (RCT). General and clinical populations, and studies of ill individuals with systemic diseases were included. Light therapy, co-supplementation (except calcium) and bipolar disorder were exclusionary. Databases Medline, PsycINFO, CINAHL and The Cochrane Library were searched to identify relevant articles in English published before April 2022. Cochrane risk-of-bias tool (RoB 2) and GRADE were used to appraise studies. Forty-one RCTs (n = 53,235) were included. Analyses based on random-effects models were performed with the Comprehensive Meta-analysis Software. Results for main outcome (n = 53,235) revealed a positive effect of vitamin D on depressive symptoms (Hedges’ g = −0.317, 95% CI [−0.405, −0.230], p < 0.001, I2 = 88.16%; GRADE: very low certainty). RoB assessment was concerning in most studies. Notwithstanding high heterogeneity, vitamin D supplementation ≥ 2,000 IU/day appears to reduce depressive symptoms. Future research should investigate possible benefits of augmenting standard treatments with vitamin D in clinical depression. PROSPERO registration number: CRD42020149760. Funding: Finnish Medical Foundation, grant 4120 and Juho Vainio Foundation, grant 202100353.
Introduction
Rationale
Depression is the leading cause of disability worldwide, affecting more than 320 million people every year (World Health Organization Citation2017, Citation2020). Antidepressants can be an effective treatment for depression (Cipriani et al. Citation2018) but their therapeutic efficacy is not always sufficient for all individuals. Further, treatment relapses are common, and most patients require multiple antidepressant trials to achieve adequate response (Rush et al. Citation2006). Thus, adjunctive treatment options for those with depression require further investigation.
Vitamin D is known for its role in bone ossification and is formed in the skin by endogenous synthesis by sunlight UVB radiation or obtained from dietary sources. Sources of vitamin D in the diet include oily fish, eggs, meat, mushrooms and fortified dairy products. In some countries, vitamin D supplementation on regular basis in the form of fish liver oil or synthesized vitamin D is recommended for specific populations that are vulnerable to vitamin D deficiency, such as young children and the elderly (Prentice Citation2008). Following the discovery of vitamin D receptors in the brain, it has been suggested that vitamin D could regulate neurophysiological processes associated with depression as a neuroactive steroid (Eyles et al. Citation2005). Low circulating vitamin D [25-hydroxyvitamin D3; 25(OH)D] levels have been linked to depression in cross-sectional studies (Anglin et al. Citation2013). Biologically active vitamin D (1,25-dihydroxyvitamin D3), nuclear vitamin D receptor (VDR), and enzymes activating and metabolizing vitamin D are present in neurons, glial cells, and brain macrophages, and vitamin D is considered to have various autocrine or paracrine actions in the brain (Kalueff and Tuohimaa Citation2007). Hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis and hypersecretion of the stress hormone cortisol due to decreased sensitivity of glucocorticoid receptors in the brain are common physiological abnormalities in patients with depression (Willner, Scheel-Krüger, and Belzung Citation2013). Depression has also been associated with low-grade inflammation in the form of elevated cytokine levels and stress-induced abnormal lipid and glucose metabolism (Berk et al. Citation2013). Vitamin D has been suggested to act as an antagonist of glucocorticoids and to protect the vulnerable hippocampus in HPA axis dysregulation (Obradovic et al. Citation2006). Vitamin D may regulate the formation and maturation of new neurons in the hippocampus (Brown et al. Citation2003), the secretion of serotonin and dopamine by neurons (Sabir et al. Citation2018; Sedaghat et al. Citation2019), and the mechanisms underlying synaptic plasticity via genomic and nongenomic pathways (Groves, McGrath, and Burne Citation2014). The body’s innate and adaptive immune responses have also been shown to be partially dependent on circulating 25(OH)D levels (Di Rosa et al. Citation2011).
Several systematic reviews and meta-analyses have investigated with mixed results a possible causal link between circulating 25(OH)D levels, vitamin D supplementation, and depression (Anglin et al. Citation2013; Cheng, Huang, and Huang Citation2020; Gowda et al. Citation2015; Li et al. Citation2014; Sarris et al. Citation2016; Shaffer et al. Citation2014; Sparling et al. Citation2017; Spedding Citation2014; Vellekkatt and Menon Citation2019). A systematic review by Guzek et al. (Citation2021) examined how vitamin D supplementation affects mental health more broadly, with outcomes including depression as well as well-being, quality of life, mood, general mental component, and anxiety. In that qualitative review, studies were heterogeneous (N = 14). The majority of studies provided no evidence for a positive effect of vitamin D supplementation on overall mental health in adults. Of the studies associated with low risk of bias (N = 7), only two reported protective effects of vitamin D. Likewise, no benefit emerged in high-quality studies that assessed outcomes other than depression (N = 4). Inconsistent results were reported in high-quality studies (N = 5) investigating supplementation and depressive symptoms, with some demonstrating either evidence of a positive effect (N = 2) or non-significant results (N = 3) (Guzek et al. Citation2021). Another systematic review reported no evidence of an association between vitamin D supplementation and changes in quality of life (N = 15). The exception was a small- or medium-sized effect in separate short-term (≤ 6 months) studies (N = 3) that included clinical populations with various systemic diseases (Hoffmann, Senior, and Mager Citation2015).
Due to between-study heterogeneity, previous meta-analyses were relatively small-scale and yielded mainly ambiguous results. Some recent, more extensive meta-analyses on vitamin D and depressive symptoms provide stronger indications of possible benefits. A meta-analysis by Cheng, Huang, and Huang (Citation2020), for instance, included trials with both depression and anxiety or bipolar disorder as the main outcomes. They found a positive overall vitamin D effects, and also a positive effect in the subsets of trials evaluating depressive and anxiety symptoms. A recent systematic review of the efficacy of vitamin D in depressive and anxiety disorders focused on studies published in the decade ending with September 2021. Between vitamin D monotherapy and symptom reduction in major depressive disorder (MDD), mild to severe depressive symptoms, and generalized anxiety disorder (GAD), significant correlations emerged. However, that review contained no meta-analysis, and also included trials with standard treatment as comparator instead of placebo (Borges-Vieira and Cardoso 2022).
Despite fewer studies on individuals experiencing MDD, since previous meta-analyses, the number of related publications has increased. Also, due to possible issues with the analytic approach of previous meta-analyses, an updated systematic review and meta-analysis is crucial (Erhard et al. Citation2017). As such, for more precise estimates, a comprehensive meta-analysis investigating the effect of vitamin D supplementation on depressive symptoms in general and clinical populations, using different dosages for comparison and treatment durations is necessary.
Objectives
To determine the efficacy of vitamin D supplementation on depressive symptoms, we systematically reviewed and meta-analyzed the current relevant literature of randomized controlled trials (RCTs) that examined the efficacy of vitamin D (cholecalciferol, ergocalciferol, calcitriol) or vitamin D-calcium-supplementation in reducing depressive symptoms compared with placebo in adults from general and clinical populations. In addition, we investigated how the effect of vitamin D supplementation varied in different subgroups, including individuals experiencing MDD, and whether the supplementation effect differed based on intervention duration or vitamin D dosage. A detailed statement of the comparisons made is presented in the PICO framework (Population, Intervention, Comparator, Outcome) in Table S1 (supplementary material).
Table 1. Results of statistical syntheses.
Methods
The search strategy, selection of studies and data synthesis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines with 2020 updates (Moher et al. Citation2009; Page et al. Citation2020) in accordance with a pre-registered protocol (PROSPERO: www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42020149760).
Eligibility criteria and information sources
Detailed inclusion and exclusion criteria for article screening are presented in Table S1 (supplementary material). Studies including adults from general and clinical populations were considered for eligibility.
Studies were identified by searching electronic databases and scanning reference lists of included primary articles and systematic reviews on the same topic. No restrictions for publication year were applied but publication printed in languages other than English were excluded. MEDLINE (via Ovid), EMBASE (via EMBASE), PsycINFO (via EBSCO), CINAHL (via EBSCO) and The Cochrane Library (via Wiley InterScience) were searched with similar search strategies from database inception to present. The database searches were completed between September 2019 and April 2022. Primary database searches were updated in August 2020, May 2021, and April 2022. EMBASE was searched only in the primary database searches in September 2019. Reference lists of eligible studies and systematic reviews on the same topic were manually examined for additional relevant studies.
Systematic search and study selection
The search strategy was developed in consultation with an experienced medical librarian. The free text search terms were the same for every database (see supplementary material for the search string for database searches). The literature search was conducted by TM. The titles and abstracts identified by the search and those from additional sources were reviewed independently by two of the reviewers (AR and TM). Articles were screened and selected for full-text review if they met the selection criteria independently by the two reviewers. Finally, any disagreements were discussed and resolved between the two reviewers and if necessary, a third reviewer (WM) was consulted.
Data collection process
A standardized, pre-piloted form was used to extract data from the included studies for assessment of study quality and evidence synthesis. Two reviewers (TM and AR, MH, AL, TR, SR, or JR) extracted data from each report independently and discrepancies were identified and resolved through discussion (with a third reviewer WM where necessary). When needed, primary study authors were contacted via email to obtain missing data for the analysis.
Data items
Reviewers extracted data using a spreadsheet that recorded study inclusion and exclusion criteria, intervention and control group sizes, sex proportions, and mean age. Serum 25(OH)D levels at baseline and post-treatment and proportions of study subjects with baseline serum 25(OH)D level < 50 nmol/L were extracted when available. Reviewers extracted the assessment tool used for evaluating depressive symptoms and change in depressive symptoms as reported. Also, the type of depressive symptoms the tool evaluated (unipolar, postpartum/antenatal or seasonal affective disorder) and whether the study participants had a diagnosis of depression as determined by a clinician or self-evaluated their symptoms, was extracted. When multiple assessment tools for depressive symptoms were reported in a single study, all tool results were extracted. When 25(OH)D concentrations or depressive symptoms were examined at multiple time points, data were extracted from time points closest to the beginning and ending of the vitamin D supplementation. Vitamin D supplementation details included intervention duration, dose, type, frequency, and possible calcium supplementation or add-on medications. If reported, also compliance rate for the vitamin D or placebo, attrition, and observed adverse events were extracted.
Risk of bias assessment
Information for assessment of risk of bias was extracted independently by two reviewers (TM and ML or AR) from each study using a standardized form provided by version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) (Cochrane Collaboration Citation2020). RoB 2 form includes data items for assessment of risk of bias arising from the randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Disagreements in risk of bias judgements were resolved between the reviewers. Following the guidance manual given for RoB 2, reviewers derived an overall summary “Risk of bias judgment” (low, some concerns, or high) for each of the included studies.
Effect measures and synthesis methods
The meta-analyses were performed with the Comprehensive Meta-analysis Software (Biostat Inc., Englewood, NJ, USA) using a random-effects model due to differences between the individual RCTs, with standardized mean differences (Hedges’ g) for continuous outcomes (depression scores). A negative value was indicative of a larger improvement in the intervention versus control group. For each outcome, 95% confidence intervals (CIs) and two-sided P values for each outcome were calculated.
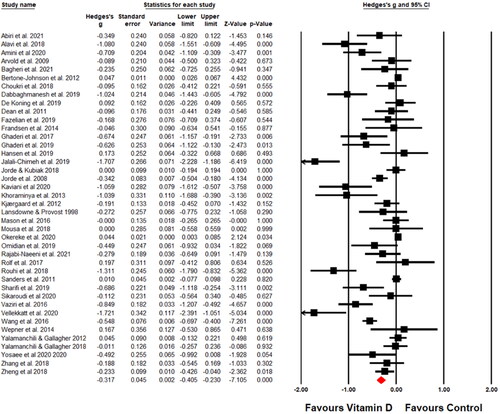
Heterogeneity was assessed using the I2 test. We considered an I2 statistic value greater than 75% indicative of substantial heterogeneity. For studies that had reported the results with median and range or median and inter-quartile range, means with standard deviations (SD) were computed from available reported data using methods described elsewhere (Hozo, Djulbegovic, and Hozo Citation2005; Luo et al. Citation2018; Wan et al. Citation2014). When required, scores on depressive symptom scales were reversed to ensure that all scales were aligned. If the studies reported change in depressive symptoms using several depression scales, a combined effect size was calculated. If depressive symptoms had been assessed at multiple time points, we extracted data for baseline and the time point closest to the end of vitamin D consumption. We synthesized evidence both narratively and graphically using standard forest plot. Studies were ordered in forest plot alphabetically according to the first author.
In addition to the primary meta-analysis, we performed sensitivity analyses with specific subgroups to address whether the summary effects varied in relation to different population characteristics. Studies were categorized into subgroups based on the criteria presented in Table S2 (supplementary material). If the required data were not reported and missing data could not be obtained from the original authors, the study was excluded from the subgroup analyses. Sensitivity analyses were also performed based on RoB 2 overall risk of bias judgment. We originally planned to run separate analyses for lower versus higher vitamin D supplementation studies, but due to heterogeneity in study designs and vitamin D doses these studies were excluded from our meta-analysis.
Reporting bias assessment
Risk of publication bias was examined statistically by applying Egger’s regression and via visual inspection of a funnel plot from the primary analyses.
Certainty assessment
The Grading of Recommendation, Assessment, Development and Evaluation (GRADE) assessment tool was used to assess the certainty in the body of evidence. Certainty was judged by considering study design, risk of bias, inconsistency, indirectness, imprecision, publication bias, and effect size. Following the GRADE guidelines, the pooled outcome was rated as having high, moderate, low, or very low certainty (Guyatt et al. Citation2011). Certainty was assessed by one investigator (WM) and judgements were confirmed by a second and a third author (TM and AR). The results of assessments of certainty were reported in summary of findings table.
Results
Study selection
The identification, screening and inclusion of the studies is presented as a PRISMA flow diagram in Figure S1. Our primary database searches identified a total of 4,621 records. After removing duplicates (N = 1,382), titles and abstracts were screened and 3,164 papers were excluded as they did not meet the inclusion criteria. Excluded full text articles and conference abstracts (Shahi et al. Citation2017; Fedotova Citation2018; Abdul-Razzak et al. Citation2018; Abou-Raya, Abou-Raya, and Helmii Citation2014; Tella, Yalamanchili, and Gallagher Citation2015; Rolf et al. Citation2016; Williams et al. Citation2016; Yalamanchili and Gallagher Citation2016; Fedotova and Dudnichenko Citation2017; Zheng et al. Citation2018; Far, Rahnema, and Qafelehbashi Citation2018; Raja-Khan et al. Citation2012; Aucoin et al. Citation2018; Kusmiyati et al. Citation2020; CitationTabra, Abu-Zaid, and Hablas 2020; Zhu et al. Citation2020; Paduchová et al. Citation2021; Gaughran et al. Citation2021; Missaoui et al. Citation2021; Torrisi et al. Citation2021; Sepehrmanesh et al. Citation2016) with reasons for exclusion are presented in PRISMA flow diagram (Figure S1). Two additional eligible studies meeting our criteria were identified through screening of reference lists of the included articles (Arvold et al. Citation2009; Vieth et al. Citation2004). After removing an additional five studies with an ineligible study design (low versus high vitamin D supplementation without placebo) (Vieth et al. Citation2004; Mozaffari-Khosravi et al. Citation2013; Narula et al. Citation2017; Gugger et al. Citation2019; Penckofer et al. Citation2022), a total of 41 full text papers were determined eligible for inclusion (Amini et al. Citation2022; Kaviani et al. Citation2020; Sikaroudi et al. Citation2020; Yosaee et al. 2020; Zheng et al. Citation2019; Arvold et al. Citation2009; Alavi et al. Citation2019; Bertone-Johnson et al. Citation2012; Choukri et al. Citation2018; Dabbaghmanesh et al. Citation2019; de Koning et al. Citation2019; Dean et al. Citation2011; Fazelian et al. Citation2019; Frandsen et al. Citation2014; Ghaderi et al. Citation2017; Citation2020; Hansen et al. Citation2019; Jalali-Chimeh et al. Citation2019; Jorde et al. Citation2008; Jorde and Kubiak Citation2018; Khoraminya et al. Citation2013; Kjaergaard et al. Citation2012; Lansdowne and Provost Citation1998; Mason et al. Citation2016; Mousa et al. Citation2018; Omidian et al. Citation2019; Rolf et al. Citation2017; Rouhi et al. Citation2018; Sanders et al. Citation2011; Sharifi et al. Citation2019; Vaziri et al. Citation2016; Wang et al. Citation2016; Wepner et al. Citation2014; Yalamanchili and Gallagher Citation2012; Citation2018; Zhang et al. Citation2018; Okereke et al. Citation2020; Vellekkatt et al. Citation2020; Abiri, Sarbakhsh, and Vafa Citation2021; Bagheri et al. Citation2022; Rajabi‐Naeeni et al. Citation2021). Supplementary list of “near-misses” (studies that met many but not all inclusion criteria) provides an explanation for exclusion of each study. The report by Sepehrmanesh et al. (Citation2016) was excluded due to its having been retracted in updated database search.
Study characteristics
Table S3 summarizes the study characteristics and key findings of the included studies (supplementary material). All 41 included studies were RCTs published in English between 1998 and December 2021. The trials were conducted in Iran (18 studies) (Amini et al. Citation2022; Kaviani et al. Citation2020; Sikaroudi et al. Citation2020; Yosaee et al. 2020; Alavi et al. Citation2019; Dabbaghmanesh et al. Citation2019; Fazelian et al. Citation2019; Ghaderi et al. Citation2017; Citation2020; Jalali-Chimeh et al. Citation2019; Khoraminya et al. Citation2013; Omidian et al. Citation2019; Rouhi et al. Citation2018; Sharifi et al. Citation2019; Vaziri et al. Citation2016; Abiri, Sarbakhsh, and Vafa Citation2021; Bagheri et al. Citation2022; Rajabi‐Naeeni et al. Citation2021), United States of America (6 studies) (Arvold et al. Citation2009; Bertone-Johnson et al. Citation2012; Mason et al. Citation2016; Okereke et al. Citation2020; Yalamanchili and Gallagher Citation2012, Citation2018), Australia (5 studies) (Dean et al. Citation2011; Lansdowne and Provost Citation1998; Mousa et al. Citation2018; Sanders et al. Citation2011; Zheng et al. Citation2019;), Norway (3 studies) (Jorde et al. Citation2008; Jorde and Kubiak Citation2018; Kjaergaard et al. Citation2012), Denmark (2 studies) (Frandsen et al. Citation2014; Hansen et al. Citation2019), China (2 studies) (Wang et al. Citation2016; Zhang et al. Citation2018), Netherlands (2 studies) (de Koning et al. Citation2019; Rolf et al. Citation2017), New Zealand (1 study) (Choukri et al. Citation2018), Austria (1 study) (Wepner et al. Citation2014), and India (1 study) (Vellekkatt et al. Citation2020). A total of 53,235 participants were involved in the 41 studies and most of the included participants were women (approximately 84%). Sample sizes ranged from n = 42 to n = 36,282. The duration of interventions varied between five days to five years. The single doses of vitamin D varied from 400 to 500,000 IU and as calculated per intervention day from 400 IU to approximately 14,000 IU. Depression was measured with various questionnaires and instruments. Ten study papers (24%) did not report the inclusion or numbers of participants using concurrent antidepressants. Thirty-four percent of studies (N = 14) allowed antidepressant usage outside the study protocol or had an antidepressant as a part of the intervention.
Risk of bias in studies
The results of risk of bias assessment of the trials are presented in Figure S2 (supplementary material).
Results of individual studies
Results of individual studies are presented in Table S4 with means, SDs and sample sizes for each group (supplementary material). Results that were not reported directly in the original studies and had to be computed or estimated from other information are indicated in the table.
Results of syntheses
The effect estimates of vitamin D supplementation on depressive symptoms for all participants, and for particular subgroups, are presented in . Effect size estimates and their precision are reported for each study as a forest plot in . The primary meta-analysis, which included 41 randomized controlled trials (n = 53,235), produced a small-to-moderate effect of vitamin D on symptoms of depression (Hedges’ g = −0.317, 95% CI −0.405 to −0.230, p < 0.001, I2 = 88.16%; GRADE: very low certainty).
In subgroup analyses, an effect favoring vitamin D supplementation was observed in patients (total n = 1,166) diagnosed with MDD (Hedges’ g = −0.729, 95% CI −1.100 to −0.358, p < 0.001, I2 = 82.3%), whereas subgroup analyses in healthy people (total n = 47,400) yielded a minimal effect favoring placebo (Hedges’ g = 0.043, 95% CI 0.025 to 0.061, p < 0.001, I2 = 0%). In the small subset of trials (N = 4) where participants (total n = 407) were mothers with perinatal depressive symptoms, a large effect for vitamin D supplementation was observed (Hedges’ g = −0.930, 95% CI −1.229 to −0.632, p < 0.001, I2 = 44.6%). Two of the studies followed mothers from 26–28 weeks of gestation until four to eight weeks after childbirth (Dabbaghmanesh et al. Citation2019; Vaziri et al. Citation2016), while one study investigated postnatal depressive symptoms for six months starting from four to ten months after childbirth (Rouhi et al. Citation2018).
In a further subgroup analysis with the three trials focused on symptoms of seasonal affective disorder (SAD), no significant effect was seen (Hedges’ g = −0.443, 95% CI −0.982 to 0.096, p = 0.107, I2 = 77.3%). We examined whether reported mean scores of depression scales in study populations exceeded the common cutoff values for clinically relevant symptoms used by the original studies and ran subgroup analyses for populations with and without at least mild clinically relevant depressive symptoms. Subgroup analysis in participants with clinically relevant depressive symptoms (including trials with patients diagnosed with MDD) yielded a moderate effect favoring vitamin D supplementation (Hedges’ g = −0.604, 95% CI −0.802 to −0.406, p < 0.001, I2 = 78.4%). In participants without clinically relevant depressive symptoms, the vitamin D effect estimate was smaller (Hedges’ g = −0.157, 95% CI −0.284 to −0.030, p = 0.015, I2 = 77.9%).
Vitamin D had a mostly similar small-to-moderate effect in both subsets of trials including people with reported baseline average serum 25(OH)D levels ≤ 50 nmol/L (considered the cutoff for normal levels; Hedges’ g = −0.380, 95% CI −0.561 to −0.198, p < 0.001, I2 = 85.02%) and > 50 nmol/L (Hedges’ g = −0.236, 95% CI −0.342 to −0.131, p < 0.001, I2 = 88.0%). The observed effects of vitamin D supplementation were on average larger (Hedges’ g = −0.659, 95% CI −0.955 to −0.364, p < 0.001, I2 = 81.2%) in shorter trials with duration of less than 12 weeks than in trials lasting at least 12 weeks (Hedges’ g = −0.205, 95% CI −0.291 to −0.119, p < 0.001, I2 = 86.4%). The effect of vitamin D did not clearly depend on the supplementation dose. A slightly larger effect was observed at doses of over 2,000 IU/day (Hedges’ g = −0.407, 95% CI −0.556 to −0.259, p < 0.001, I2 = 75.8%) than at doses of at most 2,000 IU/day (Hedges’ g = −0.183, 95% CI −0.286 to −0.079, p < 0.001, I2 = 87.7%). Similarly, the effect size seemed to be larger in the subgroup with the highest doses of over 4,000 IU/day (Hedges’ g = −0.392, 95% CI −0.605 to −0.180, p < 0.001, I2 = 78.4%) compared to the subgroup with doses of at most 4,000 IU/day (Hedges’ g = −0.279, 95% CI −0.371 to −0.189, p < 0.001, I2 = 85.4%).
Subgroup analysis with samples solely consisting of female participants produced a similar effect size estimate as the main meta-analysis (Hedges’ g = −0.372, 95% CI −0.540 to −0.204, p < 0.001, I2 = 90.9%). No significant vitamin D effect occurred in the subset of trials (N = 6) including only older adults (reported sample mean age over 65 years) (Hedges’ g = −0.030, 95% CI −0.155 to 0.095, p = 0.636, I2 = 77.6%), whereas in younger people (reported sample mean age 65 years or less), a significant effect was seen (Hedges’ g = −0.421, 95% CI −0.552 to −0.289, p < 0.001, I2 = 77.1%).
Subgroup analyses for trials (N = 14) that reportedly allowed participants to use antidepressants outside the trial protocol (including both general population trials with patients with depression in minor proportions and trials with patients with MDD with ongoing not-specified standard antidepressants) provided a small but statistically significant vitamin D effect (Hedges’ g = −0.162, 95% CI −0.286 to −0.039, p = 0.010, I2 = 82.5%). For the subset of studies (N = 17) that excluded participants using antidepressants outside trial protocol (including both general population trials with depression as one exclusion criteria and trials focusing on patients with depression without ongoing antidepressants), a significant medium-size vitamin D effect occurred (Hedges’ g = −0.461, 95% CI −0.673 to −0.250, p < 0.001, I2 = 91.3%). Vitamin D did not have a significant effect in the subset of interventions (N = 5) that included calcium co-supplementation (Hedges’ g = −0.102, 95% CI −0.315 to 0.111, p = 0.346, I2 = 84.2%). However, the effect estimate was significant when calcium co-supplementation trials were excluded (N = 37) (Hedges’ g = −0.391, 95% CI −0.513 to −0.268, p < 0.001, I2 = 87.3%).
The effect size estimate for vitamin D supplementation in studies adopting the most frequently used tool, the Beck Depression Inventory (BDI or BDI-II) was significant (Hedges’ g = −0.461, 95% CI −0.629 to −0.292, p < 0.001, I2 = 75.7%). Finally, there was a significant effect favoring vitamin D both in the subset of trials (N = 15) with high overall risk of bias judgment (Hedges’ g = −0.366, 95% CI −0.595 to −0.137, p = 0.002, I2 = 88.9%) and with some concerns (N = 21) (Hedges’ g = −0.430, 95% CI −0.601 to −0.259, p < 0.001, I2 = 86.3%). In the subset of studies with low overall risk of bias (N = 5), a small effect favoring placebo occurred (Hedges’ g = 0.037, 95% CI −0.002 to 0.077, p = 0.061, I2 = 0). Most of the included studies had minor methodological issues or the reported information did not allow proper evaluation of the risk of bias. Most commonly the trial protocol or statistical analysis plan was not available and possible deviations from the intended intervention or selection of the reported results was not evaluable.
Safety
Based on the minimal reported side effects, the administered doses of vitamin D were considered safe. In the majority of studies, no adverse reactions were reported, and the reported vitamin D related adverse reactions were predominantly mild (e.g., nausea, mild hypercalcemia) (Jorde and Kubiak Citation2018; Vaziri et al. Citation2016; Zhang et al. Citation2018; Zheng et al. Citation2018) or were not study group specific. In single studies, adverse events such as bone and joint pain, nausea, diarrhea, and itching led to discontinuation of five (Wang et al. Citation2016) and two (Zhang et al. Citation2018) participants from the vitamin D group. In a general population study with a large annual single oral dose (500,000 IU), more bone fractures and cardiovascular events were reported in the vitamin D group than in the placebo group (Sanders et al. Citation2011).
Results of investigations of heterogeneity
The I2 test showed substantial heterogeneity in the primary meta-analysis. In subgroups analyses, the I2 statistic mainly corresponded to the result of the primary analysis. However, low heterogeneity was found in the subgroup analysis with healthy participants (total n = 47,400) and with the group of studies with low overall risk of bias (N = 5).
Reporting biases
Visual inspection of the funnel plot (Figure S3) and Egger’s regression test for the primary analysis detected significant evidence for potential publication bias (p < 0.0001) (supplementary material).
Certainty of evidence
Using the GRADE framework, there was very low certainty that the effect size estimate of our main analysis represents the true effect (supplementary Table S5). Reasons for rating down the certainty of evidence were a large percentage (36.6%) of individual studies reporting high risk of bias in at least one domain and a large degree of detected statistical heterogeneity. Statistical heterogeneity was largely unresolved by subgroup analyses. In addition, funnel plot and Egger’s regression test found significant evidence of publication bias.
Discussion
Our analyses showed that vitamin D supplementation appeared to reduce depressive symptoms, especially among individuals diagnosed with MDD (Hedges’ g = −0.729) and in women with perinatal depressive symptoms (Hedges’ g = −0.930). However, our result favored placebo in the subset of healthy individuals without a diagnosis of depression or without other major psychiatric or physical conditions. The effect of vitamin D supplementation appeared larger when supplements were taken for less than 12 weeks compared to longer than 12 weeks. Moreover, vitamin D doses up to 2,000 IU/day had a similar small-to-moderate size effect as doses up to 4,000 IU/day, but doses of over 4,000 IU/day produced a larger effect. Our results also show that vitamin D supplementation had a similar small-to-moderate effect size in people with low (≤ 50 nmol/L) and sufficient (> 50 nmol/L) serum levels of vitamin D at baseline. The effect size estimate was slightly larger in the subset with 25(OH)D levels ≤ 50 nmol/L. However, vitamin D supplementation did not seem to affect depressive symptoms in older adults (age > 65 years). Finally, the overall effect of vitamin D supplementation did not change when trials with high overall risk of bias were excluded from the analysis. Subgroup analysis of trials with low overall risk of bias showed a small but statistically non-significant effect favoring placebo. It should be noted that a high degree of heterogeneity was observed across most analyses except for those with healthy participants and with low overall risk of bias, and significant evidence of publication bias was detected.
These findings appear to be largely in line with the most recent systematic review and meta-analysis on the same topic with 25 studies (Cheng, Huang, and Huang Citation2020). In an earlier meta-analysis of nine studies by Gowda et al. (Citation2015), no significant reduction in depressive symptoms occurred after vitamin D supplementation. However, it has been suggested that results of Gowda et al. should be interpreted with caution due to some possible errors in their data extraction and/or confounding related to the differences in mean baseline depression values between the vitamin D and placebo groups of two individual studies (Dean et al. Citation2011; Jorde et al. Citation2008) included in their meta-analysis (Erhard et al. Citation2017). Both our results and the findings of Cheng, Huang, and Huang (Citation2020) support this suggestion.
An important difference between results of Cheng, Huang, and Huang (Citation2020) and our analysis is that the former included trials with anxiety as the main outcome, and also participants with bipolar depression. Given the addition of participants with anxiety (six studies) and bipolar depression (one study) in the former review, our results may not be readily comparable, as our findings focus on samples with unipolar depression and hence are more useful for guiding treatment in this population. Despite these differences in study inclusion criteria, the main analysis by Cheng, Huang, and Huang (Citation2020) revealed results similar to ours, with a small-to-moderate effect size −0.499 (95% CI, −0.845 to −0.153) favoring vitamin D for negative emotions. Although our meta-analyses, which excluded trials involving anxiety and bipolar disorders, consist largely of the same older studies, we also included the most recently published studies.
Cheng, Huang, and Huang (Citation2020) performed a subgroup analysis with individuals experiencing MDD (total n = 650), with a significant result favoring vitamin D (Hedges’ g = −1.10, 95% CI −1.55 to −0.64). Similarly, an earlier meta-analysis by Vellekkatt and Menon (Citation2019) (total n = 948) found vitamin D effective in individuals diagnosed with MDD (mean effect size 0.58, 95% CI from 0.45 to 0.72). Our larger subgroup analysis with individuals experiencing MDD may explain the differing results in prior smaller meta-analyses. Most of the studies with participants diagnosed with MDD reported whether vitamin D was given alone or in combination with other treatments, such as psychiatric medications or psychotherapy (Alavi et al. Citation2019; Hansen et al. Citation2019; Khoraminya et al. Citation2013; Zhang et al. Citation2018; Vellekkatt et al. Citation2020). Not all of these studies, however, reported precise details of other ongoing treatments, but most allowed standard antidepressant usage outside the study protocol. Only one trial used vitamin D in a protocol-planned combination with an antidepressant (Khoraminya et al. Citation2013). The overall importance of combining vitamin D supplementation with antidepressant treatment is hard to interpret based on our meta-analysis results. Most trials in which antidepressant usage outside study protocol was allowed represented general population samples with low prevalence of depressive symptoms (and therefore antidepressant treatment) at baseline. Future interventional research should further investigate the possible benefits of augmenting evidence-based psychiatric medication with vitamin D supplementation.
For vitamin D deficiency, a standard criterion has been a serum level of 25(OH)D lower than 50 nmol/L (20 ng/mL), with calls to revise the criterion upwards (Prentice Citation2008). Serum vitamin D concentrations of 75–100 nmol/L (30–40 ng/mL) are most advantageous for various skeletal and extra-skeletal health outcomes (Bischoff-Ferrari et al. Citation2006). Observational studies of the general adult population have documented an inverse association between serum 25(OH)D level and depression risk (Anglin et al. Citation2013; Ju, Lee, and Jeong Citation2013). Additionally, maternal 25(OH)D levels below 50 nmol/L have been associated with an increased risk of postpartum depression (Wang et al. Citation2018). In our review, subgroup analyses in people with low and with sufficient baseline vitamin D status, showed significant positive effects of vitamin D supplementation on depressive symptoms. Compared to the sufficient (> 50 nmol/L) level subset, the low (≤ 50 nmol/L) baseline serum 25(OH)D level subset displayed a larger effect size estimate. Whether the effect of vitamin D supplementation on depression varies in adults with various baseline 25(OH)D levels needs further exploration.
Observational studies have reported an association between vitamin D deficiency and high body mass index (BMI) (BMI at or above 30). The most likely explanation for this association is volumetric dilution of vitamin D into greater volumes of adipose tissue, serum, the liver, and muscle. Another suggested mechanism for lower serum 25(OH)D in people with obesity is impaired hepatic 25-hydroxylation due to nonalcoholic fatty liver disease (NAFLD) (Vranić, Mikolašević, and Milić Citation2019). In response to vitamin D supplementation, compared to normal-weight individuals, individuals with obesity exhibit smaller increases in serum 25(OH)D levels. Especially in trial settings focusing on raising serum 25(OH)D by a desired amount, supplementation dose could be adjusted according to individuals’ body mass (Drincic et al. Citation2013). Our review included studies in which being overweight or obese (BMI > 25) was an inclusion criterion (Jorde et al. Citation2008; Mason et al. Citation2016; Mousa et al. Citation2018; Yosaee et al. 2020; Abiri, Sarbakhsh, and Vafa Citation2021). Numerous other studies examined general population samples in which the proportion of individuals with obesity was presumably noteworthy, unless excluded from the study. Whether 25(OH)D concentration in the central nervous system (CNS) or the potential effects of vitamin D on depressive symptoms are altered due to volumetric dilution is unclear. Given the global prevalence of obesity, future vitamin D trials should evaluate and report BMI association with change in depressive symptoms.
Congruent with the finding of Cheng, Huang, and Huang (Citation2020), our meta-analysis demonstrated that, in healthy individuals, vitamin D produced no beneficial effect. In contrast to that in their results, in healthy individuals, our significant result minimally favored a placebo. Similarly, in the subset of populations without clinically relevant depressive symptoms, effect size decreased. These findings could represent a floor effect: when the baseline levels of depressive symptoms are very low, a reduction in depressive symptoms is challenging to detect through statistical analysis. In addition, participants in the subset of trials with healthy subjects may have been more likely to have a sufficient vitamin D status at baseline. The benefits of raising sufficient serum vitamin D levels are likely to be small. Most of the included studies that reported vitamin D sufficiency at baseline were conducted in healthy populations. However, in studies included in our meta-analysis, the baseline serum 25(OH)D levels both in MDD and other populations varied widely, with no clear relationship to supplementation efficacy.
The largest single trials of our meta-analysis included several hundred to thousands of participants (Bertone-Johnson et al. Citation2012; Jorde et al. Citation2008; Kjaergaard et al. Citation2012; Mason et al. Citation2016; Okereke et al. Citation2020; Sanders et al. Citation2011; Yalamanchili and Gallagher Citation2012, Citation2018; Zheng et al. Citation2019). Compared to the overall effect, these studies produced mainly unclear results (Kjaergaard et al. Citation2012; Mason et al. Citation2016; Sanders et al. Citation2011;Yalamanchili and Gallagher Citation2012, Citation2018) or even favored placebo (Bertone-Johnson et al. Citation2012; Okereke et al. Citation2020) as a result. However, most of the largest trials (Bertone-Johnson et al. Citation2012; Kjaergaard et al. Citation2012; Mason et al. Citation2016; Okereke et al. Citation2020; Yalamanchili and Gallagher Citation2012, Citation2018) included healthy participants without clinically relevant depressive symptoms or other health issues and, despite all the trials lasting 12 weeks or more, only two (Jorde et al. Citation2008; Kjaergaard et al. Citation2012) administered vitamin D doses over 4,000 IU/day.
The vitamin D doses used in the studies included in our meta-analysis varied widely. As calculated per intervention day, the highest vitamin D doses were over 14,000 IU/day (Rolf et al. Citation2017; Zhang et al. Citation2018), while the lowest were 400 IU/day (Bertone-Johnson et al. Citation2012; Lansdowne and Provost Citation1998). The most reported type of vitamin D and administration was cholecalciferol orally. Few trials used intramuscular administration (Jalali-Chimeh et al. Citation2019; Sharifi et al. Citation2019; Vellekkatt et al. Citation2020) or reported other forms of vitamin D (ergocalciferol or calcitriol) (Omidian et al. Citation2019; Yalamanchili and Gallagher Citation2012). Most of the vitamin D doses in our meta-analysis were large enough to bring serum vitamin D levels up to sufficient from long-term use. For achieving and maintaining a serum 25(OH)D concentration of 50 nmol/L, an intake of 15 µg/day (600 IU/day) of cholecalciferol is estimated to be adequate for most adults and for pregnant or lactating women (European Food Safety Authority Citation2016a). On the other hand, it is estimated that at least 40 µg of cholecalciferol per day (≥ 1,000 IU/day) is needed to bring serum 25(OH)D concentrations up to 75 nmol/l in at least half of the adult population (Bischoff-Ferrari et al. Citation2006). The half-life of 25(OH)D in the circulation is one to two months, and with daily supplementation of oral vitamin D, serum 25(OH)D concentration begins to reach pharmacological equilibrium after approximately one month of use (Vieth Citation1999). Compared to this, the supplementation durations in our review were mostly long enough for reaching the highest achievable 25(OH)D concentrations. Many of the dosages exceeded 4,000 IU/day, and reported adverse events were rare. The tolerable upper intake level for adults and pregnant or lactating women is set at 100 µg of vitamin cholecalciferol/ergocalciferol per day (4,000 IU/day), although, as with our review, even higher daily doses have been administered in studies without reported toxicity (European Food Safety Authority Citation2016b).
There is not yet consensus about the optimal serum 25(OH)D concentration for alleviating symptoms of depression. Hypothetically, if vitamin D supplementation has direct causal therapeutic effects in the human central nervous system, the standard cutoff level of vitamin D deficiency may not be adequate in neuropsychiatric disorders. However, the evidence for this is not strong. For example, as the study of multiple sclerosis (MS) has suggested, it appears that serum 25(OH)D concentrations significantly higher than 50 nmol/L are needed to achieve immunomodulatory and neuroprotective benefits in the brain (Häusler and Weber Citation2019). In addition, the upper limit of the potential therapeutic window for vitamin D in relieving depressive symptoms is yet to be determined. As suggested by the MS research, extremely high doses of vitamin D may limit its benefits, for example through immunomodulatory effects of vitamin D-induced secondary hypercalcaemia in the CNS (Häusler and Weber Citation2019).
Cheng, Huang, and Huang (Citation2020) found that a supplementation duration of at least eight weeks could be viewed as sufficient for observing a response to vitamin D intervention. In our analyses, the effects of vitamin D supplementation with intervention duration less than 12 weeks and at least 12 weeks were both significantly better than placebo. Compared to an intervention duration of 12 weeks or more (N = 27), a larger effect size was observed in the subset of interventions lasting less than 12 weeks (N = 14). A possible short-term placebo effect due to trial participation could partly explain the larger effect size estimates in shorter trials. It is important to note, however, that the mean vitamin D dose was more than 2,900 IU/day in interventions lasting 12 weeks or more versus approximately 5,700 IU/day in shorter interventions. Higher doses, despite shorter interventions, could therefore explain the differences in effect size estimates. Only two of the studies in our review included an intervention duration of less than 8 weeks (Dean et al. Citation2011; Lansdowne and Provost Citation1998). As such, the possible efficacy of short-term vitamin D supplementation should be determined in future studies. As with other neurosteroid hormones, genomic responses to vitamin D are not rapid. Transcriptional changes in the nucleus leading to autocrine or paracrine actions in central nervous system cells may take a long period to show benefits (Groves, McGrath, and Burne Citation2014). The onset of neurobiological benefits of vitamin D should not be expected to be significantly faster than the action of antidepressants since the potential antidepressant effects of vitamin D are based on similar genomic responses. Antidepressants indirectly affect processes associated with inherently slow changes in gene expression, such as neurogenesis and synaptic remodeling, and most standard antidepressants often take roughly two months to show benefits (Cipriani et al. Citation2018; Willner, Scheel-Krüger, and Belzung Citation2013).
Our results should not be generalized to children or adolescents, since our review included only studies with young to middle-age and older adult participants. Further, the generalizability of our results to older adults is limited. In our subset of studies, most of the study samples were from the general population and only two (Alavi et al. Citation2019; Okereke et al. Citation2020) included both men and women. Two of these studies of older adults included participants with MDD or at least milder clinically relevant depressive symptoms (Alavi et al. Citation2019; de Koning et al. Citation2019). The potential benefits of vitamin D in relieving depressive symptoms should be further investigated in larger clinical trials in older adults, as the prevalence of depression in individuals aged 65 and older is estimated to be higher than in the general population (Zhao et al. Citation2012). In addition, geriatric depression is associated with poor functioning, perception of poor health, greater utilization of medical services, higher healthcare costs, and poorer prognosis (Zhao et al. Citation2012). Higher daily intake of vitamin D is recommended for older adults due to commonly insufficient sunlight exposure and the decline with age of endogenous vitamin D synthesis (European Food Safety Authority Citation2016c). Also, the potential effects of vitamin D on the neurobiology of geriatric depression and adequate dosing could possibly differ from younger adults due to physiological aging changes in the central nervous system.
Most of the trials with calcium co-supplementation had samples of general populations and three had samples of older adults aged 50 years or more (Bertone-Johnson et al. Citation2012; de Koning et al. Citation2019; Yalamanchili and Gallagher Citation2018). Study by Amini et al. (Citation2022) had post-partum depression outpatients. The calcium doses (400–1000 mg/day) in our review were standard and considered safe (Yao et al. Citation2019). To the best of our knowledge, previous meta-analyses of the effects of calcium supplementation on depressive symptoms have not been published. The limited size of the subgroup limits the conclusions, but hypothetically, supplementation-induced hypercalcaemia could alter the neurophysiological effects of vitamin D in the CNS.
Our results suggest that vitamin D has beneficial effects in reducing perinatal depressive symptoms. All of the populations of pregnant mothers included in our review also had mean baseline serum vitamin D levels indicating deficiency (Dabbaghmanesh et al. Citation2019; Rouhi et al. Citation2018; Vaziri et al. Citation2016; Amini et al. Citation2022). During pregnancy, as a part of various other hormonal changes, maternal circulating levels of active vitamin D 1,25(OH)D normally increase while 25(OH)D levels remain constant across pregnancy. Considering the important regulatory effects of calcitriol on pregnancy, there have been attempts to investigate the importance of adequate vitamin D status for maternal and fetal health (Olmos-Ortiz et al. Citation2015). For example, a higher rate of adverse perinatal outcomes such as preeclampsia and preterm birth have been reported among women with co-occurring prenatal vitamin D deficiency and clinically relevant depressive symptoms (Accortt et al. Citation2018). However, further research is needed to draw more precise conclusions about the efficacy of vitamin D supplementation targeting perinatal depressive symptoms. Due to the small size of the subgroup, it is not possible to assess whether vitamin D could have beneficial effects only in either antenatal or postnatal depressive symptoms.
In addition, we performed a subgroup analysis in the subset of trials with participants with SAD symptoms (Frandsen et al. Citation2014; Lansdowne and Provost Citation1998). Also, a study by Kjaergaard et al. (Citation2012) examined SAD symptoms and was included in the analysis. SAD occurs in autumn or winter during shortage of daylight and includes atypical depressive symptoms, which usually resolve spontaneously in spring and summer and recur the following winter. SAD is considered to be a distinct diagnosis with specific symptoms and characteristic treatment, but the etiology of SAD and the mechanisms of light therapy as the primary treatment are not yet fully understood (Meesters and Gordijn Citation2016). Given the number of included studies for this subgroup analysis was only three (total n = 324), and our results non-significant, the possibility of alleviating SAD symptoms with vitamin D supplementation remains unclear.
Strengths and limitations of evidence
Our review provides a comprehensive and up-to-date summary of the available research data provided by RCTs on the use of vitamin D in alleviating depressive symptoms. Our results appear to resolve the differing results of previous meta-analyses by replicating the favorable results noted in the most recent meta-analysis within a larger, recent dataset. The strengths of our review and meta-analysis are its scope and our efforts to systematically assess the methodological quality of available research data. Our broad inclusion criteria considered both general and clinical populations. In addition, we aimed to evaluate the safety of different doses of vitamin D.
However, our findings should be interpreted with respect to the following limitations. First, the total amount of evidence is still limited for clinical populations such as those with a diagnosis of MDD. Second, the studies in our meta-analysis were heterogeneous in many aspects, reflected by the large I2 values in almost all analyses. In addition to the high degree of heterogeneity, the certainty of evidence was assessed as very low due to the proportion of individual studies reporting high risk of bias and potential publication bias. Most of the studies provided limited descriptions on factors that could affect responses to vitamin D supplementation, such as participants’ ethnicity, diet, BMI, sunlight exposure, amount of exercise, or adherence to supplementation. In addition, studies in which vitamin D supplementation was administered as part of multi-nutrient supplements were outside the scope of our review. Third, the sensitivity and specificity to detect MDD or milder clinically relevant depressive symptoms may depend on the assessment tool being used. For example, Hospital Anxiety and Depression Scale (HADS) has been developed for the detection of anxiety and depression symptoms in people with physical health problems, while BDI and BDI-II screen the features of depression more broadly (Zigmond and Snaith Citation1983; Beck et al. Citation1961). However, to manage heterogeneity, we performed a subgroup analysis with studies (N = 17) including the most frequently used assessment tool BDI or BDI-II with a significant result.
Fourth, the quality of evidence varied. In most studies it was not possible to fully judge the overall level of risk of bias. The small placebo-favoring effect seen in the subgroup of trials with low risk of overall bias is interesting, but the small number of such studies (N = 5) limits the conclusions. None of the trials with only participants with MDD had low overall risk of bias in our assessment. Two of the studies of individuals with MDD had high risk of bias (Kaviani et al. Citation2020; Hansen et al. Citation2019) due to missing outcome data or possible deviations from the intended intervention, but the remaining trials in this subset were of average study quality and possible sources of bias were inadequate reporting of the protocol and statistical analysis plan (Alavi et al. Citation2019; Khoraminya et al. Citation2013; Wang et al. Citation2016; Zhang et al. Citation2018; Vellekkatt et al. Citation2020). In addition, we found significant evidence of the file-drawer effect. The possibility that null results are less likely published in this research field, can lead to a bias in published research with strong consequences on the results of our meta-analysis.
Fifth, more accurate reporting of other ongoing therapies, such as antidepressant usage, for depressive symptoms would be important to allow the efficacy of vitamin D to be more accurately distinguished from that of other therapies.
Finally, our meta-analyses focused on the change in depressive symptom scores rather than examining the associations between serum vitamin D levels and depressive symptoms. A certain dosage of vitamin D may not increase the circulating concentrations of 25-hydroxyvitamin D3 similarly in all participants in heterogeneous populations (European Food Safety Authority Citation2016d). Nevertheless, the results of our meta-analysis both confirm the earlier evidence and provide interesting new information about the potential therapeutic utility of vitamin D in nutritional psychiatry.
Conclusions
Our results suggest that vitamin D supplementation has beneficial effects in both individuals with MDD as well as in those with milder, clinically significant depressive symptoms. There was, however, evidence of high heterogeneity and publication bias that needs to be considered in the interpretation of findings. Our results encourage the enactment of future RCTs focused on clinical samples of individuals diagnosed with MDD or perinatal depression and to favor vitamin D doses of at least 2,000 IU/day. More well-documented and high-quality trials are needed to draw accurate conclusions about the possibilities of complementing conventional treatments of depression with vitamin D supplementation.
Amendments
There were some minor differences between our protocol and review: We did not use Mesh terms or Emtree keywords in the EMBASE database for database searches. The EMBASE was used only in the primary database searches. We did not include studies which compared lower vitamin D supplementation to higher vitamin D supplementation without placebo control group. The decision to exclude these studies was made before meta-analysis phase. Risk of bias was assessed using the Cochrane Collaboration’s tool updated version 2 (ROB 2.0). Updated PRISMA 2020 guidelines were used in writing this report.
Author contribution
AR and TM designed this study. TM conducted the literature search and screened articles with AR. TM, AR, MH, AL, TR, and SR reviewed the literature and extracted data. TM, ML, and AR assessed the risk of bias. WM analyzed the data. WM made the certainty assessment with TM and AR. All authors contributed to interpreting the results and drafting the manuscript. SR passed away before the manuscript was completed.
Funding and acknowledgements
This review study received financial support in the form of scholarships from The Finnish Medical Foundation (TM, grant number 4120) and The Juho Vainio Foundation (TM, grant number 202100353) for research, writing, and publishing this article. The literature search strategy was developed in consultation with an experienced librarian (Ms. Tuulevi Ovaska) at Kuopio University Hospital Medical library. Ms. Josephine Russell assisted in the data extraction phase. Supporters had no input into the study design.
Registration and protocol
Methods of the systematic review and inclusion criteria were specified in advance and documented in a study protocol published in in the international prospective register of systematic reviews (PROSPERO) under the registration number: CRD42020149760. Details of the protocol can be accessed at www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42020149760.
Supplemental Material
Download Zip (303.3 KB)Availability of data, code and other materials
The data, code and other materials that support the findings of this review study are available from the corresponding author, TM, upon reasonable request.
Declaration of interest
The authors declare that they have no competing of interests.
TM has received grants from The Finnish Medical Foundation (grant number 4120) and The Juho Vainio Foundation (grant number 202100353).
WM is currently funded by an Alfred Deakin Postdoctoral Research Fellowship and a Multiple Sclerosis Research Australia early-career fellowship. He has previously received funding from the NHMRC, Clifford Craig Foundation, Cancer Council Queensland and university grants/fellowships from La Trobe University, Deakin University, University of Queensland, and Bond University, received industry funding and has attended events funded by Cobram Estate Pty. Ltd, received travel funding from Nutrition Society of Australia, received consultancy funding from Nutrition Research Australia, and has received speakers honoraria from The Cancer Council Queensland and the Princess Alexandra Research Foundation.
MML is supported by a Deakin University Scholarship and has received research funding support from Be Fit Foods.
MH is supported by an Australian Rotary Health PhD Scholarship and has received research support from The a2 Milk Company.
AL has received grants, fellowships and research support from the University of New South Wales, the University of Melbourne, RMIT University, Deakin University, the National Health and Medical Research Council (NHMRC), Australian Academy of Science, National Institutes of Health (NIH), and The Jack Brockhoff Foundation. AL has received honoraria and travel funds from Sydney University, the University of Technology Sydney, American Epilepsy Society, Epilepsy Society of Australia, International Human Microbiome Congress, European Society of Neurogastroenterology, Australian and New Zealand College of Anesthetists, Falk Foundation and Fonds de la Recherche Scientifique (FNRS).
SR has been funded by the Doctoral School of the University of Eastern Finland. Previously she has received grants from the Finnish Cultural Foundation and travel grants from the Finnish Association for the Study of Obesity and the Finnish Society for Nutrition Research.
TR has received grants, fellowships and research support from University of the Sunshine Coast, Australian Postgraduate Awards, Fernwood Foundation, Wilson Foundation, Robertsa Family Foundation, and Be Fit Food. TR received consultancy, honoraria and travel funds from Oxford University Press, the University of Melbourne, the University of Sydney, Bond University, University of Southern Queensland, Dietitians Association of Australia, Nutrition Society of Australia, The Royal Australian and New Zealand College of Psychiatrists, Academy of Nutrition and Dietetics, Black Dog Institute, Australian Rotary Health, Australian Disease Management Association, Department of Health and Human Services, Primary Health Networks, Barwon Health, West Gippsland Healthcare Group, Central West Gippsland Primary Care Partnership, Parkdale College, City of Greater Geelong and Global Age.
AO is supported by a Heart Foundation Future Leader Fellow (#101160) from the Heart Foundation, Australia.
DM has received research support from Nordic Naturals and heckel medizintechnik GmbH. He has received honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy. He also works with the MGH Clinical Trials Network and Institute (CTNI), which has received research funding from multiple pharmaceutical companies and NIMH.
MV-K: None.
SML: None.
AR: None.
References
- Abdul-Razzak, K. K., S. O. Almanasrah, B. A. Obeidat, and A. G. Khasawneh. 2018. Vitamin D is a potential antidepressant in psychiatric outpatients. International Journal of Clinical Pharmacology and Therapeutics 56 (12):585–96. doi: 10.5414/CP203309.
- Abiri, B., P. Sarbakhsh, and M. Vafa. 2021. Randomized study of the effects of vitamin D and/or magnesium supplementation on mood, serum levels of BDNF, inflammation, and SIRT1 in obese women with mild to moderate depressive symptoms. Nutritional Neuroscience (ahead of print). doi: 10.1080/1028415X.2021.1945859.
- Abou-Raya, S., A. Abou-Raya, and M. Helmii. 2014. THU0327 efficacy of vitamin D supplementation in the treatment of fibromyalgia: randomized controlled trial. Annals of the Rheumatic Diseases 73 (Suppl 2):295. doi: 10.1136/annrheumdis-2014-eular.2767.
- Accortt, E. E., A. Lamb, J. Mirocha, and C. J. Hobel. 2018. Vitamin D deficiency and depressive symptoms in pregnancy are associated with adverse perinatal outcomes. Journal of Behavioral Medicine 41 (5):680–9. doi: 10.1007/s10865-018-9924-9.
- Alavi, N. M., S. Khademalhoseini, Z. Vakili, and F. Assarian. 2019. Effect of Vitamin D supplementation on depression in elderly patients: a randomized clinical trial. Clinical Nutrition (Edinburgh, Scotland) 38 (5):2065–70. doi: 10.1016/j.clnu.2018.09.011.
- Amini, S., R. Amani, S. Jafarirad, B. Cheraghian, M. Sayyah, and A. A. Hemmati. 2022. The effect of vitamin D and calcium supplementation on inflammatory biomarkers, estradiol levels and severity of symptoms in women with postpartum depression: a randomized double-blind clinical trial. Nutritional Neuroscience 25 (1):22–11. doi: 10.1080/1028415X.2019.1707396.
- Anglin, R. E. S., Z. Samaan, S. D. Walter, and S. D. McDonald. 2013. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. The British Journal of Psychiatry 202 (2):100–7. doi: 10.1192/bjp.bp.111.106666.
- Arvold, D. S., M. J. Odean, M. P. Dornfeld, R. R. Regal, J. G. Arvold, G. C. Karwoski, D. J. Mast, P. B. Sanford, and R. J. Sjoberg. 2009. Correlation of symptoms with vitamin D deficiency and symptom response to cholecalciferol treatment: a randomized controlled trial. Endocrine Practice 15 (3):203–12. doi: 10.4158/EP.15.3.203.
- Aucoin, M., K. Cooley, L. Anand, M. Furtado, A. Canzonieri, A. Fine, K. Fotinos, R. Chandrasena, L. J. Klassen, I. Epstein, et al. 2018. Adjunctive vitamin D in the treatment of non-remitted depression: lessons from a failed clinical trial. Complementary Therapies in Medicine 36:38–45. doi: 10.1016/j.ctim.2017.09.011.
- Bagheri, S., A. R. Saghazade, S. Abbaszadeh-Mashkani, H. R. Banafshe, F. S. Ghoreishi, A. Mesdaghinia, and A. Ghaderi. 2022. The effect of vitamin D supplementation on tobacco-related disorders in individuals with a tobacco use disorder: a randomized clinical trial. Journal of Addictive Diseases 40 (3):382–93. doi: 10.1080/10550887.2021.2010971.
- Beck, A. T., C. H. Ward, M. Mendelson, J. Mock, and J. Erbaugh. 1961. An inventory for measuring depression. Archives of General Psychiatry 4 (6):561–71. doi: 10.1001/archpsyc.1961.01710120031004.
- Berk, M., L. J. Williams, F. N. Jacka, A. O’Neil, J. A. Pasco, S. Moylan, N. B. Allen, A. L. Stuart, A. C. Hayley, M. L. Byrne, et al. 2013. So depression is an inflammatory disease, but where does the inflammation come from? BMC Medicine 11 (1):200. doi: 10.1186/1741-7015-11-200.
- Bertone-Johnson, E. R., S. I. Powers, L. Spangler, J. Larson, Y. L. Michael, A. E. Millen, M. N. Bueche, E. Salmoirago-Blotcher, S. Wassertheil-Smoller, R. L. Brunner, et al. 2012. Vitamin D supplementation and depression in the women’s health initiative calcium and vitamin D trial. American Journal of Epidemiology 176 (1):1–13. doi: 10.1093/aje/kwr482.
- Bischoff-Ferrari, H. A., E. Giovannucci, W. C. Willett, T. Dietrich, and B. Dawson-Hughes. 2006. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. The American Journal of Clinical Nutrition 84 (1):18–28. doi: 10.1093/ajcn/84.1.18.
- Borges-Vieira, J. G., and C. K. S. Cardoso. 2022. Efficacy of B-vitamins and vitamin D therapy in improving depressive and anxiety disorders: a systematic review of randomized controlled trials. Nutritional Neuroscience (ahead of print). doi: 10.1080/1028415X.2022.2031494.
- Brown, J., J. I. Bianco, J. J. McGrath, and D. W. Eyles. 2003. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neuroscience Letters 343 (2):139–43. doi: 10.1016/S0304-3940(03)00303-3.
- Cheng, Y.-C., Y.-C. Huang, and W.-L. Huang. 2020. The effect of vitamin D supplement on negative emotions: a systematic review and meta-analysis. Depression and Anxiety 37 (6):549–64. doi: 10.1002/da.23025.
- Choukri, M. A., T. S. Conner, J. J. Haszard, M. J. Harper, and L. A. Houghton. 2018. Effect of vitamin D supplementation on depressive symptoms and psychological wellbeing in healthy adult women: a double-blind randomised controlled clinical trial. Journal of Nutritional Science 7:E23. doi: 10.1017/jns.2018.14.
- Cipriani, A., T. A. Furukawa, G. Salanti, A. Chaimani, L. Z. Atkinson, Y. Ogawa, S. Leucht, H. G. Ruhe, E. H. Turner, J. P. T. Higgins, et al. 2018. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. The Lancet 391 (10128):1357–66. doi: 10.1016/S0140-6736(17)32802-7.
- Cochrane Collaboration. 2020. RoB 2: a revised cochrane risk-of-bias tool for randomized trials. Accessed 4 December 2020. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials.
- Dabbaghmanesh, M. H., F. Vaziri, F. Najib, S. Nasiri, and S. Pourahmad. 2019. The effect of vitamin D consumption during pregnancy on maternal thyroid function and depression: a randomized, placebo-controlled, clinical trial. Jundishapur Journal of Natural Pharmaceutical Products 154:e65328. doi: 10.5812/jjnpp.65328.
- Dean, A. J., M. A. Bellgrove, T. Hall, W. M. J. Phan, D. W. Eyles, D. Kvaskoff, and J. J. McGrath. 2011. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults – a randomised controlled trial. PLoS One 6 (11):e25966. doi: 10.1371/journal.pone.0025966.
- Di Rosa, M., M. Malaguarnera, F. Nicoletti, and L. Malaguarnera. 2011. Vitamin D3: a helpful immuno-modulator. Immunology 134 (2):123–39. doi: 10.1111/j.1365-2567.2011.03482.x.
- Drincic, A., E. Fuller, R. P. Heaney, and L. A. G. Armas. 2013. 25-hydroxyvitamin D Response to graded vitamin D3 supplementation among obese adults. The Journal of Clinical Endocrinology and Metabolism 98 (12):4845–51. doi: 10.1210/jc.2012-4103.
- Erhard, S. M., S. Knitter, R. Westphale, S. Roll, and T. Keil. 2017. Re: “Vitamin D Supplementation to Reduce Depression in Adults: Meta-Analysis of Randomized Controlled Trials.” Gouda U et al., Nutrition 2015;31:421–429. Nutrition (Burbank, Los Angeles County, Calif.) 38:94. doi: 10.1016/j.nut.2016.12.002.
- European Food Safety Authority. 2016a. Dietary reference values for vitamin D. EFSA Journal 14 (10): 4–5. doi: 10.2903/j.efsa.2016.4547.
- European Food Safety Authority. 2016b. Dietary reference values for vitamin D. EFSA Journal 14 (10): 14. doi: 10.2903/j.efsa.2016.4547
- European Food Safety Authority. 2016c. Dietary reference values for vitamin D. EFSA Journal 14 (10): 21–23. doi: 10.2903/j.efsa.2016.4547.
- European Food Safety Authority. 2016d. Dietary reference values for vitamin D. EFSA Journal 14 (10): 65–66. doi: 10.2903/j.efsa.2016.4547.
- Eyles, D. W., S. Smith, R. Kinobe, M. Hewison, and J. J. McGrath. 2005. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. Journal of Chemical Neuroanatomy 29 (1):21–30. doi: 10.1016/j.jchemneu.2004.08.006.
- Far, Z. M., M. Rahnema, and H. Qafelehbashi. 2018. The effect of vitamin D3 on depression in Iranian women. Journal of Clinical and Diagnostic Research 12 (8):13–6. doi: 10.7860/JCDR/2018/35716.11870.
- Fazelian, S., R. Amani, Z. Paknahad, S. Kheiri, and L. Khajehali. 2019. Effect of vitamin D supplement on mood status and inflammation in vitamin D deficient type 2 diabetic women with anxiety: a randomized clinical trial. International Journal of Preventive Medicine 10 (1):17. doi: 10.4103/ijpvm.IJPVM_174_18.
- Fedotova, J., and T. Dudnichenko. 2017. Effects of different doses of vitamin D3 supplementation in young premenopausal women on depression and hormonal status. Psychoneuroendocrinology 83:35. doi: 10.1016/j.psyneuen.2017.07.331.
- Fedotova, Y. O. 2018. The effect of cholecalciferol on anxiety-related depression and hormonal state in women of reproductive age with premature ovarian failure. Eksperimental’naya i Klinicheskaya Farmakologiya 81 (12):7–14. doi: 10.30906/0869-2092-2018-81-12-7-14.
- Frandsen, T. B., M. Pareek, J. P. Hansen, and C. T. Nielsen. 2014. Vitamin D supplementation for treatment of seasonal affective symptoms in healthcare professionals: a double-blind randomised placebo-controlled trial. BMC Research Notes 7:528. doi: 10.1186/1756-0500-7-528.
- Gaughran, F., D. Stringer, G. Wojewodka, S. Landau, S. Smith, P. Gardner-Sood, D. Taylor, H. Jordan, E. Whiskey, A. Krivoy, et al. 2021. Effect of vitamin D supplementation on outcomes in people with early psychosis: the DFEND randomized clinical trial. JAMA Network Open 4 (12):e2140858. doi: 10.1001/jamanetworkopen.2021.40858.
- Ghaderi, A., H. R. Banafshe, M. Motmaen, M. Rasouli-Azad, F. Bahmani, and Z. Asemi. 2017. Clinical trial of the effects of vitamin D supplementation on psychological symptoms and metabolic profiles in maintenance methadone treatment patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry 79:84–9. doi: 10.1016/j.pnpbp.2017.06.016.
- Ghaderi, A., M. Rasouli-Azad, M. H. Farhadi, N. Mirhosseini, M. Motmaen, E. Pishyareh, A. Omidi, and Z. Asemi. 2020. Exploring the effects of vitamin D supplementation on cognitive functions and mental health status in subjects under methadone maintenance treatment. Journal of Addiction Medicine 14 (1):18–25. doi: 10.1097/ADM.0000000000000550.
- Gowda, U., M. P. Mutowo, B. J. Smith, A. E. Wluka, and A. M. N. Renzaho. 2015. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition (Burbank, Los Angeles County, Calif.) 31 (3):421–9. doi: 10.1016/j.nut.2014.06.017.
- Groves, N. J., J. J. McGrath, and T. H. J. Burne. 2014. Vitamin D as a neurosteroid affecting the developing and adult brain. Annual Review of Nutrition 34 (1):117–41. doi: 10.1146/annurev-nutr-071813-105557.
- Gugger, A., A. Marzel, E. J. Orav, W. C. Willett, B. Dawson‐Hughes, R. Theiler, G. Freystätter, A. Egli, and H. A. Bischoff‐Ferrari. 2019. Effect of monthly high-dose vitamin D on mental health in older adults: secondary analysis of a RCT. Journal of the American Geriatrics Society 67 (6):1211–7. doi: 10.1111/jgs.15808.
- Guyatt, G., A. D. Oxman, E. A. Akl, R. Kunz, G. Vist, J. Brozek, S. Norris, Y. Falck-Ytter, P. Glasziou, H. DeBeer, et al. 2011. GRADE guidelines: 1. introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 64 (4):383–94. doi: 10.1016/j.jclinepi.2010.04.026.
- Guzek, D., A. Kołota, K. Lachowicz, D. Skolmowska, M. Stachoń, and D. Głąbska. 2021. Association between vitamin D supplementation and mental health in healthy adults: a systematic review. Journal of Clinical Medicine 10 (21):5156. doi: 10.3390/jcm10215156.
- Hansen, J. P., M. Pareek, A. Hvolby, A. Schmedes, T. Toft, E. Dahl, and C. T. Nielsen. 2019. Vitamin D3 supplementation and treatment outcomes in patients with depression (D3-Vit-Dep). BMC Research Notes 12 (1):203. doi: 10.1186/s13104-019-4218-z.
- Häusler, D., and M. S. Weber. 2019. Vitamin D supplementation in central nervous system demyelinating disease—enough is enough. International Journal of Molecular Sciences 20 (1):218. doi: 10.3390/ijms20010218.
- Hoffmann, M. R., P. A. Senior, and D. R. Mager. 2015. Vitamin D supplementation and health-related quality of life: a systematic review of the literature. Journal of the Academy of Nutrition and Dietetics 115 (3):406–18. doi: 10.1016/j.jand.2014.10.023.
- Hozo, S. P., B. Djulbegovic, and I. Hozo. 2005. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 5 (1):13. doi: 10.1186/1471-2288-5-13.
- Jalali-Chimeh, F., A. Gholamrezaei, M. Vafa, M. Nasiri, B. Abiri, T. Darooneh, and G. Ozgoli. 2019. Effect of vitamin D therapy on sexual function in women with sexual dysfunction and vitamin D deficiency: a randomized, double-blind, placebo controlled clinical trial. Journal of Urology 201 (5):987–93. doi: 10.1016/j.juro.2018.10.019.
- Jorde, R., and J. Kubiak. 2018. No improvement in depressive symptoms by vitamin D supplementation: results from a randomised controlled trial. Journal of Nutritional Science 7:E30. doi: 10.1017/jns.2018.19.
- Jorde, R., M. Sneve, Y. Figenschau, J. Svartberg, and K. Waterloo. 2008. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. Journal of Internal Medicine 264 (6):599–609. doi: 10.1111/j.1365-2796.2008.02008.x.
- Ju, S.-Y., Y.-J. Lee, and S.-N. Jeong. 2013. Serum 25-hydroxyvitamin D levels and the risk of depression: a systematic review and meta-analysis. The Journal of Nutrition, Health & Aging 17 (5):447–55. doi: 10.1007/s12603-012-0418-0.
- Kalueff, A. V., and P. Tuohimaa. 2007. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Current Opinion in Clinical Nutrition and Metabolic Care 10 (1):12–9. doi: 10.1097/MCO.0b013e328010ca18.
- Kaviani, M., B. Nikooyeh, H. Zand, P. Yaghmaei, and T. R. Neyestani. 2020. Effects of vitamin D supplementation on depression and some involved neurotransmitters. Journal of Affective Disorders 269:28–35. doi: 10.1016/j.jad.2020.03.029.
- Khoraminya, N., M. Tehrani-Doost, S. Jazayeri, A. Hosseini, and A. Djazayery. 2013. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. The Australian and New Zealand Journal of Psychiatry 47 (3):271–5. doi: 10.1177/0004867412465022.
- Kjaergaard, M., K. Waterloo, C. E. A. Wang, B. Almås, Y. Figenschau, M. S. Hutchinson, J. Svartberg, and R. Jorde. 2012. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case—control study and randomised clinical trial. British Journal of Psychiatry 201 (5):360–8. doi: 10.1192/bjp.bp.111.104349.
- Koning, E. J. d., P. Lips, B. W. J. H. Penninx, P. J. M. Elders, A. C. Heijboer, M. den Heijer, P. M. Bet, H. W. J. van Marwijk, and N. M. van Schoor. 2019. Vitamin D supplementation for the prevention of depression and poor physical function in older persons: the D-vitaal study, a randomized clinical trial. The American Journal of Clinical Nutrition 110 (5):1119–30. doi: 10.1093/ajcn/nqz141.
- Kusmiyati, Y., E. Suryani, L. Herawati, and A. Firdausi. 2020. Vitamin D and reduced academic stress of health students. Kesmas: Jurnal Kesehatan Masyarakat Nasional (National Public Health Journal) 15 (3): 128–133. doi: 10.21109/kesmas.v15i3.3274.
- Lansdowne, A. T. G., and S. C. Provost. 1998. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology 135 (4):319–23. doi: 10.1007/s002130050517.
- Li, G., L. Mbuagbaw, Z. Samaan, M. Falavigna, S. Zhang, J. D. Adachi, J. Cheng, A. Papaioannou, and L. Thabane. 2014. Efficacy of vitamin D supplementation in depression in adults: a systematic review. The Journal of Clinical Endocrinology and Metabolism 99 (3):757–67. doi: 10.1210/jc.2013-3450.
- Luo, D., X. Wan, J. Liu, and T. Tong. 2018. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Statistical Methods in Medical Research 27 (6):1785–805. doi: 10.1177/0962280216669183.
- Mason, C., J. de Dieu Tapsoba, C. Duggan, C.-Y. Wang, L. Korde, and A. McTiernan. 2016. Repletion of vitamin D associated with deterioration of sleep quality among postmenopausal women. Preventive Medicine 93:166–70. doi: 10.1016/j.ypmed.2016.09.035.
- Meesters, Y., and M. C. M. Gordijn. 2016. Seasonal affective disorder, winter type: current insights and treatment options. Psychology Research and Behavior Management 9:317–27. doi: 10.2147/PRBM.S114906.
- Missaoui, M., A. Hassine, S. Naija, J. Emna, and S. Ben Amor. 2021. Effect of vitamin D supplementation on anxiety-depressive disorder at six months of stroke: a randomized clinical trial. European Journal of Neurology 28 (Suppl 1): 864. doi: 10.1002/central/CN-02293557.
- Moher, D., A. Liberati, J. Tetzlaff, and D. G. Altman. and for the PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical Research ed.) 339:b2535. doi: 10.1136/bmj.b2535.
- Mousa, A., N. Naderpoor, M. P. J. de Courten, and B. de Courten. 2018. Vitamin D and symptoms of depression in overweight or obese adults: a cross-sectional study and randomized placebo-controlled trial. The Journal of Steroid Biochemistry and Molecular Biology 177:200–8. doi: 10.1016/j.jsbmb.2017.08.002.
- Mozaffari-Khosravi, H., L. Nabizade, S. M. Yassini-Ardakani, H. Hadinedoushan, and K. Barzegar. 2013. The effect of 2 different single injections of high dose of vitamin D on improving the depression in depressed patients with vitamin d deficiency: a randomized clinical trial. Journal of Clinical Psychopharmacology 33 (3):378–85. doi: 10.1097/JCP.0b013e31828f619a.
- Narula, N., M. Cooray, R. Anglin, Z. Muqtadir, A. Narula, and J. K. Marshall. 2017. Impact of high-dose vitamin D3 supplementation in patients with crohn’s disease in remission: a pilot randomized double-blind controlled study. Digestive Diseases and Sciences 62 (2):448–55. doi: 10.1007/s10620-016-4396-7.
- Obradovic, D., H. Gronemeyer, B. Lutz, and T. Rein. 2006. Cross-talk of vitamin D and glucocorticoids in hippocampal cells. Journal of Neurochemistry 96 (2):500–9. doi: 10.1111/j.1471-4159.2005.03579.x.
- Okereke, O. I., C. F. Reynolds, D. Mischoulon, G. Chang, C. M. Vyas, N. R. Cook, A. Weinberg, V. Bubes, T. Copeland, G. Friedenberg, et al. 2020. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA 324 (5):471–80. doi: 10.1001/jama.2020.10224.
- Olmos-Ortiz, A., E. Avila, M. Durand-Carbajal, and L. Díaz. 2015. Regulation of calcitriol biosynthesis and activity: focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients 7 (1):443–80. doi: 10.3390/nu7010443.
- Omidian, M., M. Mahmoudi, M. Abshirini, M. R. Eshraghian, M. H. Javanbakht, M. Zarei, H. Hasani, and M. Djalali. 2019. Effects of vitamin D supplementation on depressive symptoms in type 2 diabetes mellitus patients: randomized placebo-controlled double-blind clinical trial. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 13 (4):2375–80. doi: 10.1016/j.dsx.2019.06.011.
- Paduchová, Z., B. Katrenčíková, M. Vaváková, L. Laubertová, Z. Nagyová, I. Garaiova, Z. Ďuračková, and J. Trebatická. 2021. The effect of omega-3 fatty acids on thromboxane, brain-derived neurotrophic factor, homocysteine, and vitamin D in depressive children and adolescents: randomized controlled trial. Nutrients 13 (4):1095. doi: 10.3390/nu13041095.
- Page, M. J. J. McKenzie, P. Bossuyt, I. Boutron, T. Hoffmann, C. D. Mulrow, and L. Shamseer. 2020. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. MetaArXiv Preprints, September. https://osf.io/preprints/metaarxiv/v7gm2/.
- Penckofer, S., M. Ridosh, W. Adams, M. Grzesiak, J. Woo, M. Byrn, J. Kouba, P. Sheean, C. Kordish, R. Durazo-Arvizu, et al. 2022. Vitamin D supplementation for the treatment of depressive symptoms in women with type 2 diabetes: a randomized clinical trial. Journal of Diabetes Research 2022:1–10. doi: 10.1155/2022/4090807.
- Prentice, A. 2008. Vitamin D deficiency: a global perspective. Nutrition Reviews 66 (10 Suppl 2):S153–S64. doi: 10.1111/j.1753-4887.2008.00100.x.
- Rajabi‐Naeeni, M., M. Dolatian, M. Qorbani, and A. A. Vaezi. 2021. Effect of omega‐3 and vitamin D co‐supplementation on psychological distress in reproductive‐aged women with pre‐diabetes and Hypovitaminosis D: a randomized controlled trial. Brain and Behavior 11 (11):e2342. doi: 10.1002/brb3.2342.
- Raja-Khan, N., C. Stetter, M. Lott, A. R. Kunselman, W. C. Dodson, and R. Legro. 2012. High-dose vitamin D fails to improve insulin sensitivity in women with polycystic ovary syndrome. Endocrine Reviews 33 (3): SUN-36.
- Rolf, L., A.-H. Muris, Y. Bol, J. Damoiseaux, J. Smolders, and R. Hupperts. 2016. P761. High dose vitamin D3 supplementation does not ameliorate depressive symptoms in relapsing remitting multiple sclerosis: results of a randomized placebo-controlled trial. Multiple Sclerosis Journal 22 (S3):379. doi: 10.1177/1352458516663081.
- Rolf, L., A.-H. Muris, Y. Bol, J. Damoiseaux, J. Smolders, and R. Hupperts. 2017. Vitamin D3 supplementation in multiple sclerosis: symptoms and biomarkers of depression. Journal of the Neurological Sciences 378:30–5. doi: 10.1016/j.jns.2017.04.017.
- Rouhi, M., N. Rouhi, S. Mohamadpour, and H. P.-R. Tajrishi. 2018. Vitamin D reduces postpartum depression and fatigue among Iranian women. British Journal of Midwifery 26 (12):787–93. doi: 10.12968/bjom.2018.26.12.787.
- Rush, A. J., M. H. Trivedi, S. R. Wisniewski, A. A. Nierenberg, J. W. Stewart, D. Warden, G. Niederehe, M. E. Thase, P. W. Lavori, B. D. Lebowitz, et al. 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. American Journal of Psychiatry 163 (11):1905–17. doi: 10.1176/ajp.2006.163.11.1905.
- Sabir, M. S., M. R. Haussler, S. Mallick, I. Kaneko, D. A. Lucas, C. A. Haussler, G. K. Whitfield, and P. W. Jurutka. 2018. Optimal vitamin D spurs serotonin: 1,25-dihydroxyvitamin d represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes & Nutrition 13 (1): 19–19. doi: 10.1186/s12263-018-0605-7.
- Sanders, K. M., A. L. Stuart, E. J. Williamson, F. N. Jacka, S. Dodd, G. Nicholson, and M. Berk. 2011. Annual high-dose vitamin D3 and mental well-being: randomised controlled trial. The British Journal of Psychiatry: The Journal of Mental Science 198 (5):357–64. doi: 10.1192/bjp.bp.110.087544.
- Sarris, J., J. Murphy, D. Mischoulon, G. I. Papakostas, M. Fava, M. Berk, and C. H. Ng. 2016. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. The American Journal of Psychiatry 173 (6):575–87. doi: 10.1176/appi.ajp.2016.15091228.
- Sedaghat, K., Z. Yousefian, A. A. Vafaei, A. Rashidy-Pour, H. Parsaei, A. Khaleghian, and S. Choobdar. 2019. Mesolimbic dopamine system and its modulation by vitamin D in a chronic mild stress model of depression in the rat. Behavioural Brain Research 356:156–69. doi: 10.1016/j.bbr.2018.08.020.
- Sepehrmanesh, Z., F. Kolahdooz, F. Abedi, N. Mazroii, A. Assarian, Z. Asemi, and A. Esmaillzadeh. 2016. Vitamin D supplementation affects the beck depression inventory, insulin resistance, and biomarkers of oxidative stress in patients with major depressive disorder: a randomized, controlled clinical trial. The Journal of Nutrition 146 (2):243–8. doi: 10.3945/jn.115.218883.
- Shaffer, J. A., D. Edmondson, L. T. Wasson, L. Falzon, K. Homma, N. Ezeokoli, P. Li, and K. W. Davidson. 2014. Vitamin D supplementation for depressive symptoms: a systematic review and meta-analysis of randomized controlled trials. Psychosomatic Medicine 76 (3):190–6. doi: 10.1097/PSY.0000000000000044.
- Shahi, M. M., S. A. Hosseini, B. Helli, M. H. Haghighyzade, and M. Abolfathi. 2017. The effect of vitamin D supplement on quality of sleep in adult people with sleep disorders. Tehran University Medical Journal TUMS Publications 75 (6):443–8.
- Sharifi, A., H. Vahedi, S. Nedjat, A. Mohamadkhani, and M. J. Hosseinzadeh Attar. 2019. Vitamin D decreases beck depression inventory score in patients with mild to moderate ulcerative colitis: a double-blind randomized placebo-controlled trial. Journal of Dietary Supplements 16 (5):541–9. doi: 10.1080/19390211.2018.1472168.
- Sikaroudi, M. K., M. Mokhtare, F. Shidfar, L. Janani, A. Faghihi Kashani, M. Masoodi, S. Agah, A. Dehnad, and S. Shidfar. 2020. Effects of vitamin D3 supplementation on clinical symptoms, quality of life, serum serotonin (5-hydroxytryptamine), 5-hydroxy-indole acetic acid, and ratio of 5-HIAA/5-HT in patients with diarrhea-predominant irritable bowel syndrome: a randomized clinical trial. EXCLI Journal 19:652–67. doi: 10.17179/excli2020-2247.
- Sparling, T. M., N. Henschke, R. C. Nesbitt, and S. Gabrysch. 2017. The role of diet and nutritional supplementation in perinatal depression: a systematic review. Maternal & Child Nutrition 13 (1).doi: 10.1111/mcn.12235.
- Spedding, S. 2014. Vitamin D and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients 6 (4):1501–18. doi: 10.3390/nu6041501.
- Tabra, S. A. A., M. H. Abu-Zaid, and S. Hablas. 2020. Vitamin D supplementation; is it effective in fibromyalgia patients? Annals of the Rheumatic Diseases 79 (Suppl 1):472–3. doi: 10.1136/annrheumdis-2020-eular.487.
- Tella, H., V. Yalamanchili, and J. C. Gallagher. 2015. Effect of vitamin D3 supplementation on depression in post-menopausal women: A multi-dose randomized placebo controlled trial. Endocrine Reviews 36 (2 Suppl). doi: 10.1002/central/CN-01293796.
- Torrisi, M., L. Bonanno, C. Formica, F. A. Arcadi, D. Cardile, V. Cimino, P. Bramanti, and E. Morini. 2021. The role of rehabilitation and vitamin D supplementation on motor and psychological outcomes in poststroke patients. Medicine 100 (45):e27747. doi: 10.1097/MD.0000000000027747.
- Vaziri, F., S. Nasiri, Z. Tavana, M. H. Dabbaghmanesh, F. Sharif, and P. Jafari. 2016. A randomized controlled trial of vitamin d supplementation on perinatal depression: in Iranian pregnant mothers. BMC Pregnancy and Childbirth 16 (1):239. doi: 10.1186/s12884-016-1024-7.
- Vellekkatt, F., and V. Menon. 2019. Efficacy of vitamin D supplementation in major depression: a meta-analysis of randomized controlled trials. Journal of Postgraduate Medicine 65 (2):74–80. doi: 10.4103/jpgm.JPGM_571_17.
- Vellekkatt, F., V. Menon, M. Rajappa, and J. Sahoo. 2020. Effect of adjunctive single dose parenteral vitamin D supplementation in major depressive disorder with concurrent vitamin D deficiency: a double-blind randomized placebo-controlled trial. Journal of Psychiatric Research 129:250–6. doi: 10.1016/j.jpsychires.2020.07.037.
- Vieth, R. 1999. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. The American Journal of Clinical Nutrition 69 (5):842–56. doi: 10.1093/ajcn/69.5.842.
- Vieth, R., S. Kimball, A. Hu, and P. G. Walfish. 2004. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 Mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutrition Journal 3 (1): 8. doi: 10.1186/1475-2891-3-8.
- Vranić, L., I. Mikolašević, and S. Milić. 2019. Vitamin D deficiency: consequence or cause of obesity? Medicina 55 (9):541. doi: 10.3390/medicina55090541.
- Wan, X., W. Wang, J. Liu, and T. Tong. 2014. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 14 (1):135. doi: 10.1186/1471-2288-14-135.
- Wang, J., N. Liu, W. Sun, D. Chen, J. Zhao, and W. Zhang. 2018. Association between vitamin D deficiency and antepartum and postpartum depression: a systematic review and meta-analysis of longitudinal studies. Archives of Gynecology and Obstetrics 298 (6):1045–59. doi: 10.1007/s00404-018-4902-6.
- Wang, Y., Y. Liu, Y. Lian, N. Li, H. Liu, and G. Li. 2016. Efficacy of high-dose supplementation with oral vitamin D3 on depressive symptoms in dialysis patients with vitamin D3 insufficiency: a prospective, randomized, double-blind study. Journal of Clinical Psychopharmacology 36 (3):229–35. doi: 10.1097/JCP.0000000000000486.
- Wepner, F., R. Scheuer, B. Schuetz-Wieser, P. Machacek, E. Pieler-Bruha, H. S. Cross, J. Hahne, and M. Friedrich. 2014. Effects of vitamin D on patients with fibromyalgia syndrome: a randomized placebo-controlled trial. Pain 155 (2):261–8. doi: 10.1016/j.pain.2013.10.002.
- Williams, T. G., R. Ehsanian, K. L. Shem, J. Wright, L. Isaac, and J. Crew. 2016. The effect of vitamin D supplementation on pain, mood, depression, and strength in patients with spinal cord injury. PM & R: The Journal of Injury, Function, and Rehabilitation 8 (9S):S153. doi: 10.1016/j.pmrj.2016.07.024.
- Willner, P., J. Scheel-Krüger, and C. Belzung. 2013. The neurobiology of depression and antidepressant action. Neuroscience and Biobehavioral Reviews 37 (10 Pt 1):2331–71. doi: 10.1016/j.neubiorev.2012.12.007.
- World Health Organization. 2017. Depression and other common mental disorders. Global health estimates. https://www.who.int/publications-detail/depression-global-health-estimates.
- World Health Organization. 2020. Depression. Newsroom Fact Sheets, January. https://www.who.int/news-room/fact-sheets/detail/depression.
- Yalamanchili, V., and J. C. Gallagher. 2012. Treatment with hormone therapy and calcitriol did not affect depression in older postmenopausal women: no interaction with estrogen and vitamin D receptor genotype polymorphisms. Menopause (New York, N.Y.) 19 (6):697–703. doi: 10.1097/gme.0b013e31823bcec5.
- Yalamanchili, V., and J. C. Gallagher. 2016. Comparison of the effect of vitamin D and 1,25 dihydroxyvitamin D treatment on depression scores in older women. Endocrine Reviews 37 (2)
- Yalamanchili, V., and J. C. Gallagher. 2018. Dose ranging effects of vitamin D3 on the geriatric depression score: a clinical trial. The Journal of Steroid Biochemistry and Molecular Biology 178 (4):60–4. doi: 10.1016/j.jsbmb.2017.10.025.
- Yao, P., D. Bennett, M. Mafham, X. Lin, Z. Chen, J. Armitage, and R. Clarke. 2019. Vitamin D and calcium for the prevention of fracture. JAMA Network Open 2 (12):e1917789. doi: 10.1001/jamanetworkopen.2019.17789.
- Yosaee, S., S. Soltani, A. Esteghamati, S. A. Motevalian, M. Tehrani-Doost, C. C. T. Clark, and S. Jazayeri. 2020. Effects of zinc, vitamin D, and their co-supplementation on mood, serum cortisol, and brain-derived neurotrophic factor in patients with obesity and mild to moderate depressive symptoms: a phase II, 12-Wk, 2 × 2 factorial design, double-blind, randomized, placebo-controlled trial. Nutrition 71:110601. doi: 10.1016/j.nut.2019.110601.
- Zhang, L., S. Wang, Y. Zhu, and T. Yang. 2018. Vitamin D3 as adjunctive therapy in the treatment of depression in tuberculosis patients: a short-term pilot randomized double-blind controlled study. Neuropsychiatric Disease and Treatment 14:3103–9. doi: 10.2147/NDT.S183039.
- Zhao, K.-X., C.-Q. Huang, Q. Xiao, Y. Gao, Q.-X. Liu, Z.-R. Wang, Y.-H. Li, and Y.-Z. Xie. 2012. Age and risk for depression among the elderly: a meta-analysis of the published literature. CNS Spectrums 17 (3):142–54. doi: 10.1017/S1092852912000533.
- Zheng, S., L. Tu, F. Cicuttini, W. Han, Z. Zhu, B. Antony, A. Wluka, T. Winzenberg, D. Aitken, L. Blizzard, et al. 2018. Factors Associated with depression and effects of vitamin d supplementation and maintainingvitamin d sufficiency on depression in patients with knee osteoarthritis. Osteoarthritis and Cartilage 26:S308–S9. doi: 10.1016/j.joca.2018.02.621.
- Zheng, S., L. Tu, F. Cicuttini, W. Han, Z. Zhu, B. Antony, A. Wluka, T. Winzenberg, T. Meng, D. Aitken, et al. 2019. Effect of vitamin D supplementation on depressive symptoms in patients with knee osteoarthritis. Journal of the American Medical Directors Association 20 (12):1634–40.e1. doi: 10.1016/j.jamda.2018.09.006.
- Zhu, C., Y. Zhang, T. Wang, Y. Lin, J. Yu, Q. Xia, P. Zhu, and D. Zhu. 2020. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain and Behavior 10 (11):e01760. doi: 10.1002/brb3.1760.
- Zigmond, A. S., and R. P. Snaith. 1983. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 67 (6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x.