ABSTRACT
The aquaculture sector faces sustainability challenges due to unsustainable feedstuffs. To address this, there’s a continuous search for alternatives to traditional aquafeed protein sources such as fishmeal. Clostridium autoethanogenum is a novel aquafeed protein source with a long-standing application in the bio-energy industry. With 80–89% crude protein content on a dry matter basis, Clostridium autoethanogenum protein (CAP) has gained attention in nutrition research for aquaculture species. This study aimed to review CAP utilization in aquafeed, examining its nutritional value and impacts on aquatic animals’ growth, feed efficiency, digestion, absorption, and immune response. Research suggests promising potential for CAP in aquafeed, showing potential for achieving complete fishmeal replacement without compromising growth performance and immune response in select aquaculture species. Nonetheless, higher inclusion levels show adverse effects on the health status of certain fish species, indicating variability among species to utilize CAP-based diets. Further investigations are required to understand this variability in outcomes reported among species.
Introduction
The aquafeed industry faces increasing pressure from the continuously expanding aquaculture sector to meet the growing demand for aquatic products. Feeding is a major determinant of aquaculture productivity since it represents the largest and most costly single input because of the dietary protein component (Hardy Citation2010; Maulu et al. Citation2021). Dietary proteins are building blocks for the body and are essential for cell growth and development, energy, metabolism, and immune functions (NRC Citation2011). Globally, fishmeal from wild fish harvests is still a major protein source in aquafeed due to its superior quality such as high digestibility, excellent amino acids profile, and mineral and vitamin compositions (Naylor et al. Citation2021). However, fishmeal prices have risen continuously in recent years owing to increasing demand as aquaculture continues to expand while wild fish production stagnates (Glencross et al. Citation2023). On the other hand, significant progress has been made globally toward reducing aquaculture production’s dependence on wild fish resources over the last 20 years (El-Ouny et al. Citation2023; Hasimuna et al. Citation2019; Naylor et al. Citation2021). This can be attributed to significant efforts made by the scientific community in identifying alternative feedstuffs in aquafeed.
Single-cell proteins (SCPs) have attracted considerable attention in animal feeds and nutrition research recently owing to their rapid growth and high protein content (Reihani and Khosravi-Darani Citation2019; Woolley et al. Citation2023). SCPs generally refer to dried cells of micro-organisms such as bacteria, fungi, and algae used mainly as supplements in human foods or animal feeds (Reihani and Khosravi-Darani Citation2019). Bacteria have shown superior protein content of all SCPs and have thus attracted wide usage in aquafeeds (Woolley et al. Citation2023). Additionally, bacteria have a higher efficiency in converting substrates to rich protein biomass compared to fungi and algae (Sillman et al. Citation2019). Earlier studies focused on gram-negative bacteria species that utilize hydrocarbon substrates, particularly methane by natural gas fermentation as carbon sources (Øverland et al. Citation2010). However, more recent research has identified a novel protein from a gram-positive bacterial species called Clostridium autoethanogenum (Maulu et al. Citation2021).
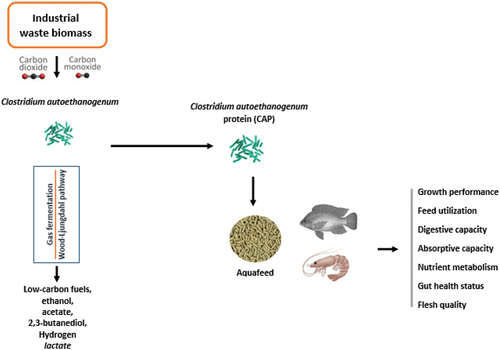
Clostridium autoethanogenum is a spore-forming anaerobic bacteria extensively used in industries to produce high-value chemicals from waste gases (Abrini, Naveau, and Nyns Citation1994; Maulu et al. Citation2021). C. autoethanogenum biomass has recently been evaluated for inclusion in aquafeed as a novel protein to potentially replace conventional proteins because of its high crude protein content estimated at over 80% on a dry basis (Chen et al. Citation2020; Ma et al. Citation2022a; Maulu et al. Citation2021). Furthermore, the bacterial meal has considerable amounts of other nutritional elements as well as essential amino acids (EAA) like that of fishmeal (Simpsons et al. Citation2016). C. autoethanogenum protein (CAP) has been investigated in several finfish and shellfish aquaculture species (Jiang et al. Citation2021; Ma et al. Citation2022a; Wu et al. Citation2022; Xu et al. Citation2023; Yao et al. Citation2022; Zhu et al. Citation2022). As a novel protein, CAP research in aquaculture has increased in recent years and the reported results are generally promising. However, a gap still exists in the literature as there is no comprehensive study consolidating these various research findings to evaluate and analyze the current knowledge regarding the use of CAP in aquaculture. Therefore, this study aimed to review existing research on the utilization of CAP in aquaculture. A compilation of findings from existing research could provide more insights into the utilization of novel proteins and present gaps in existing literature for further development.
Characteristics of Clostridium autoethanogenum bacteria
Clostridium autoethanogenum is a strictly anaerobic, gram-positive, and spore-forming bacterium belonging to the Clostridium genus (Abrini, Naveau, and Nyns Citation1994). It is a motile rod-like shaped bacterium first isolated from rabbit fecal matter using carbon monoxide as the only source of energy and carbon (Abrini, Naveau, and Nyns Citation1994; Humphreys and Minton Citation2018). Clostridium is a large, diverse genus comprising gram-positive, spore-forming, obligate anaerobic Firmicutes, most of which are industrially relevant species (Abrini, Naveau, and Nyns Citation1994). Most species in this genus are considered carboxydotrophic acetogenic because of the important roles they play in carbon capture and the production of fuels and platform chemicals from various wastes and renewable resources by synthesis of gas fermentation (Humphreys and Minton Citation2018; Liew et al. Citation2016; Marcellin et al. Citation2016; Norman et al. Citation2018a). C. autoethanogenum is often referred to as an “industrial microbe” due to its role in the commercial-scale production of ethanol from carbon monoxide (Norman et al. Citation2018b). Hence, C. autoethanogenum is extensively used in industry to produce high-value chemicals from waste gases (Drake, Gossner, and Daniel Citation2008; Song et al. Citation2018). Through this application in industry, the utilization of C. autoethanogenum presents an opportunity to mitigate climate change impacts by reducing greenhouse gas (GHG) emissions.
Production technology and potential
Industrial biomass is the second largest carbon-rich material on earth after petroleum. Therefore, utilizing this biomass to produce energy is one of the most proficient ways of mitigating industrial contribution to greenhouse gas (GHG) emissions (Wood et al. Citation2021). At the same time, the process can produce a high nutritional value protein for utilization in animal feeds. The production of animal proteins for human consumption through this approach is more sustainable given the increasing feed-food competition of the conventional proteins which makes their ability to sustain the rapidly expanding food production sector uncertain.
Despite significant progress made recently in investigating the utilization of C. autoethanogenum in aquafeed as a protein source, commercial production of the bacteria for animal feeds has not yet been developed. Current production is aimed mainly at utilizing industrial carbon-rich biomass as a feedstock to produce high-value chemicals (Heffernan et al. Citation2023). As summarized in , C. autoethanogenum grows the carbon-rich biomass and converts carbon monoxide (CO) and carbon dioxide (CO2) into low-carbon fuels and chemicals such as ethanol, acetate, 2,3-butanediol, some H2, and lactate through the Wood-Ljungdahl pathway in a gas fermentation (Heffernan et al. Citation2023; Humphreys and Minton Citation2018; Jin et al. Citation2021; Liew et al. Citation2016). Through this gas fermentation, high-value chemicals are produced in a more environmentally friendly way. Therefore, the utilization of C. autoethanogenum in the energy industry and food production sector can promote cross-sector partnerships to achieve sustainable development goals (SDGs) and the attainment of a global circular economy. Although many countries such as the USA, Germany, China, and Brazil currently produce huge amounts of biomass, existing studies on CAP utilization in aquaculture obtained the product from Chinese energy-producing companies. Furthermore, China has approved the use of bacteria for this purpose (Xu et al. Citation2023). With the expansion of the energy sector globally to meet the energy demand of the growing population, huge opportunities exist to produce CAP for animal feeds. A recent study by Xu et al (Xu et al. Citation2023) highlights these opportunities and suggests possible solutions to addressing existing challenges.
Nutritional composition
The nutritional composition of C. autoethanogenum is suitable to meet the protein requirement of most aquatic animals including carnivorous species (). Despite minor variations in the nutritional value even with the product from the same company, CAP has a very high crude protein content of 80–89% on a dry matter basis, considerable amounts of crude lipids at 0.20–2.6%, ash content of 3.3–6.4% and dry matter of 92–94% on a dry matter basis. The protein is also reported to contain appreciable amounts of carbohydrates, minerals, and vitamins (Maulu et al. Citation2021; Simpson et al. Citation2016), although the exact compositions are not provided in the literature. Furthermore, CAP is reported to contain cell wall components that may play important roles in modulating immune response in fish (Simpson et al. Citation2016). However, the digestibility of CAP has not been critically studied although a similar apparent digestibility coefficient of crude protein and dry matter in CAP-based diets with those based solely on fishmeal were reported (Yang et al. Citation2023). Notably, minor variation in the composition of the protein even for the product from the same company exists. These could be influenced by various factors including the bacterial culture substrate, fermentation process, and post-fermentation processing (Xu et al. Citation2023). Further studies are required to investigate the apparent digestibility of CAP in major aquaculture species.
Table 1. Nutritional composition (dry matter, DM basis) of Clostridium autoethanogenum protein biomass from different companies.
The amino acid profile of CAP has been documented showing a similar composition to that of fishmeal (). Furthermore, the amino acid profile also shows some degree of variation, and this is high in some amino acids. For example, CAP has higher compositions of branched amino acids (BAA), such as valine, isoleucine, and leucine compared to those of fishmeal. Although Xu et al. (Xu et al. Citation2023), reported that the levels of arginine and histidine were lower in CO-utilizing bacteria, the results in existing studies rather show significant variation without a definite trend which shows the need to optimize production technology for aquafeed. Furthermore, opportunities may exist in the future to modify the amino acid profile of CAP to meet specific requirements by modifying the bacterial culture conditions (Maulu et al. Citation2021). As technology for commercial production of the protein for aquafeed develops, there is a need to improve and optimize the amino acids profile of CAP to meet specific conditions. Besides the nutritional elements, CAP may also contain immunomodulatory compounds (such as nucleic acids, peptidoglycan, etc) that may modulate immune functions in aquaculture species. However, compounds, such as nucleic acids may also have adverse effects on the growth performance of aquatic animals, particularly at higher inclusion levels (Maulu et al. Citation2022; Glencross et al., Citation2024).
Table 2. An overview of the amino acids profile of Clostridium autoethanogenum protein (CAP) biomass against fishmeal (% diet, DM).
Utilization in aquafeed
Effects on growth performance and feed utilization
The results of the effects of CAP on growth performance and feed utilization of aquatic animals have been widely studied in aquaculture (). Notably, a large variation exists in results reported based mainly on aquaculture species and CAP inclusion level in the diet. In some species, complete replacement of fishmeal or plant-based proteins with CAP can be achieved without adverse effects on growth performance and feed utilization, while in most species, higher inclusion levels could have deleterious effects. In Abalone, Haliotis discus hannai, for example, complete fishmeal replacement with CAP could be achieved without adverse effects on growth performance (Wu et al. Citation2022). Similarly, complete FM replacement with CAP could be achieved in the diet of red swamp crayfish (Procambarus clarkii) without negatively affecting growth and feed utilization although immunity was negatively affected at 100% FM replacement (Dai et al. Citation2023). However, in largemouth bass Micropterus salmoides, 50% fishmeal could be replaced with CAP without adverse effects on growth performance or health of the fish (Ma et al. Citation2022a). In the same species, another study showed that 75% fishmeal could be replaced with CAP without negatively affecting the growth performance of the fish, but hepatic histology was adversely affected by over 63% CAP (Zhu et al. Citation2022). The authors further reported better protein efficiency ratio (PER) and feed conversion ratio (FCR) in CAP-fed fish compared with control-fed fish suggesting that CAP is highly digestible and easily utilized by fish. However, increasing the inclusion level of CAP to 100% (complete fishmeal replacement) in M. salmoides, led to poor FCR of the fish (Yang et al. Citation2022) and reduced weight gain (WG) (Yang et al. Citation2022) which was attributed to arginine deficiency in CAP used in the study. Thus, ensuring CAP’s amino acid profile matches specific species requirements is essential. In juvenile turbot, Scophthalmus maximus L, replacing fishmeal with over 45% CAP negatively affected the feed intake of the fish without affecting the specific growth rate (SGR), feed efficiency ratio (FER), and PER (Zheng et al. Citation2022). Although the cell wall of bacteria has been shown to negatively affect nutrient digestion in fish (Øverland Citation2010), CAP cell does not appear to negatively affect nutrient digestibility due to its thinner wall compared to that of yeasts (Ma et al. Citation2022a). Therefore, the reduction in feed intake observed in some fish species at higher levels could be due to some bioactive compounds such as nucleic acids reportedly present in bacteria products (Maulu et al. Citation2021; Glencross et al. Citation2024). In GIFT Oreochromis niloticus juveniles and Jian carp (Cyprinus carpio var. Jian), replacing plant proteins with 20% CAP levels significantly improved the growth performance, exhibiting an increasing trend with increasing CAP levels from 5–20% (Li et al. Citation2021; Maulu et al. Citation2021). These results were further supported by transcriptomic analysis of growth-related gene expression. There is a need to further investigate the effects at higher inclusion levels in the diets of these species.
Table 3. A summary of the effects of Clostridium autoethanogenum protein in various aquaculture species reported by numerous studies.
In grass carp, conflicting results regarding CAP inclusion levels in the diets have been reported by different authors. For example, a study by Wei et al. (Citation2018) showed that replacing SBM with as low as 10% CAP could negatively affect the growth performance and liver health status in the juvenile fish (average size 25.66 g), while a study showed by Xue et al. (Citation2023) yielded no adverse effects on fish growth performance and feed utilization even up to 100% SBM replacement with CAP in the rather adult fish (average weight 512.05 g). Besides, a study by Liu et al. (Citation2024) to investigate the optimum inclusion level of CAP in the diet of grass carp (average size 4.56 g) as replacement for FM established that only up to 37.9% CAP could be included in the diet without adverse effects on growth performance, immune response, and antioxidant capacity. The reason for the reported variations may be due to the fish size/age, suggesting that different growth stages of aquatic animals possess different capabilities to utilize CAP. Additionally, bacterial culture substrate, fermentation process, and processing technology could influence the nutritional composition of CAP (Xu et al. Citation2023). Further research is also required to critically investigate the influence of these factors on CAP utilization in grass carp and other species.
In other species, such as obscure pufferfish, Takifugu obscurus, and large yellow croaker, Larimichthys crocea, negative effects on fish growth performance and feed utilization were reported even at low CAP inclusion levels (Cui et al. Citation2022; Zhang et al. Citation2022). However, in T. obscurus, apparent nutrient digestibility only declined when 80% fishmeal was replaced by CAP (Cui et al. Citation2022), suggesting that CAP may be highly digestible like fishmeal. Similar findings were also reported in juvenile turbot, Scophthalmus maximus L (Zheng et al. Citation2022). Additionally, some studies have reported similar nutrient digestibility results of CAP to that of fishmeal (Li et al. Citation2021; Yang et al. Citation2022). This could explain the attainment of FCR of less than 1 in some species fed diets containing CAP (Li et al. Citation2021; Maulu et al. Citation2021; Yang et al. Citation2022; Zhu et al. Citation2022). However, more studies are required to investigate the digestibility of CAP and feed utilization of several other aquaculture species.
Nevertheless, some studies have shown the possibility to improve the utilization of CAP through supplementation with feed additives such as phosphorous, tributyrin, bile acids, and taurine (Wang et al. Citation2023; Xu et al. Citation2023; Zheng et al. Citation2022). Besides, the inclusion of CAP in the diets of L. vannamei reared in different water salinities enhanced the shrimp’s growth performance and feed utilization (Chen et al. Citation2022) which suggests that CAP could help the shrimp to withstand stress due to changes in culture conditions. Further studies should, however, be investigate this and the mechanism of occurrence.
Nutrients digestion, absorption, and metabolism
The digestion of nutrients is critical for absorption and utilization of nutrients for metabolic functions in the body. So far, few studies have investigated the effects of dietary CAP on the digestive and absorptive capacity of aquatic animals and how nutrients are further utilized for metabolic purposes (). In abalone, the inclusion of CAP in the diet at 25% improved the digestive capacity, and inclusion up to 100% was possible without adverse effects (Wu et al. Citation2022). Similarly, up to 75% CAP could replace fishmeal in the diet of the largemouth seabass (Zhu et al. Citation2022) and up to 80% CAP in the diets of black sea bream, Acanthopagrus schlegelii without negatively affecting the digestive enzyme activities (Chen et al. Citation2020). In GIFT juveniles, dietary CAP inclusion at 10% significantly enhanced the fish’s absorptive capacity, and up to 20% showed no adverse effects (Maulu et al. Citation2021). However, in L. vannamei, the hepatopancreas gene expression in the shrimp fed with over 30% dietary CAP revealed adverse effects on protein digestion, absorption, and synthesis (Jiang et al. Citation2021). Overall, these findings suggest that different species could respond to dietary CAP levels differently. Considering that the nutritional value of CAP is comparable to that of fishmeal, there is a need to further investigate factors that might limit CAP utilization in aquaculture species. The presence of bioactive compounds in CAP should particularly be investigated concerning protein digestibility.
An investigation of differential metabolites in CAP-fed largemouth seabass revealed enhanced lipid and protein metabolism (Yang et al. Citation2022). Generally, the utilization of CAP has shown beneficial effects on the overall metabolism of nutrients in aquaculture, although variations still exist depending on CAP inclusion levels and species. In GIFT juveniles for instance, 10% dietary CAP enhanced the fish’s overall regulation of nutrients based on demand via the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway (Maulu et al. Citation2021). The fish showed the ability to control some genes regulating protein and glucolipid metabolism through the target of rapamycin (TOR) and phosphoinositide 3-kinase (PI3K) signaling pathways, respectively. Similarly, replacing fishmeal with 50% CAP in abalone diets enhanced the expressions of TOR, PI3K, and threonine kinase/protein B genes while decreasing the regulation of AMPK-α2 in the muscle of the fish (Wu et al. Citation2022). As the MPK signaling pathway is a master energy sensor and regulator, its activation indicates the status of the energy level in the animal (Maulu et al. Citation2021). Therefore, its activation suggests the ability of an animal to regulate body energy levels. Besides, 80% fishmeal could be replaced with CAP in obscure pufferfish without adversely affecting the muscle and liver protein synthesis despite the observed effects on digestibility and fish growth (Cui et al. Citation2022). However, in juvenile turbot fish, replacing fishmeal with over 45% CAP may lower protein metabolism (Zheng et al. Citation2022), suggesting that different fish species may possess different capabilities to utilize CAP probably due to their digestive physiology.
Antioxidant status, immunity, and disease resistance
With the increasing search for novel proteins in aquafeed, there is a growing consensus that feedstuffs must not only promote animal growth performance but also maintain prime health status. CAP has shown beneficial effects on aquatic health status and most attention has been focused on gut health, likely because of the important role it plays in determining overall animal health status. The inclusion of CAP in aquatic animal diets has shown potential to enhance intestinal integrity, immunity, antioxidant capacity, gut microbiota, and disease resistance although higher inclusion levels could have adverse effects in some species. In juvenile Jian carp and GIFT juvenile, partial CAP inclusion enhanced intestinal histology, immune responses, and antioxidant capacity besides promoting growth performance (Li et al. Citation2021; Maulu et al. Citation2021). Partial fishmeal replacement with CAP enhanced intestinal and hepatic histology, antioxidant capacity, immunochemistry barrier performance, and gut health status in juvenile largemouth bass (Li et al. Citation2022, Citation2023; Ma et al. Citation2022a; Zhu et al. Citation2022). However, complete fishmeal replacement with CAP could negatively affect fish growth and intestinal morphology by lowering protease activity, villi width, and height (Yang et al. Citation2022). In large yellow croaker, Larimichthys crocea replacing fishmeal with over 30% CAP did not only negatively affect fish growth but also gut microbiota and immune response (Wu et al. Citation2022; Zhang et al. Citation2022). Similarly, the mucosal fold length and immune system of the Pacific white shrimp Litopenaeus vannamei were affected negatively by replacing fishmeal with at least 30% CAP (Jiang et al. Citation2021). Interestingly, however, supplementing CAP with phosphorous in fishmeal-replaced diets enhances gut histology, immune response, and healthy gut microbiota and improves the shrimp’s resistance against pathogens (Zheng et al. Citation2022). Supplementation of bile acids in fishmeal replaced diets with CAP also improved the intestinal health status of L. vannamei (Xu et al. Citation2023). Supplementation of tributyrin could ameliorate the negative effects of higher CAP diets on intestinal health status in the large yellow croaker (Wang et al. Citation2023). Therefore, the negative effects of CAP at higher inclusion levels in some aquaculture species could be ameliorated by supplementation with feed additives.
Although studies investigating the effect of CAP in aquaculture species exposed to different culture conditions are rare, a study by Chen et al. (Citation2022) showed that CAP could enhance the response against pathogens in L. vannamei. Analysis of gut health status further showed that CAP enhanced the abundance of beneficial bacteria while inhibiting pathogenic species. Besides, the shrimp fed with CAP had better stress coping capacity compared to those in the control group. These results suggest that CAP could also be used as a functional feed additive in aquaculture to improve animal health status.
Fresh quality and safety issues
Novel feedstuffs in aquaculture must not deteriorate product quality and threaten consumer safety and health status. While most consumers are generally skeptical when it comes to consuming food fish grown on new feedstuffs, research must prove the safety of such products to guarantee human safety and health. Limited studies have investigated the effect of dietary CAP on the product quality of aquaculture species. As in other aspects discussed above, the results on flesh quality have also been conflicting. For example, in the abalone Haliotis discus hannai, complete fishmeal replacement with CAP could be achieved without affecting the animal’s flesh quality and growth performance negatively (Wu et al. Citation2022). In the large yellow croaker, while muscle amino acids composition and skin color remained unchanged by complete fishmeal replacement with CAP, the muscle firmness, water-holding capacity, myofiber diameter and myofiber density were affected negatively by at least 75% fishmeal replacement (Wu et al. Citation2022). Similarly, 70% fishmeal replacement with CAP could be achieved in the diets of largemouth sea bass without adversely fish growth performance and overall flesh quality despite lowering muscle lipid metabolism (Yang et al. Citation2022). Furthermore, partial (40%) fishmeal replacement with CAP in A. schlegelii did not affect the growth performance, muscle, and whole-body composition of the fish (Chen et al. Citation2020). In adult C. idellus, although a study by Xue et al. (Citation2023) demonstrated the possibility of achieving complete fishmeal with CAP in the diet without affecting growth and feed utilization, a study by Fan et al. (Citation2022) rather suggested negative effects on the flesh quality characteristics. Replacing fishmeal with over 30% CAP in L. vannamei lowered not only growth performance but also flesh quality by decreasing flesh hardness, shear force, steaming and cooking loss, and a decline in the metabolism of amino acids and fatty acids (Yao et al. Citation2022). Lowered muscle hardness gumminess and taurine amino acids were also observed in T. obscurus fed diets containing at least 40% CAP (Cui et al. Citation2022).
Challenges and prospects
Despite many potential benefits of utilizing CAP in aquafeed, several challenges and opportunities must be considered going forward. Currently, CAP has only been investigated on a limited number of aquaculture species with a bias toward the early stages of development. Future studies must also investigate the response of aquatic animals in their adult stages. Besides, the large variations existing within and between aquatic species merits further investigation. Although all existing studies obtained the CAP from Chinese energy companies, opportunities to produce this protein in other countries especially those leading in biomass production such as the USA, Brazil, Germany, and Argentina exist. This highlights an opportunity to accelerate transitioning into a circular economy while mitigating climate change by reducing GHG emissions. It must also be noted that commercial production of CAP for utilization in aquafeeds does not currently exist. Besides cost consideration, there is need for enhanced investigations on various aspects of aquatic animals fed CAP diets. Furthermore, there may be several regulatory frameworks regarding product safety and quality to guarantee human safety in many countries before CAP can be approved for use to produce food fish. Expanding the scope of investigations across various species could facilitate the resolution of conflicting findings concerning aquatic animals, particularly regarding their impact on the health status and resultant product quality, leading to a clearer understanding of the effects involved. The potential properties of CAP as a functional ingredient in aquaculture diets requires a thorough investigation into its probiotic effects in aquaculture. Additionally, it is imperative to critically examine the potential negative impacts associated with higher dietary inclusion levels of CAP in certain aquaculture species, while isolating limiting factors to enhance protein utilization. Concurrently, exploring the role of bioactive compounds present in bacterial protein is also essential for a comprehensive understanding of its effects.
Conclusion
Clostridium autoethanogenum protein (CAP) is a promising novel protein in aquaculture and its utilization in aquafeed at an appropriate level could promote growth performance, feed utilization, digestive and absorptive capacity, nutrients metabolism, antioxidant capacity, gut health status, and flesh quality of aquatic animals. However, existing findings are still inconsistent mainly depending on aquaculture species, development stage, and CAP inclusion level in aquafeed. More research is required to investigate factors limiting its utilization in some aquaculture species at higher inclusion levels. Furthermore, bioactive compounds potentially present in the bacteria must be investigated further in aquaculture. As commercial production of the CAP does not currently exist, there is a need to investigate the potential of producing this bacteria using various substrates under different conditions to promote commercial production.
Acknowledgments
This work was supported by the Centre for innovative Approach Zambia (CIAZ), Lusaka Zambia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abrini, J., H. Naveau, and E. J. Nyns. 1994. Clostridium autoethanogenum, sp. nov. an anaerobic bacterium that produces ethanol from carbon monoxide. Archives of Microbiology 161 (4):345–51. doi:10.1007/BF00303591.
- Cai, Y. W., H. F. Huang, W. X. Yao, H. Yang, M. Xue, X. Q. Li, and X. J. Leng. 2022. Effects of fish meal replacement by three protein sources on physical pellet quality and growth performance of Pacific white shrimp (Litopenaeus vannamei). Aquaculture Reports 25:101210. doi:10.1016/j.aqrep.2022.101210.
- Chen, Y., G. Sagada, B. Y. Xu, W. Chao, F. Q. Zou, W. K. Ng, Y. X. Sun, L. Wang, Z. W. Zhong, and Q. J. Shao. 2020. Partial replacement of fishmeal with Clostridium autoethanogenum single-cell protein in the diet for juvenile black sea bream (Acanthopagrus schlegelii). Aquaculture Research 51 (3):1000–11. doi:10.1111/are.14446.
- Chen, J., H. M. Wang, H. Yuan, N. J. Hu, F. Q. Zou, C. Y. Li, L. L. Shi, B. P. Tan, and S. Zhang. 2022. Effects of dietary Clostridium autoethanogenum protein on the growth, disease resistance, intestinal digestion, immunity and microbiota structure of Litopenaeus vannamei reared at different water salinities. Frontiers in Immunology 13. doi:10.3389/fimmu.2022.1034994.
- Cui, X. S., Q. Ma, M. Duan, H. G. Xu, M. Q. Liang, and Y. L. Wei. 2022. Effects of fishmeal replacement by Clostridium autoethanogenum protein on the growth, digestibility, serum free amino acid and gene expression related to protein metabolism of obscure pufferfish (Takifugu obscurus). Animal Feed Science and Technology 292:115445. doi:10.1016/j.anifeedsci.2022.115445.
- Dai, J., T. Chen, X. Guo, Z. Dai, Z. He, and Y. Hu. 2023. Evaluation of fish meal replacement by Clostridium autoethanogenum protein in diets for juvenile red swamp crayfish (Procambarus clarkii). Aquaculture 570:739379. doi:10.1016/j.aquaculture.2023.739379.
- Drake, H. L., A. S. Gossner, and S. L. Daniel. 2008. Old acetogens, new light. Annals of the New York Academy of Sciences 1125 (1):100–28. doi:10.1196/annals.1419.016.
- El-Ouny, Y. M., S. Maulu, M. A. A. Zaki, A. A. Helaly, A. A. M. Nour, M. F. ElBasuini, E. M. H. Labib, R. H. Khalil, A. H. Gouda, A. A. A. Hessein, et al. 2023. Effect of fishmeal replacement with dried red wigglers (Eisenia fetida) worm meal on growth and feed utilization, production efficiency, and serum biochemistry in Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture Reports 29:101518. doi:10.1016/j.aqrep.2023.101518.
- Fan, Z., C. H. Li, D. Wu, J. N. Li, L. S. Wang, D. C. Cao, L. H. Miao, and S. Q. Xie. 2022. Evaluation of four novel protein sources as alternatives to soybean meal for two specifications of cage-farmed grass carp (Ctenopharyngodon idellus) deeds: Effect on growth performance, flesh quality, and expressions of muscle-related genes. Frontiers in Marine Science. 9. doi:10.3389/fmars.2022.935651.
- Glencross, B., D. M. Fracalossi, K. Hua, M. Izquierdo, K. S. Ma, M. Overland, D. Robb, R. Roubach, J. Schrama, B. Small, et al. 2023. Harvesting the benefits of nutritional research to address global challenges in the 21st century. Journal of the World Aquaculture Society 54 (2):343–63. doi:10.1111/jwas.12948.
- Glencross, B., X. Ling, D. Gatlin, S. Kaushik, M. Øverland, R. Newton, and L. M. P. Valente. 2024. A SWOT analysis of the use of Marine, grain, terrestrial-animal and novel protein ingredients in aquaculture feeds. Reviews in Fisheries Science & Aquaculture 1–39. doi:10.1080/23308249.2024.2315049.
- Hall, H. N., H. V. Masey O’Neill, D. Scholey, E. Burton, M. Dickinson, and E. C. Fitches. 2018. Amino acid digestibility of larval meal (Musca domestica) for broiler chickens. Poultry Science 97:1290–97.
- Hardy, R. W. 2010. Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquaculture Research 41 (5):770–76. doi:10.1111/j.1365-2109.2009.02349.x.
- Hasimuna, O. J., S. Maulu, C. Monde, and M. Mweemba. 2019. Cage aquaculture production in Zambia: Assessment of opportunities and challenges on Lake Kariba, Siavonga district. The Egyptian Journal of Aquatic Research 45 (3):281–85. doi:10.1016/j.ejar.2019.06.007.
- Heffernan, J. K., C. Y. Lai, R. A. Gonzalez-Garcia, L. K. Nielsen, J. H. Guo, and E. Marcellin. 2023. Biogas upgrading using Clostridium autoethanogenum for value-added products. Chemical Engineering Journal 452:138950. doi:10.1016/j.cej.2022.138950.
- Humphreys, C. M., and N. P. Minton. 2018. Advances in metabolic engineering in the microbial production of fuels and chemicals from C1 gas. Current Opinion in Biotechnology 50:174–81. doi:10.1016/j.copbio.2017.12.023.
- Jiang, X. R., W. X. Yao, H. Yang, S. M. Tan, X. J. Leng, and X. Q. Li. 2021. Dietary effects of Clostridium autoethanogenum protein substituting fish meal on growth, intestinal histology and immunity of Pacific white shrimp (Litopenaeus vannamei) based on transcriptome analysis. Fish & Shellfish Immunology 119:635–44. doi:10.1016/j.fsi.2021.10.005.
- Jin, S., Y. Jeon, M. S. Jeon, J. Shin, Y. S. Song, S. Kang, J. Bae, S. Cho, J. K. Lee, D. R. Kim, et al. 2021. Acetogenic bacteria utilize light-driven electrons as an energy source for autotrophic growth. Proceedings of the National Academy of Sciences 118 (9). doi:10.1073/pnas.2020552118.
- Li, X. Y., Y. K. Chen, C. Z. Zheng, S. Y. Chi, S. Zhang, B. P. Tan, and S. W. Xie. 2022. Evaluation of six novel protein sources on apparent digestibility in Pacific White Shrimp, Litopenaeus vannamei. Aquaculture Nutrition 2022:1–11. doi:10.1155/2022/8225273.
- Liew, F., A. M. Henstra, K. Winzer, M. Kopke, S. D. Simpson, N. P. Minton, and S. Y. Lee. 2016. Insights into CO2 Fixation Pathway of Clostridium autoethanogenum by Targeted Mutagenesis. Mbio 7 (3). doi:10.1128/mBio.00427-16.
- Liew, F., M. E. Martin, R. C. Tappel, B. D. Heijstra, C. Mihalcea, and M. Kopke. 2016. Gas fermentation a flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Frontiers in Microbiology 7. doi:10.3389/fmicb.2016.00694.
- Li, M. Y., H. L. Liang, J. Xie, W. Chao, F. Q. Zou, X. P. Ge, and M. C. Ren. 2021. Diet supplemented with a novel Clostridium autoethanogenum protein have a positive effect on the growth performance, antioxidant status and immunity in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture Reports 19:100572. doi:10.1016/j.aqrep.2020.100572.
- Li, L. K., X. J. Liu, Y. Wang, Y. Q. Huang, and C. F. Wang. 2022. Effects of alternate feeding between fish meal and novel protein diets on the intestinal health of juvenile largemouth bass (Micropterus salmoides). Aquaculture Reports 23:101023. doi:10.1016/j.aqrep.2022.101023.
- Liu, G., M. Zhou, X. Mao, D. Gu, W. Chen, X. Long, S. Xie, and Q. Tan. 2024. Evaluation of the appropriate Clostridium autoethanogenum protein level in grass carp (Ctenopharyngodon idellus) diets by growth performance, health status, and intestinal microbiota. Aquaculture International 32:31–59. doi:10.1007/s10499-023-01163-y.
- Li, L. K., Y. Wang, Z. Zhang, and C. F. Wang. 2023. Microbiomic and metabonomic analysis provide new insights into the enhanced intestinal health in large-size largemouth bass (Micropterus salmoides) when fed novel proteins: Novel proteins are promising foods for future aquaculture. Aquaculture 563:739019. doi:10.1016/j.aquaculture.2022.739019.
- Ma, S., X. Liang, P. Chen, J. Wang, X. Gu, Y. Qin, C. Blecker, and M. Xue. 2022a. A new single-cell protein from clostridium autoethanogenum as a functional protein for largemouth bass (Micropterus salmoides). Animal Nutrition 10:99–110. doi:10.1016/j.aninu.2022.04.005.
- Marcellin, E., J. B. Behrendorff, S. Nagaraju, S. DeTissera, S. Segovia, R. W. Palfreyman, J. Daniell, C. Licona-Cassani, L. E. Quek, R. Speight, et al. 2016. Low carbon fuels and commodity chemicals from waste gases – systematic approach to understand energy metabolism in a model acetogen. Green Chemistry 18 (10):3020–28. doi:10.1039/C5GC02708J.
- Maulu, S., S. Langi, O. J. Hasimuna, D. Missinhoun, B. P. Munganga, B. M. Hampuwo, N. N. Gabriel, M. Elsabagh, H. Van Doan, Z. Abdul Kari, et al. 2022. Recent advances in the utilization of insects as an ingredient in aquafeeds: A review. Animal Nutrition 11:334–49. doi:10.1016/j.aninu.2022.07.013.
- Maulu, S., H. L. Liang, X. P. Ge, H. Yu, D. Y. Huang, J. Ke, M. C. Ren, and H. F. Mi. 2021. Effect of dietary Clostridium autoethanogenum protein on growth, body composition, plasma parameters and hepatic genes expression related to growth and AMPK/TOR/PI3K signaling pathway of the genetically improved farmed tilapia (GIFT: Oreochromis niloticus) juveniles. Animal Feed Science and Technology. 276 (114914):1–13. doi:10.1016/j.anifeedsci.2021.114914.
- Maulu, S., H. L. Liang, J. Ke, M. C. Ren, X. P. Ge, D. Y. Huang, and H. Yu. 2021. Dietary Clostridium autoethanogenum protein modulates intestinal absorption, antioxidant status, and immune response in GIFT (Oreochromis niloticus) juveniles. Aquaculture Research 52 (11):5787–99. doi:10.1111/are.15454.
- Ma, S. F., H. Wang, J. Yang, J. G. Li, M. Xue, H. Y. Cheng, F. Q. Zou, and C. Blecker. 2022b. Effects of clostridium autoethanogenum protein inclusion levels and processing parameters on the physical properties of low-starch extruded floating feed. Aquaculture Reports 23:101030. doi:10.1016/j.aqrep.2022.101030.
- Naylor, R. L., R. W. Hardy, A. H. Buschmann, S. R. Bush, L. Cao, D. H. Klinger, D. C. Little, J. Lubchenco, S. E. Shumway, and M. Troell. 2021. Publisher Correction: A 20-year retrospective review of global aquaculture (vol 591, pg 551, 2021). Nature 595 (7868):E36–36. doi:10.1038/s41586-021-03736-4.
- Norman, R. O. J., T. Millat, K. Winzer, N. P. Minton, and C. Hodgman. 2018a. Progress towards platform chemical production using Clostridium autoethanogenum. Biochemical Society Transactions 46 (3):523–35. doi:10.1042/BST20170259.
- Norman, R. O. J., T. Millat, K. Winzer, N. P. Minton, and C. Hodgman. 2018b. Progress towards platform chemical production using Clostridium autoethanogenum. Biochemical Society Transactions 46 (3):523–35. doi:10.1042/BST20170259.
- NRC, N. R. C. 2011. Nutrient requirements of fish and shrimp. Washington DC: The National Academies Press.
- Øverland, M., A. H. Tauson, K. Shearer, and A. Skrede. 2010. Evaluation of methane-utilising bacteria products as feed ingredients for monogastric animals. Archives of Animal Nutrition 64 (3):171–89. doi:10.1080/17450391003691534.
- Reihani, S. F. S., and K. Khosravi-Darani. 2019. Influencing factors on single-cell protein production by submerged fermentation: A review. Electronic Journal of Biotechnology 37:34–40. doi:10.1016/j.ejbt.2018.11.005.
- Sillman, J., L. Nygren, H. Kahiluoto, V. Ruuskanen, A. Tamminen, C. J. Bajamundi, M. Nappa, M. Wuokko, T. Lindh, P. Vainikka, et al. 2019. Bacterial protein for food and feed generated via renewable energy and direct air capture of CO2: Can it reduce land and water use? Global Food Security 22:25–32. doi:10.1016/j.gfs.2019.09.007.
- Simpson, S., W. E. Allen, R. J. Conrado, and S. Molloy. 2016. Gas fermentation for the production of protein or feed (Patent No. US0338380 A1). United States Patent Publication.
- Song, Y., J. Shin, S. Jin, J. K. Lee, D. R. Kim, S. C. Kim, S. Cho, and B. K. Cho. 2018. Genome-scale analysis of syngas fermenting acetogenic bacteria reveals the translational regulation for its autotrophic growth. Bmc Genomics 19 (1):837. doi:10.1186/s12864-018-5238-0.
- Wang, X. E., M. Wan, Z. Wang, H. T. Zhang, S. Zhu, X. F. Cao, N. Xu, J. C. Zheng, X. Y. Bu, W. Xu, et al. 2023. Effects of tributyrin supplementation on growth performance, intestinal digestive enzyme activity, antioxidant capacity, and inflammation-related gene expression of large yellow croaker (Larimichthys crocea) fed with a high level of Clostridium autoethanogenum protein. Aquaculture Nutrition 2023 (2687734): 1–12. doi:10.1155/2023/2687734.
- Wang, D., S. W. Zhai, C. X. Zhang, Y. Y. Bai, S. H. An, and Y. N. Xu. 2005. Evaluation on nutritional value of field crickets as a poultry feedstuff. Asian-Australasian Journal of Animal Sciences 18:667–70.
- Wei, H. C., H. H. Yu, X. M. Chen, W. Chao, F. Q. Zou, P. Chen, Y. H. Zheng, X. F. Wu, X. F. Liang, and M. Xue. 2018. Effects of soybean meal replaced by Clostridium autoethanogenum protein on growth performance, plasma biochemical indexes and hepatopancreas and intestinal histopathology of grass carp (Ctenopharyngodon idllus). Chinese Journal of Animal Nutrition 30:4190–201.
- Wood, J. C., J. Grove, E. Marcellin, J. K. Heffernan, S. Hu, Z. Yuan, and B. Virdis. 2021. Strategies to improve viability of a circular carbon bioeconomy-A techno-economic review of microbial electrosynthesis and gas fermentation. Water Research 201:117306. doi:10.1016/j.watres.2021.117306.
- Woolley, L., M. R. Chaklader, L. Pilmer, F. Stephens, C. Wingate, M. Salini, and G. Partridge. 2023. Gas to protein: Microbial single cell protein is an alternative to fishmeal in aquaculture. Science of the Total Environment 859:160141. doi:10.1016/j.scitotenv.2022.160141.
- Wu, Y., S. J. Tian, J. Yuan, Z. Y. Zhang, H. H. Zhou, W. H. Gao, W. B. Zhang, and K. S. Mai. 2022. Effects of Clostridium autoethanogenum protein as substitute for dietary fishmeal on the growth, feed utilization, intestinal health and muscle quality of large yellow croaker Larimichthys crocea. Aquaculture 561:738591. doi:10.1016/j.aquaculture.2022.738591.
- Wu, Z. H., X. J. Yu, J. S. Guo, Y. H. Fu, Y. L. Guo, M. Z. Pan, W. B. Zhang, and K. S. Mai. 2022. Replacement of dietary fish meal with Clostridium autoethanogenum protein on growth performance, digestion, mTOR pathways and muscle quality of abalone Haliotis discus hannai. Aquaculture 553:738070. doi:10.1016/j.aquaculture.2022.738070.
- Xue, R. R., H. D. Li, S. Liu, Z. C. Hu, Q. Wu, and H. Ji. 2023. Substitution of soybean meal with Clostridium autoethanogenum protein in grass carp (Ctenopharygodon idella) diets: Effects on growth performance, feed utilization, muscle nutritional value and sensory characteristics. Animal Feed Science and Technology 295:115547. doi:10.1016/j.anifeedsci.2022.115547.
- Xu, J., J. Wang, C. L. Ma, Z. X. Wei, Y. D. Zhai, N. Tian, Z. G. Zhu, M. Xue, and D. M. Li. 2023. Embracing a low-carbon future by the production and marketing of C1 gas protein. Biotechnology Advances 63:108096. doi:10.1016/j.biotechadv.2023.108096.
- Xu, J., C. Z. Zheng, S. Y. Chi, S. Zhang, J. M. Cao, B. P. Tan, and S. W. Xie. 2023. Clostridium autoethanogenum protein substitution and bile acids addition altered intestinal health and transcriptome profiles of hepatopancreas in Litopenaeus vannamei. Aquaculture Reports 28:101432. doi:10.1016/j.aqrep.2022.101432.
- Yang, P., X. Li, B. Song, M. He, C. Wu, and X. Leng. 2023. The potential of clostridium autoethanogenum, a new single cell protein, in substituting fish meal in the diet of largemouth bass (micropterus salmoides): Growth, feed utilization and intestinal histology. Aquaculture and Fisheries 8 (1):67–75. doi:10.1016/j.aaf.2021.03.003.
- Yang, P. X., X. Q. Li, W. X. Yao, M. L. Li, Y. Y. Wang, and X. J. Leng. 2022. Dietary effect of Clostridium autoethanogenum protein on growth, Intestinal histology and flesh lipid metabolism of Largemouth Bass (Micropterus salmoides) Based on Metabolomics. Metabolites 12 (11):1088. doi:10.3390/metabo12111088.
- Yang, P. X., W. X. Yao, Y. Y. Wang, M. L. Li, X. Q. Li, and X. J. Leng. 2022. Dietary effects of fish meal substitution with Clostridium autoethanogenum on flesh quality and metabolomics of largemouth bass (Micropterus salmoides). Aquaculture Reports 23:101012. doi:10.1016/j.aqrep.2022.101012.
- Yao, W., X. Li, X. Zhang, M. Li, Y. Wang, H. He, and X. Leng. 2024. The complete replacement of fish meal with Clostridium autoethanogenum protein in practical diet did not affect the growth, but reduced the flesh quality of Pacific white shrimp, Litopenaeus vannamei. Animal Feed Science and Technology 310:115919. doi:10.1016/j.anifeedsci.2024.115919.
- Yao, W. X., P. X. Yang, X. Zhang, X. Y. Xu, C. Y. Zhang, X. Q. Li, and X. J. Leng. 2022. Effects of replacing dietary fish meal with Clostridium autoethanogenum protein on growth and flesh quality of Pacific white shrimp (Litopenaeus vannamei). Aquaculture 549:737770. doi:10.1016/j.aquaculture.2021.737770.
- Zhang, J., Y. Z. Dong, K. Song, L. Wang, X. S. Li, K. L. Lu, B. P. Tan, and C. X. Zhang. 2022. Substituting fish meal with a bacteria protein (Clostridium autoethanogenum protein) derived from industrial-scale gas fermentation: effects on growth and gut health of juvenile large yellow croakers (Larimichthys crocea). Fishes-Basel 7 (5):228. doi:10.3390/fishes7050228.
- Zheng, C. Z., J. M. Cao, S. Y. Chi, X. H. Dong, Q. H. Yang, H. Y. Liu, S. Zhang, S. W. Xie, and B. P. Tan. 2022. Dietary phosphorus supplementation in the diet of Pacific white shrimp (Litopenaeus vannamei) alleviated the adverse impacts caused by high Clostridium autoethanogenum protein. Fish & Shellfish Immunology 131:137–49. doi:10.1016/j.fsi.2022.10.005.
- Zheng, J. C., W. C. Zhang, Z. J. Dan, Y. W. Zhuang, Y. T. Liu, K. S. Mai, and Q. H. Ai. 2022. Replacement of dietary fish meal with Clostridium autoethanogenum meal on growth performance, intestinal amino acids transporters, protein metabolism and hepatic lipid metabolism of juvenile turbot (Scophthalmus maximus L.). Frontiers in Physiology 13. doi:10.3389/fphys.2022.981750.
- Zhu, S. J., W. H. Gao, Z. Y. Wen, S. Y. Chi, Y. H. Shi, W. Hu, and B. P. Tan. 2022. Partial substitution of fish meal by Clostridium autoethanogenum protein in the diets of juvenile largemouth bass (Micropterus salmoides). Aquaculture Reports 22:22. doi:10.1016/j.aqrep.2021.100938.