Abstract
Muscarinic receptor binding activity was tested on 224 plant extracts obtained from more than 50 plant families found in Malaysia. The plant extracts were evaluated by a 96-well microplate filtration–based radioligand competitive assay, centered on the ability of the plant extracts to competitively displace the radioligand, [3H]N.-methylscopolamine, from binding to the muscarinic membrane receptors. The screening assay was initially carried out at 50 µg/assay point, and those showing inhibition at and above 61% were retested at 10 µg/assay point. The extracts of Ficus septica. Burm. f. (Moraceae) [65.85±3.75% inhibition; mean (n = 3)±SD], Polyalthia microtus. Miq. (Annonaceae) (32.63±1.38% inhibition), and Popowia odoardoi. Diels (Annonaceae) (35.79±7.11% inhibition) at 10 µg/assay point exhibited muscarinic properties, which are worthy of further investigation.
Introduction
Since ancient times, human being have used natural products, including plants, as remedies to treat illnesses. Today, about one-third of the top 25 prescription drugs in the world are natural products or their derivatives (Manly et al., Citation2002). The sources of natural products include plants, marine and microorganisms (Zhu, Citation1996; Cragg et al., Citation1997; Shu, Citation1997; Manly et al., Citation2002), and historically, plants have been an important source of pharmacologically active compounds providing for a number of muscarinic receptor antagonists. For example, atropine from the plant Atropa belladonna. L. and scopolamine from Datura. sp. are the most commonly used anticholinergic drugs (Evans & Evans, Citation2002).
Anticholinergic drugs act on two distinct classes of receptors, muscarinic and nicotinic. The muscarinic class of acetylcholine receptors are widely distributed throughout the body and subserve numerous vital functions in both the brain and autonomic nervous system (Lefkowitz et al., Citation1990). Heterogeneity of muscarinic receptor was shown in the late 1980s when five subtypes (M1–M5) were identified using molecular biological techniques (Kubo et al., Citation1986; Bonner et al., Citation1987; Liao et al., Citation1989; Sokolovsky, Citation1989; Kashihara et al., Citation1992).
Activation of muscarinic receptors in the periphery causes a decrease in heart rate, relaxation of blood vessels, constriction in the airways of the lung, an increase in secretions, and motility of various organs of the gastrointestinal tract, increase in the secretions of lacrimal and sweat glands, constriction in the iris sphincter and ciliary muscles of the eye (Lefkowitz et al., Citation1990). In the brain, muscarinic receptors influence many important functions such as learning, memory, and the control of posture (Lefkowitz et al., Citation1990).
Classic muscarinic receptor antagonists such as atropine do not distinguish between muscarinic receptor subtypes. These nonselective compounds cover therapeutic indications such as antispasmodic, antitussive, and antibronchospastic, but their therapeutic utility is limited by the presence of side effects including mydriasis, CNS disturbances, tachycardia, and constipation. In recent studies, it has been shown that selective M1 antagonists are useful in reducing gastric acid secretion. Selective M2 antagonists may be useful in the treatment of bradycardia and Alzheimer disease, whereas selective M3 antagonists affect smooth muscles (chronic obstructive airway disease, irritable bowel syndrome, and incontinence) (Eglen, Citation1998). To reduce side effects and to improve therapeutic activities, selective muscarinic receptor antagonists have been developed for antiulcer activity (telenzepine; M1/M4 selective; Byk), bradycardia (otenzepad; M2/M4 selective; Boehringer Ingelheim), irritable bowel syndrome (darifenacin; M3/M1 selective; Pfizer), and antibronchospastic activity (rispenzepine; M3/M1 selective; Dompe) (Eglen, Citation1998).
As part of an ongoing screening program for potential receptor agonists and antagonists Malaysian plants that showed significant muscarinic receptor competitive binding activity were identified. The active crude extracts are subjected to further bioassay-guided isolation of active constituents. Upon successful isolation, the active constituents will be evaluated for their muscarinic receptor subtype selectivity.
Materials and Methods
Chemicals and reagents
[3H]N.-Methylscopolamine was supplied by Amersham Pharmacia Biotech UK Ltd. All other reagents were of analytical grade and obtained from standard commercial sources (Little Chalfont, Buckinghamshire, UK).
Plant materials
Plant samples were collected from the Forest Research Institute Malaysia, Kuala Lumpur, Malaysia (voucher no.: 5-digits series), and the state of Sabah, Malaysia (voucher no.: 6-digits series). The voucher specimens were kept at the herbaria of Forest Research Institute Malaysia (5-digits series) and Forest Research Center, Sepilok, Sandakan, Malaysia (6-digits series). Different parts of the plants were dried separately at 40°C. The dried materials (100–200 g) were powdered and soaked with sufficient methanol in conical flasks for 7 days with sonication (5 × sufficient volume of methanol to cover the plant materials). Methanol extracts were collected and filtered at 48, 96, and 168 h, and the conical flasks with plant materials were replaced with fresh methanol to continue extraction. The pooled extracts were evaporated at 50°C in vacuo. and the residues freeze-dried and kept in sample bottles at − 20°C until use.
Crude extract dilution
DMSO (1 ml) was added to 4 mg of crude plant extracts and vortexed vigorously giving an initial concentration of 4 mg/ml. The extracts were tested at 50 and 10 µg/assay points.
Membrane preparation
Total rat brain (minus cerebellum) membrane was prepared according to the protocol described by Gattu et al. (Citation1995) with minor modifications. Male Sprague-Dawley rats (250–300 g) were decapitated and the brains removed. The cerebellum was dissected out and the rest of the brain finely chopped with scissors, homogenized in 10 volumes of ice-cold 50 mM Tris-HCl, pH 7.4, buffer using Ultra-Turax (2 × 10 s) and followed by glass-Teflon pestle homogenization at 800 rpm for 20 strokes.
The homogenate was centrifuged at 40,000 × g. using Beckman type 28 rotor at 4°C for 15 min. The pellet was retained and washed twice by resuspending in ice-cold 50 mM Tris-HCl, pH 7.4, buffer (centrifuged at 40,000 × g. for 15 min). The final pellet was suspended in 5 ml ice-cold 50 mM Tris-HCl, pH 7.4, buffer, aliquoted and kept at − 80°C until use. Protein was determined using the Sigma Total Protein Reagent using bovine serum albumin as the standard, and the protein content corresponded to about 20 mg protein/ml.
Muscarinic receptor binding assay
The muscarinic receptor binding was assayed by a modification of the method of Bockman et al. (Citation2001). Briefly, the membranes were thawed on ice and diluted to 0.75 mg/ml protein using 50 mM Tris-HCl, pH 7.4, buffer. The reference ligand (atropine) and radioligand ([3H]N.-methylscopolamine) were diluted to 100 µM and 5 nM final concentration using 50% DMSO in deionized water and binding buffer, respectively.
With its cover removed, the Multiscreen plate (GF-B, Millipore Billerica, MA, USA) was placed on vacuum manifold and the filter of each well prewetted with 200 µl of 50 mM Tris-HCl, pH 7.4, buffer. Radioligand (25 µl) was added to each well of the Multiscreen plate, followed by addition of 25 µl of 50% DMSO (total binding, 3 wells), reference compound (nonspecific binding, 3 wells), or crude extracts to the corresponding well in the plate. The reaction was initiated by adding 200 µl of diluted membranes to each well. The plate was then covered, vortexed gently, and incubated at 22°C for 90 min.
The reaction mixture was filtered on the vacuum manifold and washed four-times with 200 µl of ice-cold 50 mM Tris-HCl, pH 7.4. The plate was cleaned with tissues to remove excess buffer and air-dried. The filters were punched out and transferred into 5-ml scintillation vials and 4 ml of scintillation cocktail added. The vials were capped, the content shaken for a few minutes, and radioactivity counted for 3 min per vial, using a scintillation counter (Packard, Meriden, CT, USA).
Data analysis
The percentage inhibitory specific binding in the presence of the test compounds was calculated using a standard data reduction algorithm, and it is as follows:
where B = binding in the presence of test extract, NSP = nonspecific binding in the presence of excess inhibitor (reference ligand), and T = total binding.
Results and Discussion
The crude extracts were initially screened at 100 µg/assay point and above, and under this condition, some of the extracts were not soluble in the assay buffers, which prevented proper filtration of the plates. Consequently, the extracts were screened at 50 µg/assay point (), and extracts that showed inhibition at and above 61% were retested at 10 µg/assay point. Under this condition, the samples remained soluble, and proper filtration was achieved. The extracts were screened in triplicate and the percentage inhibition averaged ().
Table 1.. Percent inhibition of extracts (50 µg/well) on specific binding to muscarinic receptor.
Table 2.. Percent inhibition of extracts (10 µg/well) on specific binding to muscarinic receptor.
Of these 224 extracts tested at 50 µg/assay point, 42 (19%) exhibited 41–60% inhibition and 12 (5%) exhibited 61% or higher inhibition. To reduce false-positive results, extracts exhibiting above 61% inhibition and comparators were then tested at 10 µg/well. Of these, only Ficus septica. gave more than 60% inhibition (65.85±3.75%). Other extracts such as Polyalthia microtus. (Annonaceae) and Popowia odoardoi. (Annonaceae) showed 32.63±1.38% and 35.79±7.11% inhibition, respectively.
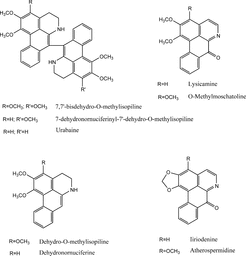
Our preliminary chemical studies and the literature indicate these plants, Ficus septica. Burm. f., Polyalthia microtus. Miq., and Popowia odoardoi. Diels, and related species contain alkaloidal compounds, and some of the reported structures are as shown in , , and . It is conceivable that the active constituents, which inhibited binding of the radioligand, [3H]N.-methylscopolamine to muscarinic receptors, are probably alkaloids, as known muscarinic receptor agonists and antagonists possess nitrogen moieties. We have, therefore, selected Ficus septica. Burm. f., Polyalthia microtus. Miq., and Popowia odoardoi. Diels for bioassay-guided fractionation to identify the muscarinic active constituents and to ascertain their activities.
Figure 1 Some alkaloids isolated from Ficus. species (Baumgartner et al., Citation1990; Buckingham, Citation1994; Peraza-Sanchez et al., Citation2002; Wu et al., Citation2002).
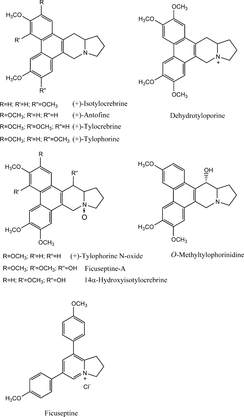
Figure 2 Some alkaloids isolated from Popowia. species (Jossang et al., Citation1986).
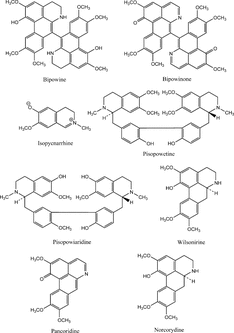
Figure 3 Some alkaloids isolated from Polyalthia. species (Jossang et al., Citation1977; Marsaioli et al., 1977; Connolly et al., Citation1996; Said et al., Citation2003).
Acknowledgments
The project was funded by the Ministry of Science, Technology and the Environment, Malaysia (IRPA 26-02-06-0127). K.F. Yap is supported by the National Science Fellowship, Malaysia.
References
- Baumgartner B, Erdermeier CAJ, Wright AD, Rali T, Sticher O (1990): An antimicrobial alkaloid from Ficus septica.. Phytochemistry 29: 3327–3330. [CSA]
- Bockman CS, Bradley ME, Dang HK, Zeng W, Scofield MA, Dowd FJ (2001): Molecular and pharmacological characterization of muscarinic receptor subtypes in a rat parotid gland cell line: Comparison with native parotid gland. J Pharmacol Exp Ther 297: 718–726. [PUBMED], [INFOTRIEVE], [CSA]
- Bonner TI, Buckley NJ, Young AC, Brann MR (1987): Identification of a family of muscarinic acetycholine receptor genes. Science 237: 527–532. [PUBMED], [INFOTRIEVE], [CSA]
- Buckingham J, ed. (1994): Dictionary of Natural Products. New York, Chapman & Hall, p. 769.
- Connolly JD, Haque MDE, Kadir AA (1996): Two 7,7′-bis.dehydroaporphine alkaloids from Polyalthia bullata.. Phytochemistry 43: 295–297. [CSA], [CROSSREF]
- Cragg GM, Newman DJ, Snader KM (1997): Natural products in drug discovery and development. J Nat Prod 60: 52–60. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Eglen RM (1998): Muscarinic receptor antagonists. Pharmacological and therapeutic utility. In: Leff P, ed., Receptor-based Drug Design. New York, Marcel Dekker, pp. 273–296.
- Evans WC, Evans D (2002): Trease & Evan's Pharmacognosy, 15th ed. London, WB Saunders.
- Gattu M, Terry AV, Buccafusco JJ (1995): A microtechnique for estimation of muscarinic and nicotinic receptor binding parameters using 96-well filtration plates. J Neurosci Methods 63: 121–125. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jossang A, Leboeuf M, Cave A (1977): Alkaloids of Annonaceae XVII: Alkaloids of Enantia polycarpa. Engl et. Diels. Planta Med 32: 249–257. [PUBMED], [INFOTRIEVE], [CSA]
- Jossang A, Leboeuf M, Cave A (1986): Alcaloides des Annonacées, 65. Alkaloides de Popowia pisocarpa., première partie: Nouvelles bis.benzylisoquinoléines. J Nat Prod 49: 1018–1027. [CSA], [CROSSREF]
- Kashihara K, Varga EV, Waite SL, Roeske WR, Yamamura HI (1992): Cloning of the rat M3, M4 and M5 muscarinic acetylcholine receptor genes by the polymerase chain reaction (PCR) and the pharmacological characterization of the expressed genes. Life Sci 51: 955–971. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kubo T, Fukuda K, Mikami A, Maeda A, Takahashi H, Mishina M, Haga T, Haga K, Ichiyama A, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S (1986): Cloning, sequencing and expression complementary DNA encoding the muscarinic acetycholine receptor. Nature 323: 411–416. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lefkowitz RJ, Hoffman BB, Taylor P (1990): Neurohumoral transmission: The autonomic and somatic motor nervous syatems. In: Gilman AG, Rall TW, Nites AS, Taylor P, eds., The Pharmacological Basis of Therapeutics. New York, Pergamon Press, pp. 84–121.
- Liao C-F, Themmen APN, Joho R, Barberis C, Birnbaumer M, Birnbaumer L (1989): Molecular cloning and expression of the fifth muscarinic acetylcholine receptor. J Biol Chem 264: 7328–7337. [PUBMED], [INFOTRIEVE], [CSA]
- Manly SP, Padmanabha R, Lowe SE (2002): Natural products or not? How to screen for natural products in the emerging HTS paradigm. In: Janzen WP, ed., High Throughput Screening. Methods and Protocols. New Jersey, Humana Press, pp. 153–168.
- Marsaioli AJ, Magalhães AF, Rúveda EA, Francisco de AM Reis (1980): 13C NMR analysis of some oxoaporphine alkaloids. Phytochemistry 19: 995–997. [CSA], [CROSSREF]
- Peraza-Sanchez SR, Chai HB, Shin YG, Santisuk T, Reutrakul V, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD (2002): Constituents of the leaves and twigs of Ficus hispida.. Planta Med 68: 186–188. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Said M, Hadi AA, Awang K (2003): Alkaloids from Polylthia sclerophylla.. In: Hamzah AS, Ismail NH, Ariffin ZZ, Hamzah Z, eds., Fine Chemicals from Natural Resources. Malaysia, Pusat Penerbitan Universiti (UPENA) UiTM, pp. 275–279.
- Shu YZ (1998): Review: Recent natural products based drug development: A pharmaceutical industry perspective. J Nat Prod 61: 1053–1071. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sokolovsky M (1989): Muscarinic cholinergic receptors and their interactions with drugs. In: Testa B, ed., Advances in Drug Research, Vol. 18. London, Academic Press, pp. 431–509.
- Wu PL, Rao KV, Su CH, Kuoh CS, Wu TS (2002): Phenanthroindolizidine alkaloids and their cytotoxicity from the leaves of Ficus septica.. Heterocycles 57: 2401–2408. [CSA]
- Zhu M, Bowery NG, Greengrass PM, Phillipson JD (1996): Application of radioligand receptor binding assays in the search for CNS active principles from Chinese medicinal plants. J Ethnopharmcol 54: 153–164. [CSA], [CROSSREF]
