Abstract
Introduction
Metformin toxicity following therapeutic use or overdose may result in metabolic acidosis with hyperlactatemia. This study aims to assess the relationship between serum lactate concentration, arterial pH, and ingested dose with severity of poisoning, and to identify if serum lactate concentration is a useful marker of severity in metformin toxicity.
Methods
A retrospective study of telephone enquiries relating to metformin exposures to the National Poisons Information Service between 2010 and 2019 from hospitals in the United Kingdom.
Results
Six-hundred and thirty-seven cases were identified; 117 involved metformin only and 520 involved metformin with other drugs. The majority of cases involved acute (87%) and intentional (69%) exposures. There was a statistically significant difference in doses between the Poisoning Severity Scores, as well as between intentional and unintentional or therapeutic error doses (P < 0.0001). The distribution of cases for each Poisoning Severity Score differed between the metformin only and metformin with other drugs cases (P < 0.0001). Lactic acidosis was reported in 232 cases. Serum lactate concentration and arterial pH differed across Poisoning Severity Scores. Arterial pH inversely correlated with ingested dose (r=-0.3, P = 0.003), and serum lactate concentration positively correlated with ingested dose (r = 0.37, P < 0.0001). Serum lactate concentration and arterial pH did not correlate with each other. Twenty-five deaths were recorded, all following intentional overdoses.
Discussion
The dataset focuses mostly on acute, intentional overdoses. Increasing ingested metformin dose, a higher serum lactate concentration and worsening arterial pH were all associated with an unfavourable Poisoning Severity Score in patients in both metformin only and metformin with other drugs groups. As serum lactate concentration did not correlate with arterial pH, it represents an independent marker of poisoning severity.
Conclusions
Data from the present study suggest that serum lactate concentration can be used to assess severity of poisoning in patients who have reportedly ingested metformin.
Introduction
Metformin is an oral anti-hyperglycaemic biguanide used to treat patients with type 2 diabetes mellitus [Citation1,Citation2]. Normoglycaemia is achieved by increasing the peripheral utilization of serum glucose and decreasing hepatic gluconeogenesis [Citation3,Citation4]. Metformin also has a low potential for causing hypoglycaemia [Citation5].
Clinical features indicative of metformin toxicity range from mild gastrointestinal upset, metabolic acidosis with hyperlactatemia to more severe acidaemia, hyperlactatemia, and haemodynamic compromise [Citation6]. The incidence of metabolic acidosis with hyperlactatemia in the general population is approximately 3 cases per 100,000 patient-years. The incidence is reportedly higher in patients who take metformin therapeutically, up to 19 cases per 100,000 patient-years [Citation7]. Patients with kidney impairment who are prescribed metformin are at higher risk of toxicity because of drug accumulation [Citation8]. Other predisposing conditions and intercurrent precipitating events that reduce lactate clearance (e.g., liver diseases) and/or increase its production (e.g., sepsis, congestive heart failure, reduced tissue perfusion and hypoxia) also increase the risk of metformin toxicity, with resultant acidemia and elevated lactate concentration [Citation9].
Metabolic acidosis with hyperlacatemia associated with metformin toxicity can be severe or fatal [Citation10]. In acute overdose of metformin, patients who died have much lower serum pH nadirs and much higher serum lactate and metformin concentrations than those who survived [Citation11]. In metformin-associated lactic acidosis, patients usually have other illnesses such as sepsis or kidney failure and the receiver operating characteristic curves show poor predictive power of arterial pH and serum lactate concentration for mortality [Citation10]. In the case of severe metformin poisoning, extracorporeal treatments are recommended [Citation12,Citation13].
This study aimed to analyse the demographic, clinical and death data reported to the National Poisons Information Service to ascertain patient demographics and assess the relationship between serum lactate concentration, arterial pH and ingested dose with severity of poisoning. Another aim was to identify if serum lactate concentration is a useful marker of severity in metformin toxicity.
Methods
The United Kingdom National Poisons Information Service (www.npis.org) provides clinical management advice and evidence based toxicological information to healthcare professionals through its online database TOXBASE® and a 24-hour telephone service. This 24-hour telephone service covers the United Kingdom (UK) as well as the Republic of Ireland overnight.
For each patient who is discussed over the telephone, a record is created on the United Kingdom Poisons Information Database. A retrospective study of telephone enquiries from hospitals to the National Poisons Information Service relating to metformin, including combination products was undertaken. Enquiries included in the study were recorded between 1 January 2010 and 31 December 2019. Population death data related to metformin poisoning between 2010 and 2019 registered in England and Wales were obtained from the Office for National Statistics.
The data were extracted from the United Kingdom Poisons Information Database using the following search strings: enquiry date, source of the enquiry, age, gender, weight, clinical features, laboratory investigations, medical history, drug(s) name, exposure type (acute, acute on therapeutic, sub-acute, chronic), drug amount(s), exposure duration(s), incident location, circumstances, Poisoning Severity Score and enquiry outcome (including follow up data if recorded).
We included all cases that related to ingestions of metformin or metformin with other drugs (including combination products) and calls originated from a hospital. Metformin preparations included metformin, Avandamet (rosiglitazone + metformin) and metformin + gliclazide. Cases were excluded if the enquiry did not originate from a hospital or did not relate to a metformin exposure. Duplicate enquiries regarding one patient were collated. Cases with a Poisoning Severity Score of ‘None’, representing asymptomatic cases were excluded when analysing ingested dose, arterial pH and serum lactate concentration.
Clinical features and laboratory findings are defined as per the reference ranges used within the National Poisons Information Service. The term ‘lactic acidosis’ is defined herein as a metabolic acidosis with an arterial pH of less than 7.35 and a serum lactate concentration >2.2 mmol/L. Causation of symptoms cannot be determined due to lack of analytical confirmation; patient medical history and case follow up information. Therefore, any cases reported as lactic acidosis were considered as metformin-associated lactic acidosis.
The Poisoning Severity Score measures the severity of clinical features at the time of enquiry (or within an hour of the call) according to specific criteria [Citation14]. This ranges from none (asymptomatic patients) to minor (transient, mild), moderate (pronounced or prolonged) and severe (or life-threatening) clinical features, as well as fatalities. The Poisoning Severity Score was recorded by the Specialist in Poisons Information, based on the information provided by the treating clinician. The maximum Poisoning Severity Score was not used in the analysis, due to a lack of complete case follow up information.
The data were analysed using Excel (Office 365 Subscription, Microsoft Corporation, Redmond WA, United States) and GraphPad Prism (GraphPad Software, San Diego CA, United States). Cases were divided into two groups, those with reported ingestions of only metformin and those with reported ingestions of metformin with other drugs. Data were presented as count, percentages, median and interquartile range (IQR). Odds ratios were used to analyse prognostic factors of mortality. Chi-squared or Fisher’s exact test was used to compare proportions. The Mann-Whitney U test was used to compare non-parametric data. The Kruskal Wallis H test (one-way ANOVA on ranks) was used to compare ingested metformin dose, arterial pH and serum lactate concentration for each Poisoning Severity Score. Post-hoc testing was performed using Dunn’s test with Bonferonni correction for multiple comparisons of independent samples. Pearson’s coefficient was used to determine correlation. A significance level (α) of less than 0.05 was considered significant.
A national toolkit provided by the Health Research Authority in the UK (http://www.hra-decisiontools.org.uk/ethics/) indicates that approval from a Research Ethics Committee is not required for studies that use information collected routinely in any UK administration as part of usual clinical care, provided this information is passed to the researchers in a fully anonymised format.
Results
The National Poisons Information Service received 3,134 telephone enquiries involving metformin between 2010 and 2019. A total of 693 enquiries were hospital based. The number of enquiries from hospitals involving metformin poisoning fell over the ten-year period by 3.6% (P = 0.03).
Exposures
Six-hundred and thirty-seven individual cases were identified. The demographic information regarding these patients is shown (). Four cases included metformin as a combination product (metformin + gliclazide). However, all four cases also involved other drugs and were classified in the metformin with other drugs group. Medical history was recorded in 154 (24%) cases, only five patients reported pre-existing kidney impairment prior to the enquiry.
Table 1. The demographics, median ingested dose, Poisoning Severity Score and laboratory findings in patients with metformin only and metformin with other drugs exposures.
In the metformin with other drugs cases, 168 individual co-ingestants were identified. The median number of co-ingestants reported was 5 (IQR 3-7). The most common co-ingestants for each Poisoning Severity Score is reported (). The common drug classes of reported co-ingestants did not differ across the Poisoning Severity Scores (P = 0.21). There was no difference between the observed number of metformin with other drugs cases for each Poisoning Severity Score from the expected number of cases (P = 0.06).
Table 2. Most common co-ingested drugs in patients who reportedly ingested metformin with other drugs, for each Poisoning Severity Score.
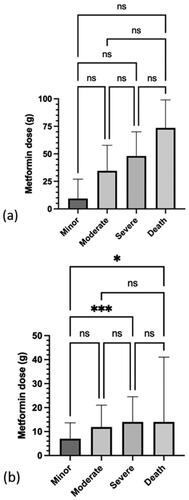
For metformin only exposures (), the trend for a dose-response relationship was not statistically significant. For metformin with other drugs exposures (), a significant difference was observed between the ingested dose for a minor versus severe Poisoning Severity Score (P = 0.002) and a minor Poisoning Severity Score versus death (P = 0.019). The median dose for intentional exposures was significantly different from the median dose for unintentional or therapeutic error cases (P < 0.0001) ().
Clinical features
Four hundred and thirty-five (63%) cases were symptomatic. The most common reported clinical features for metformin only exposures () and metformin with other drugs () exposures are reported. The reported dose required to cause clinical features in metformin only cases (including physical symptoms or abnormal laboratory results) in 50% of symptomatic cases was 36.7 g (95% confidence interval (CI) 25.5–90 g). The reported dose required to cause clinical features in metformin with other drugs cases in 50% of symptomatic cases was 10.3 g (95% CI 10–10.7 g).
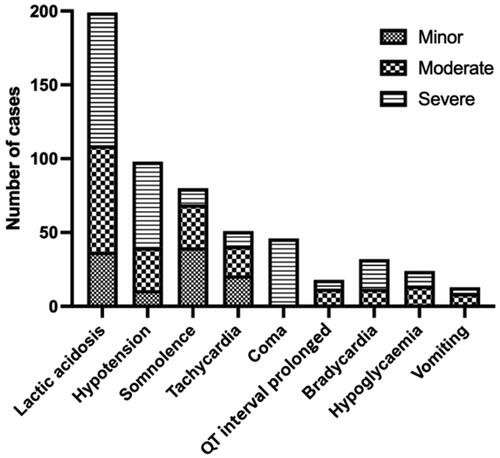
Figure 2. Frequency of the most common clinical features for each Poisoning Severity Score in patients who ingested metformin only (n = 117). Lactic acidosis (metabolic acidosis with arterial pH <7.35 and serum lactate concentration >2.2 mmol/L); abnormal kidney function (serum creatinine >120 μ/L); hypoglycaemia (blood glucose <3.9 mmol/L, 70 mg/dL); hypotension (blood pressure <90/60 mmHg); hyperkalaemia (serum potassium >5.3 mmol/L); acute kidney failure (anuria, serum creatinine >550 μmol/L).
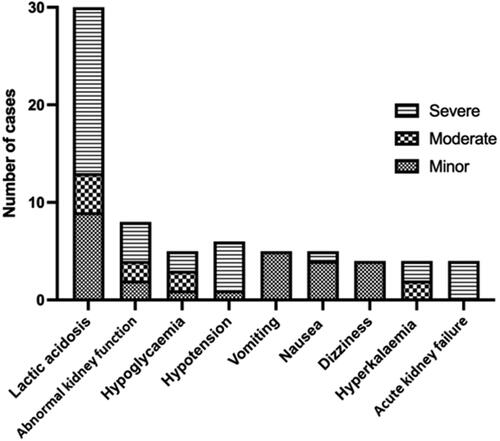
Figure 3. Frequency of the most common clinical features for each Poisoning Severity Score in patients who ingested metformin with other drugs (n = 520). Lactic acidosis (metabolic acidosis with arterial pH <7.35 and serum lactate concentration >2.2 mmol/L); hypotension (blood pressure <90/60 mmHg); somnolence (sedation, drowsiness, sleepiness); tachycardia (heart rate elevated above normal according the age of the patient); coma (unconsciousness); QT prolongation (QTc >450 ms); bradycardia (heart rate <60 beats/minute); hypoglycaemia (blood glucose <3.9 mmol/L, 70 mg/dL).
The distribution of Poisoning Severity Scores differed between the metformin only and metformin with other drugs groups, which is shown in (P < 0.0001). Lactic acidosis was reported in 232 (36%) cases; 33 were metformin only and 199 were metformin with other drugs exposures. Impaired kidney function (including kidney failure) was reported in 49 (7%) cases and was not associated with lactic acidosis following metformin exposure (P = 0.99).
Hypoglycaemia (blood glucose <3.9 mmol/L, 70 mg/dL) was reported in 38 cases. Twenty-three (71%) of the metformin with other drugs exposures included another antidiabetic drug as a co-ingestant. The median blood glucose concentration for all cases was 6.5 mmol/L (117 mg/dL) [IQR 5–12 mmol/L] (P = 0.53).
Potential biomarkers of severity of metformin poisoning
Serum metformin concentration was only reported in one case of metformin with other drugs exposure. Lactic acidosis was reported as a clinical feature in 232 cases, of which 162 had confirmed laboratory results. Median arterial pH and serum lactate concentration values were analysed separately.
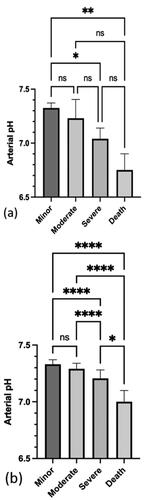
Recorded arterial pH for cases is reported (). Arterial pH inversely correlated with reported metformin dose (r =-0.3, P = 0.0003). Arterial pH did not correlate with the serum lactate concentration for either metformin only exposures (r=-0.3, P = 0.2) or metformin with other drugs exposures (r = 0.01, P = 0.9). The relationship between arterial pH and the Poisoning Severity Score is shown for patients who reportedly ingested metformin only (). There was a significant difference in arterial pH between the minor versus severe Poisoning Severity Score (P = 0.0121) and for minor versus death (P = 0.0061). For the metformin with other drugs () cases, there was a significant difference between the minor versus severe Poisoning Severity Score (P < 0.001), minor versus death (P < 0.001), moderate versus severe (P < 0.001), moderate versus death (P < 0.001) and the severe versus death cases (P = 0.0172).
Figure 4. Median and IQR of arterial pH for each Poisoning Severity Score in: (a) patients who ingested metformin only (n = 117) and (b) patients who ingested metformin and other drugs (n = 520). *P<0.05, **P<0.01, ****P<0.0001, and ns: not significant.
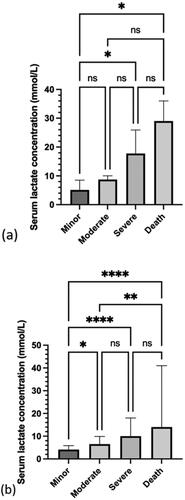
The median serum lactate concentration for metformin only and metformin with other drugs is reported for each Poisoning Severity Score (). Serum lactate concentration correlated with reported metformin dose (r = 0.37, P < 0.0001). The relationship between serum lactate concentration and Poisoning Severity Score is reported for metformin only (). There was a significant difference between the minor versus severe Poisoning Severity Score (P = 0.0319) and the minor versus death (P = 0.0176). For the metformin with other drugs cases (), there was a significant difference in serum lactate concentration between the minor versus moderate Poisoning Severity Score (P = 0.0137), the minor versus severe (P < 0.001), the minor versus death (P < 0.001) and the moderate versus death (P = 0.0072).
Figure 5. Median and IQR of serum lactate concentration for each Poisoning Severity Score in: (a) patients who ingested metformin only (n = 117) and (b) patients who ingested metformin with other drugs (n = 520). *P<0.05, **P<0.01, ****P<0.0001, and ns: not significant.
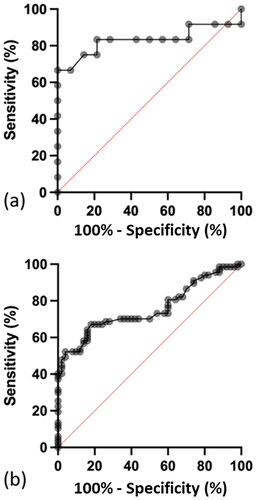
The optimum serum lactate concentration to distinguish between a Poisoning Severity Score of minor versus severe for patients with reported metformin only exposures is greater than 11.0 mmol/L () and greater than 11.4 mmol/L for patients who reported exposures to metformin with other drugs (), respectively.
Figure 6. Receiver operating characteristic (ROC) curve for serum lactate concentration for a Poisoning Severity Score of minor versus severe in: (a) patients who ingested metformin only. Sensitivity of 75% (95% CI 47–97%) and specificity of 86% (95% CI 60–97%). The optimum serum lactate concentration to distinguish between a minor versus a severe Poisoning Severity Score is 11.0 mmol/L and (b) Patients who ingested metformin with other drugs. The sensitivity was 48% (95% CI 36–60%) and specificity 98% (95% CI 90–100%). The optimum serum lactate concentration to distinguish between a minor versus a severe Poisoning Severity Score is 11.4 mmol/L.
Metformin related deaths
Three deaths involving one male and two females were recorded following acute intentional ingestions of metformin alone. Patient ages were 32, 47 and 53 years. The reported metformin doses resulting in death were 48 g and 99 g. The recorded arterial pH ranged from 6.69 to 6.9. Serum lactate concentrations were between 25–36 mmol/L.
Twenty-two deaths occurred following a metformin with other drugs overdose. The median age of the patients who died was 52.5 years (IQR 44.5–55.75). Seventeen (77%) patients were male and 5 were female. All of the deaths occurred following intentional exposures and 20 (90%) were acute exposures. The lowest recorded metformin dose resulting in death was 5.25 g (55 mg/kg). The median metformin dose, arterial pH and lactate concentration resulting in death are reported ().
Poor prognostic factors for an outcome of death versus a minor, moderate or severe Poisoning Severity Score or being asymptomatic included: male sex (OR 2.76, 95% CI 1.13–6.69), aged over 40 years (OR 2.86, 95% CI 0.96–8.47) and taking a metformin with other drugs overdose (OR 1.68, 95% CI 0.49–5.71).
The Office for National Statistics registered 66 deaths relating to metformin poisonings between 2010–2019 in England and Wales. Twenty of these deaths were due to intentional self-harm [Citation15]. Twenty-one of the deaths reported to the National Poisons Information Service were in England and Wales. The remaining three deaths were reported from hospitals in Scotland and one death was reported from the Republic of Ireland.
Discussion
A higher serum lactate concentration and worsening arterial pH were each independently associated with an unfavourable Poisoning Severity Score in both metformin only and metformin with other drugs exposures. Our dataset suggests that serum lactate concentration in patients with metformin toxicity could be a useful biomarker of poisoning severity in both single agent and multi-drug exposures.
The majority metformin exposures were acute and intentional. A minority were due to therapeutic errors (unintentional extra doses, wrong dose administration, etc.), including acute or staggered exposures. Consistent with the literature, most patients were adults. The smaller proportion of paediatric cases consisted mostly of unintentional exposures and severe cases were uncommon [Citation16,Citation17].
Previous studies have primarily investigated metformin in terms of single-drug exposure [Citation6,Citation16]. Our dataset was varied in terms of the ingested dose, circumstances of exposure, age, and often involved multiple co-ingestants. The distribution of cases between the Poisoning Severity Scores was significantly different between the metformin only and metformin with other drugs cases. A higher proportion of severe cases were metformin with other drugs cases, whilst a higher proportion of asymptomatic cases were metformin only cases.
For reported metformin only cases, the trend for a dose-response relationship between ingested metformin dose and Poisoning Severity Score was not statistically significant, possibly due to the small sample size. As for metformin with other drugs cases, some dose-response relationship occurred, with significant differences in the ingested doses for a minor versus severe Poisoning Severity Score and a minor Score versus death. The median metformin dose was higher in acute intentional overdoses than those categorised as therapeutic errors.
A positive correlation was found between serum lactate concentration and the reported ingested metformin dose in metformin with other drugs cases, suggesting a higher dose may result in elevated serum lactate concentrations. Similarly, arterial pH inversely correlated with ingested dose for all cases, suggesting the degree of metabolic acidosis is associated with dose ingested.
As with ingested dose, serum lactate concentrations correlated with a worsening Poisoning Severity Score for all cases, although this did not reach statistical significance for metformin only cases, likely due to sample size. As is expected from the Poisoning Severity Score criteria [Citation14], there is a relationship between a lower recorded arterial pH and worsening Poisoning Severity Scores. Serum lactate concentration reference ranges are not included within the Poisoning Severity Score criteria [Citation14]. However, as arterial pH and serum lactate do not correlate for metformin only or metformin with other drugs cases, the serum lactate concentration can be used as an indicator of poison severity and is independent of arterial pH.
Risk factors for metabolic acidosis with hyperlactatemia in the literature for patients taking metformin therapeutically included being elderly, decreased cardiac, hepatic or kidney function and hypoxaemia [Citation17]. The risk factors in our study were based on the risk of death versus absence of clinical features or recovery among patients. It could not be determined if the subjects in our dataset were prescribed metformin due to a lack of medical history information. Poor prognostic factors included male gender, over 40 years of age, and taking a metformin with other drugs overdose.
Limitations
Poisons Centre data is at risk of potential reporting error and bias. The National Poisons Information Service does not have access to medical records and case follow up information is limited as not all cases are routinely followed up. The ingested metformin dose is based on the patients’ own account and may be unreliable. Analytical confirmation was not routinely possible for serum metformin concentration. Serious or more complicated cases may be overrepresented. The Poisoning Severity Score is based on the clinical condition of the patient at the time of the enquiry and is not specifically based on the serum lactic concentration and, therefore other factors may be confounding the severity of poisoning. Clinicians may use other sources of information first and are not obliged to refer cases to the National Poisons Information Service.
Conclusions
The majority of cases included acute and intentional overdose with metformin, most of which also involved co-ingestants. A dose-response relationship was identified with worsening Poisoning Severity Scores, arterial pH and serum lactate concentration. Arterial pH and serum lactate concentration demonstrated independent relationships with worsening poisoning severity. Data from the present study suggest that serum lactate concentration can be used to assess severity of poisoning in metformin only and metformin with other drugs exposures.
Acknowledgements
The authors are grateful to the Office for National Statistics (ONS) for providing the data for death related to drug poisoning for England and Wales.
Disclosure statement
No specific funding was received for this research. The Authors salaries are paid in part or in full by contributions from the UK Health Security Agency. The UK Health Security Agency, however, had no input into the study or production of the manuscript.
Additional information
Funding
References
- Arroyo AM, Walroth TA, Mowry JB, et al. The MALAdy of metformin poisoning: is CVVH the cure? Am J Ther. 2010;17(1):96–100.
- Brassøe R, Elkmann T, Hempel M, et al. Fulminant lactic acidosis in two patients with type 2 diabetes treated with metformin. Diabet Med. 2005;22(10):1451–1453.
- Bebarta VS, Pead J, Varney SM. Lacticemia after acute overdose of metformin in an adolescent managed without intravenous sodium bicarbonate or extracorporeal therapy. Pediatr Emerg Care. 2015;31(8):589–590.
- Harvey B, Hickman C, Hinson G, et al. Severe lactic acidosis complicating metformin overdose successfully treated with high-volume venovenous hemofiltration and aggressive alkalinization. Pediatr Crit Care Med. 2005;6(5):598–601.
- Elshafei MN, Alamin M, Mohamed MFH. Osmolar-gap in the setting of metformin-associated lactic acidosis: case report and a literature review highlighting an apparently unusual association. Medicine (Baltimore). 2020;99(41):e22492.
- Chiew AL, Wright DFB, Dobos NM, et al. ‘Massive’ metformin overdose. Br J Clin Pharmacol. 2018;84(12):2923–2927.
- Haloob I, de Zoysa JR. Metformin associated lactic acidosis in Auckland city hospital 2005 to 2009. World J Nephrol. 2016;5(4):367–371.
- Corchia A, Wynckel A, Journet J, et al. Metformin-related lactic acidosis with acute kidney injury: results of a french observational multicenter study. Clin Toxicol (Phila). 2020;58(5):375–382.
- DeFronzo R, Fleming GA, Chen K, et al. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism. 2016;65(2):20–29.
- Blumenberg A, Benabbas R, Sinert R, et al. Do patients die with or from metformin-associated lactic acidosis (MALA)? systematic review and meta-analysis of pH and lactate as predictors of mortality in MALA. J Med Toxicol. 2020;16(2):222–229.
- Dell’Aglio DM, Perino LJ, Kazzi Z, et al. Acute metformin overdose: examining serum pH, lactate level, and metformin concentrations in survivors versus nonsurvivors: a systematic review of the literature. Ann Emerg Med. 2009;54(6):818–823.
- Calello DP, Liu KD, Wiegand TJ, et al. Extracorporeal treatment for metformin poisoning: systematic review and recommendations from the extracorporeal treatments in poisoning workgroup. Crit Care Med. 2015;43(8):1716–1730.
- Correia MS, Horowitz BZ. Continuous extracorporeal clearance in metformin-associated lactic acidosis and metformin-induced lactic acidosis: a systematic review. Clin Toxicol (Phila). 2022;60(11):1266–1276.
- Persson HE, Sjöberg GK, Haines JA, et al. Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol. 1998;36(3):205–213.
- Breen P, Manders B. Deaths related to drug poisoning in England and Wales: 2020 registrations [Dataset]. Office for National Statistics. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bu lletins/deathsrelatedtodrugpoisoninginenglandandwales/2020
- Stevens A, Hamel JF, Toure A, et al. Metformin overdose: a serious iatrogenic complication-Western France poison control Centre data analysis. Basic Clin Pharmacol Toxicol. 2019;125(5):466–473.
- Bryant SM, Cumpston K, Lipsky MS, et al. Metformin-associated respiratory alkalosis. Am J Ther. 2004;11(3):236–237.