ABSTRACT
Introduction: The use of steroids has been proposed as a pharmacological approach to treat the SARS-CoV-2 infection to improve outcomes. However, there are doubts about safety against the development of superinfections and their worse outcomes.
Objective: To establish the relative frequency of superinfection associated with using steroids in patients with SARS-CoV-2 infection.
Materials and methods: We conducted a systematic literature review and meta-analysis using PRISMA standards in 5 databases (PubMed/Scopus/Cochrane/EMBASE/Google Scholar). The search was carried out between February 2020 and May 2023. The search terms were ‘steroids’ or ‘superinfection’ ‘and’ followed by ‘SARS-CoV-2’ or ‘COVID-19’.
Results: We found 77 studies, but only 10 with 3539 patients were included in the systematic review. All patients developed severe disease. The documented OR for superinfection through the meta-analysis was 1.437 (95% IC 0.869–2.378) with a p-value of 0.158 without showing a risk attributed to steroids and the development of superinfections. In the Funnel-plot analysis, no publication biases were found.
Conclusion: No relationship was found between using steroids and superinfection in patients with SARS-CoV-2.
Introduction
More than 20 million cases of SARS-CoV-2 have been reported globally since the end of 2019 when the new coronavirus was identified as a cause of viral pneumonia in Wuhan, a city in China’s Hubei province [Citation1,Citation2.] However, the reported cases underestimate the total number of them because almost 80% of patients with the infection are asymptomatic, and the remaining 20% may have mild symptoms in such a way that they do not access the services of health and are not included in the epidemiological surveillance of local authorities. The seropositivity estimated according to European and United States studies suggests that it exceeds the number of reported cases up to 10 times [Citation3]. Approximately 20% of individuals who contract SARS-CoV-2 experience a decline in their condition that necessitates hospitalization and the use of oxygen therapy, either through and oxygen cannula or a non-rebreather mask. For the infected patients, up to 15% can present a severe version of the disease and 5% a critical illness requiring invasive ventilatory support; However, these numbers have varied between the different reported cohorts, and this difference has been attributed to the distribution of the population pyramid and the prevalence and risk control levels of non-communicable diseases in each country [Citation4,Citation5].
Virus identification has evolved during the pandemic to more sophisticated ways [Citation6]. These advances made it possible to identify genes involved and their association with clinical manifestations from mild to some more severe. Although initially it was believed that the compromise was just in the respiratory system, it has also been possible to identify the affectation generated in other organs and its implications in the progression of the disease [Citation7,Citation8].
The general mortality reported has varied between 2–4%; however, in hospitalized patients, this value can reach 26% and 37% in critical patients [Citation9]. Given the severity and implications of the outcomes, if the period of illness is exceeded, different treatment schemes have been evaluated to reduce hospital stays since the deterioration in the quality of life and functionality of patients who are admitted to an intensive care unit (ICU) represent a consequence that severely affects individuals with a critical illness [Citation10].
Many studies have described coinfection in the group of patients hospitalized for COVID-19, but the reasons have only been speculated. Among the variables analyzed, the role of the bacterial and fungal microbiota with the outcomes in the disease process has been described but so far with inconclusive information [Citation11,Citation12].
Among the strategies that have emerged as potentially beneficial have been the use of steroids, particularly in the RECOVERY study, which included an intervention arm with dexamethasone, which showed a reduction in the progression to critical illness in hospitalized patients and mortality in critical patients; representing so far the only pharmacological intervention with evidence of benefit in patients with SARS-CoV-2 infection [Citation13]. According to the literature review, there is insufficient evidence to establish an association between steroid use and superinfection in patients with SARS-CoV-2.
Materials and methods
Research question: What is the frequency of bacterial, viral, or fungal superinfection in patients with SARS-CoV-2 infection hospitalized in the general ward and the critical care unit receiving oral or intravenous steroid management?
Protocol and registration
This protocol follows the recommendations established by PRISMA statement [Citation14] and has been registered in PROSPERO: CRD42020207970.
Data sources and searches
According to PRISMA standards, we conducted a systematic review and meta-analysis in five databases (PubMed/Scopus/Cochrane/EMBASE/Google Scholar) to assess the relationship between steroid use and superinfections in SARS-CoV-2 in-hospital patients. We also searched gray literature. The authors selected keywords based on MeSH terms. The search was carried out between February 2020 and May 2023. The search terms were ‘steroids’ or ‘superinfection’ ‘and’ followed by ‘SARS-CoV-2’ or ‘COVID-19’. In addition, the superinfection detection methods, the data regarding mortality, comorbidities, follow-up time, stay in the ICU, and the steroid protocol used was considered.
Definition of criteria
Infection by SARS-CoV-2 or COVID-19: Patients hospitalized in the general ward or the critical care unit with the diagnosis made by antigenic or serological detection of viral RNA by Polymerase Chain Reaction (PCR). Imaging diagnosis was not included to reduce bias by diagnostic criteria according to country of origin.
Bacterial, viral, or fungal superinfection: A patient diagnosed with SARS-CoV-2 who presents with a superinfection documented by blood cultures or processing of multiple molecular identification tests of microorganisms duly reported in the articles. Cultures of secretions were not included to reduce the bias due to overdiagnosis of superinfection.
Use of steroids: A patient hospitalized in the general ward or the critical care unit with a diagnosis of SARS-CoV-2 who is receiving any steroid orally, intravenously, or other routes.
Selection criteria
Study selection and data extraction were performed independently by two authors.
Observational and analytical studies were included that evaluated the frequency of superinfection in patients with SARS-CoV-2 infection who were also receiving steroids either in the setting of inpatient wards or in the critical care unit. Studies that did not include data on mortality outcomes, time of steroid administration, and method by which superinfection was documented were excluded. Case reports, topic reviews, protocols, opinion articles, or those in a language other than those already defined (Spanish- English) were additionally excluded. (See the full search strategy in supplementary material).
Statistical analysis
The unit of discordance was adjusted by converting the units reported to standardized measures that allow comparison. The central tendency and dispersion measurements were calculated according to the variable type. The average units used in the studies were reported since the original individual values were unavailable. A random-effects meta-analysis model was proposed. For the outcome of categorical variables such as mortality, need for mechanical ventilation, or admission to ICU, risk ratios were established with a 95% confidence interval. The heterogeneity measures I2 index was estimated, and the Tau-squared test. Since the SARS-CoV-2 publications are grouped from 2019 to 2023, estimating a retrospective search limit is not considered. The comprehensive Meta-Analysis and the Microsoft Excel spreadsheet program used for the forest plots was Stata v.15, licensed from the Universidad Tecnológica de Pereira.
Results
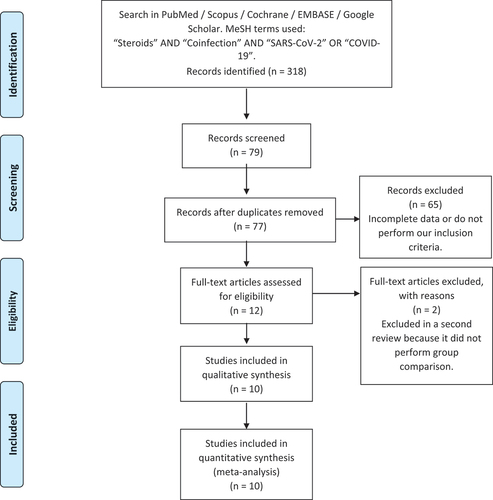
After the literature review, 77 articles were entered into the database and were reviewed in their entirety. Considering the inclusion and exclusion criteria by the researchers, 10 studies were finally used in the analysis. 3539 patients were grouped (1668 patients who received steroids and 1871 patients from the control group). The selection flowchart is shown in .
Two of the ten reviewed studies corresponded to randomized clinical trials; the other eight were observational studies. All were carried out and reported between 2020 and 2022, including papers greater than 100 subjects with different follow-up times and patients with a stay in critical care. The mean age in the 10 groups was over 62 years. The remaining study population had a mean age of 56. Most had a SARS-CoV-2 molecular diagnosis. shows the characteristics of the patients evaluated in the included studies, and shows the features according to the evaluation group (steroids and control). In addition, the number of patients on mechanical ventilation is reported.
Table 1. General characteristics of the population of patients with COVID-19 in the studies.
Table 2. General characteristics of the patients discriminated by group of steroids and control.
The comorbidities reported in the studies were mainly diabetes mellitus, arterial hypertension, a history of coronary disease or heart failure, obesity, chronic kidney disease, cancer, and chronic obstructive pulmonary disease. shows the relationship between the frequencies of comorbidities according to the group of steroids and control.
Table 3. Frequency distribution of comorbidities reported in patients with COVID-19.
It was impossible to extract precise information about hospital stay from the studies, except for the days of follow-up. However, it was not discriminated for the comparison of groups. Data on mortality are presented in .
Table 4. Mortality discriminated by steroid use and control group.
In evaluating the frequency of superinfection in the selected studies, only one study reported the method of identification of superinfection. The steroid regimens used were as follows:
Dequin, P. F., et al.: hydrocortisone 200 mg/day IV for 7 days, followed by 100 mg/day for 4 days and 50 mg/day for 3 days. The total duration of the scheme is 14 days.
Gordon, Anthony C., et al.: dexamethasone 20 mg/day IV for 5 days, followed by 10 mg/day for 5 more days. The total duration of the scheme was 10 days.
Mikulska, Malgorzata, et al.: methylprednisolone 1 mg/kg for 5 days and followed by 0.5 mg/kg for 5 days. The total duration of the scheme was 10 days.
Ramiro, Sofia, et al.: methylprednisolone 250 mg/day IV on day 1, followed by 80 mg/day from day 2 to 5. The total duration of the scheme was 5 days.
Brosnahan, Shari B, et al.: methylprednisolone with an average daily dose and duration (days) of 94.7 mg and 5.4, respectively. Dexamethasone with an average daily dose and duration (days) of 12 mg and 4.8, respectively. Hydrocortisone with an average daily dose and duration (days) of 172 mg and 5.7, respectively. Prednisone with an average daily dose and duration (days) of 28.7 mg and 7.2, respectively. This study did not report the administration route.
Almas, Talal, et al.: oral prednisone 20 mg/day, with a mean follow-up of 7 days.
Monedero, Pablo, et al.: methylprednisolone, dexamethasone, and hydrocortisone. This study did not report either the administration route or follow-up days.
Cour, Martin, et al.: dexamethasone 6 mg/day IV with a mean follow-up of 10 days.
Tran, Viet-Thi, et al.: methylprednisolone, dexamethasone, and hydrocortisone. Dose of: ≥0.8 mg/kg/day prednisone dose or ≥0.4 mg/kg/day (equivalent dose), oral, with a mean follow-up of 7 days. The total duration of the scheme was 11 days.
Søvik Signe, et al.: dexamethasone i.v. 6 mg x 1 or equivalent dose of methylprednisolone (nine patients) for a median of 11 days (8-16).
shows the frequency of superinfection discriminated by a group of steroid use and control. and present the meta-analysis evaluated by Odds Ratio (OR), which does not show statistically significant associations between the use of steroids and the risk of superinfection (p = 0.158), also with a distribution of the confidence intervals in , which shows a distribution that explains the non-finding of statistical significance given that it does not favor the outcome in any of the studies and neither in the global evaluation. Finally, shows the funnel – plot performed to analyze publication bias.
Figure 2. Confidence intervals evaluated through meta-analysis.
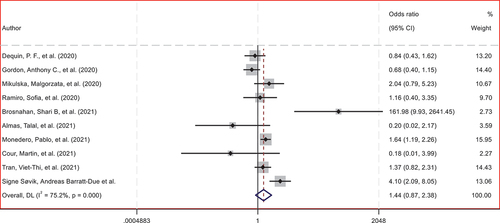
Figure 3. Analysis of publication bias using funnel-plot generated by OR.
Table 5. Frequency of superinfection discriminated by steroid use and control group.
Table 6. Meta-analysis of steroid use and risk of superinfection.
Discussion
In the present meta-analysis, which included 2 Randomized Controlled Trials, 8 Observational Studies, and 3539 patients with severe COVID-19, corticosteroid treatment was not associated with increased superinfections.
Due to their anti-inflammatory and immunosuppressive properties, corticosteroids have been widely used in various conditions, including rheumatologic, pulmonary, and infectious diseases. Additionally, these effects generate a predisposition for the development of infections [Citation15].
Observational studies are generally consistent in the risk of infection using corticosteroids, while randomized controlled trials do not show a significant association [Citation16]. The opposite case happens in chronic respiratory diseases. Various studies have shown that in conditions such as asthma and COPD, the use of inhaled corticosteroids has been associated with an increase in the incidence of upper respiratory tract infections [Citation17,Citation18], including severe pneumonia [Citation19].
In severe community-acquired pneumonia, recent systematic reviews and meta-analyses of randomized controlled trials have demonstrated its effectiveness as an adjunct therapy in adults. Corticosteroids reduce both all-cause mortality and in-hospital mortality, the need for mechanical ventilation, length of stay in the ICU, and incidence of septic shock and acute respiratory distress syndrome (ARDS) [Citation20,Citation21].
There is now evidence to support the use of corticosteroids and low-quality evidence that may go against it. However, it should be interpreted with caution to avoid bias since the information is inconclusive due to the lack of large studies. For example, an observational study concluded that patients with ARDS and COVID-19 showed reduced mortality in corticosteroid therapy patients. On the other hand, low-quality evidence was also reported where patients with severe COVID-19 and corticosteroid therapy increased the possibility of increased mortality. There is also indirect evidence from observational studies of severe COVID-19 by SARS and MERS; in the first case affecting the virus clearance and in the second case, decreased mortality associated with the use of corticosteroids [Citation22].
For its part, the possibility of viral-associated hyperinflammatory syndromes (e.g. MIS-C or MIS-A) cannot be left aside, with all the cellular and molecular implications that are still being studied and could become an argument for or against the use of corticosteroids in the future [Citation23].
So far, the effectiveness of corticosteroids in viral respiratory infections was first demonstrated in the RECOVERY trial [Citation24]. This randomized controlled trial showed decreased mortality at 28 days in critically ill patients with severe COVID-19 in the group that received dexamethasone (adjusted RR 0.83 (95% CI: 0.74–0.92) [Citation13].
The effects on reducing mortality, disease progression, and the need for mechanical ventilation in critically ill adult patients have been replicated in other randomized controlled trials [Citation25]. These beneficial effects of corticosteroids are not preserved in patients with non-severe COVID-19 [Citation26]. This may be associated with the fact that corticosteroids could prolong viral shedding in these non-severe cases, as reported by Tang X et al. in their randomized control trial [Citation27]. In contrast, it is worth mentioning that most countries have moved away from costly PCR testing for COVID-19 and only adopt antigen rapid tests or lateral flow tests especially for community-dwelling individuals. Being relevant since the testing accuracy is lower with the lateral [Citation28] flow tests, compared to rt-PCR [Citation29].
Similar findings were observed in patients with non-severe community-acquired pneumonia concerning mortality, adding to the hospital readmission of these patients [Citation30]. This suggests that corticosteroid effects depend on the degree of severity of limited respiratory diseases of infectious origin [Citation31].
Limitations
During the review, very few studies were identified that met the selected quality criteria, even though the literature generated between 2020 and 2023 related to SARS-CoV-2 has been of a significant amount. Although this finding obtained through this meta-analysis support the safety of using steroids in patients with COVID-19, the studies evaluated are few. Furthermore, it is not specifically designed to assess the incidence of superinfection associated with using steroids.
It was necessary to run a random effects model due to the heterogeneity of the studies (I-squared = 75.2%), that is, the proportion of the total variation attributable to heterogeneity, which in the case of this study is considered between moderate and high. (The fixed effects method could not be run because the heterogeneity of the studies was greater than 50%). In addition, there was also heterogeneity in terms of the type of design of the included studies, number of participants, time, and dose of steroids.
Conclusions
No statistically significant increase in superinfections was found in COVID-19 patients receiving steroids. However, within the evaluated studies, there was no standardized administration protocol for the use of steroids or a clear methodology for the identification of superinfections; This situation does not preclude the continuous evaluation of the safety of the use of intravenous steroids in the patient with moderate or severe disease due to COVID-19.
There is little comparable literature in the main databases (PubMed/Scopus/Cochrane/EMBASE/Google Scholar) evaluating a standardized protocol for administering steroids in patients with critical illness due to COVID-19 and the incidence of superinfections as the primary outcome.
The summary measure establishes an overall OR of 1.437 in favor of coinfection due to steroid use, but with a CI ranging from 0.869 to 2.378 passing through one; thus, the result is insignificant (p-value = 0.158).
Recent studies have shed light on the relationship between corticosteroid treatment and the occurrence of superinfections in patients with SARS-CoV-2, the virus responsible for COVID-19. Contrary to initial concerns, it has been found that corticosteroid treatment is not associated with an increased incidence of superinfections in these patients.
Various research studies have explored the relative frequency of superinfections, which can be bacterial, viral, or fungal, in patients with SARS-CoV-2 who received steroid therapy. The reported range of superinfection frequency due to steroid use in these patients varies significantly, from 8.3% to 88.8%. This wide range suggests that multiple factors may contribute to the likelihood of superinfection development in these individuals.
Another crucial aspect investigated in these studies is the overall mortality rate among patients with SARS-CoV-2 infection and its potential association with using steroids. The findings across different research works indicate that the overall mortality rate can vary from 8.3% to 56.3%. Interestingly, no consistent association between using steroids and the mortality rate in patients with SARS-CoV-2 infection has been identified.
These research findings provide important insights into the use of corticosteroid treatment in patients with SARS-CoV-2. While corticosteroid treatment does not appear to increase the risk of superinfections in these patients, the relative frequency of such infections can vary significantly. Moreover, the mortality rate among patients with SARS-CoV-2 infection is influenced by various factors, with no consistent link established between the use of steroids and mortality. Continued research in this area will help enhance our understanding of the complex interplay between corticosteroid therapy, superinfections, and patient outcomes in the context of SARS-CoV-2 infection.
Author contributions
MGR: Study concept and design, drafting of the manuscript, critical revision of the manuscript for important intellectual content, original material and construction of images and tables, administrative, technical, and material support, and study supervision.
JAHM: Study concept and design, drafting of the manuscript, critical revision of the manuscript for important intellectual content, original material and construction of images and tables, administrative, technical, and material support, and study supervision.
GAMG: Study concept and design, drafting of the manuscript, critical revision of the manuscript for important intellectual content, original material and construction of images and tables, administrative, technical, and material support, and study supervision.
MAF: Revision of the manuscript, English translation. Administrative, technical, and material support.
JFGG: Critical manuscript revision for important intellectual content, study supervision. Interpretation of data, and drafting of the manuscript. Administrative, technical, and material support.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (12.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20008686.2023.2277000
Additional information
Funding
References
- Bedford J, Enria D, Giesecke J, et al. COVID-19: towards controlling of a pandemic. Lancet. 2020;395(10229):1015–10. doi: 10.1016/S0140-6736(20)30673-5
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1
- Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313–319. doi: 10.1016/S0140-6736(20)31304-0
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
- Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335–. doi: 10.1001/jama.2020.4344
- Halaji M, Farahani A, Ranjbar R, et al. Emerging coronaviruses: first SARS, second MERS and third SARS-CoV-2: epidemiological updates of COVID-19. Infez Med. 2020;28(suppl 1):6–17.
- Zandi M, Farahani A, Zakeri A, et al. Clinical symptoms and types of samples are critical factors for the molecular diagnosis of symptomatic COVID-19 patients: a systematic literature review. Int J Microbiol. 2021 Sep 6;2021:1–20. doi: 10.1155/2021/5528786.
- Hosseini P, Afzali S, Karimi M, et al. The coronavirus disease 2019 and effect on liver function: a hidden and vital interaction beyond the respiratory system. Rev Med Microbiol. 2021 Jan 19;33(1):e161–e179. doi: 10.1097/MRM.0000000000000267
- Murthy S, Gomersall CD, Fowler RA. Care for critically ill patients with COVID-19. JAMA. 2020;323(15):1499–1500. doi: 10.1001/jama.2020.3633
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648
- Malekifar P, Pakzad R, Shahbahrami R, et al. Viral coinfection among COVID-19 patient groups: an update systematic review and meta-analysis. Bio Med Res Int. 2021 Sep 3;2021:1–10. doi: 10.1155/2021/5313832.
- Soltani S, Zakeri A, Zandi M, et al. The role of bacterial and fungal human respiratory microbiota in COVID-19 patients. Bio Med Res Int. 2021 Feb 23;2021:1–13. doi: 10.1155/2021/6670798.
- Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19-preliminary report. N Engl J Med. 2020. doi: 10.1056/NEJMoa2021436
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement BMJ. BMJ. 2009;339(jul21 1):b2535. doi: 10.1136/bmj.b2535
- Williams DM. Clinical Pharmacology of Corticosteroids. Respir Care. 2018;63(6):655–670. doi: 10.4187/respcare.06314
- Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am. 2016;42(1):157-76, ix–x. doi: 10.1016/j.rdc.2015.08.004
- Yang M, Zhang Y, Chen H, et al. Inhaled corticosteroids and risk of upper respiratory tract infection in patients with asthma: a meta-analysis. Infection. 2019;47(3):377–385. doi: 10.1007/s15010-018-1229-y
- Chen H, Feng Y, Wang K, et al. Association between inhaled corticosteroids and upper respiratory tract infection in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. BMC Pulm Med. 2020;20(1):282. doi: 10.1186/s12890-020-01315-3
- Yang M, Du Y, Chen H, et al. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Int Immunopharmacol. 2019;77:105950. doi: 10.1016/j.intimp.2019.105950
- Jiang S, Liu T, Hu Y, et al. Efficacy and safety of glucocorticoids in the treatment of severe community-acquired pneumonia: a meta-analysis. Medicine (Baltimore). 2019;98(26):e16239. doi: 10.1097/MD.0000000000016239
- Huang J, Guo J, Li H, et al. Efficacy and safety of adjunctive corticosteroids therapy for patients with severe community-acquired pneumonia: a systematic review and meta-analysis. Medicine (Baltimore). 2019;98(13):e14636. doi: 10.1097/MD.0000000000014636
- Zhikang YE, Wang Y, Colunga-Lozano LE, et al. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2020;192(27):E756–E767. doi: 10.1503/cmaj.200645
- Wee Song YEO, Qin Xiang NG. Distinguishing between typical Kawasaki disease and multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2. Med Hypotheses. 2020;144:110263. doi: 10.1016/j.mehy.2020.110263
- Rafiullah M, Siddiqui K. Corticosteroid use in viral pneumonia: experience so far and the dexamethasone breakthrough in coronavirus disease-2019. J Comp Eff Res. 2020;9(18):1247–1254. doi: 10.2217/cer-2020-0146
- Ma S, Xu C, Liu S, et al. Efficacy and safety of systematic corticosteroids among severe COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials. Signal Transduct Target Ther. 2021;6(1):83. doi: 10.1038/s41392-021-00521-7
- Pasin L, Navalesi P, Zangrillo A, et al. Corticosteroids for patients with coronavirus disease 2019 (COVID-19) with different disease severity: a meta-analysis of randomized Clinical trials. J Cardiothorac Vasc Anesth. 2021;35(2):578–584. doi: 10.1053/j.jvca.2020.11.057
- Tang X, Feng YM, Ni JX, et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized Control trial. Respiration. 2021;100(2):116–126. doi: 10.1159/000512063
- Søvik S, Barrat-Due A, Kåsine T, et al. Corticosteroids and superinfections in COVID-19 patients on invasive mechanical ventilation. J Infect. 2022 Jul;85(1):57–63. doi: 10.1016/j.jinf.2022.05.015
- Ng QX, Lim YL, Han MX, et al. The performance of lateral flow tests in the age of the omicron: a rapid systematic review. Life (Basel). 2022 Nov 21;12(11):1941. doi: 10.3390/life12111941
- Briel M, Spoorenberg SMC, Snijders D, et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018;66(3):346–354. doi: 10.1093/cid/cix801
- Martin-Loeches I, Torres A. Corticosteroids for CAP, influenza and COVID-19: when, how and benefits or harm? Eur Respir Rev. 2021;30(159):200346. doi: 10.1183/16000617.0346-2020