ABSTRACT
Background: Changes in oral and hand hygiene behaviors have been reported during the COVID-19 pandemic in 2020 which may be associated with the incidence of the norovirus infection, a common cause of gastroenteritis.
Objective: To estimate the trends of detection rates of norovirus gastroenteritis and associated factors before COVID-19 in 2015–2019 and during the COVID-19 in 2020 in Guangxi, China.
Methods: A secondary analysis of Guangxi surveillance data of gastroenteritis patients was conducted. The detection rate in 2020 was predicted using an autoregressive integrated moving average modeland associated factors were analyzed using multiple logistic regression adjusted for interaction effects.
Results: Of 7,903 gastroenteritis patients, the overall detection rate of norovirus gastroenteritis was 12.8%, (14.3% before and 6.1% during COVID-19). Detection rates gradually decreased from 2015 to 2020, of which the slope of predicted line was slightly flatter than the actual line. The odds ratios of detection were double to triple increase during COVID-19 in the younger age group and having food intake outside their homes. Tourist city, season, and types of food were independent associated factors.
Conclusion: The detection rates were higher during the COVID-19 year among the population aged 45 years or less and those who consumed food outside their home.
Introduction
Acute gastroenteritis is a major cause of morbidity and mortality in children [Citation1], the 2nd leading cause of death and high burden of hospital admissions [Citation2,Citation3]. Viral gastroenteritis accounts for 70% of acute gastroenteritis in children [Citation4], and norovirus is the main pathogen causing 219,000 annual deaths [Citation5] and has contributed to major gastroenteritis outbreaks worldwide [Citation6,Citation7]. The Centers for Disease Control and Prevention (CDC) reported that norovirus gastroenteritis was common in both children and adults, varying across populations and geographic environments, and was associated with contaminated foods and poor hygiene behaviors [Citation8]. Following the appearance of the COVID-19 pandemic in 2020, changes in oral and hand hygiene behaviors have been reported [Citation9–11].
In China, due to an improving economy over the last few decades, the mortality from infectious diarrhea has declined, but the morbidity has remained stable [Citation12]. The norovirus is more prevalent not only in children but also in adults and the elderly [Citation13]. A study in the north of China reported that children aged 6–35 months had high rates [Citation12]. Previous studies and systematic reviews have found that a norovirus infection was associated with food-handling hygiene [Citation14], eating outside/uncooked foods [Citation15], drinking untreated water [Citation15], lack of personal hygiene [Citation15], household density [Citation16], and person-to-person was the most common form of transmission during a norovirus outbreak [Citation17]. However, all these related factors might have changed following the COVID-19 outbreak in January 2020. The Foodborne Disease Surveillance System of Guangxi Zhuang Autonomous Region has been in operation since 2012 with the aim to monitor the magnitude of gastroenteritis within the province.
During the COVID-19 outbreak, COVID-19 policies including wearing masks, social distancing, improved personal hygiene, and locking down public places and schools have been widely implemented worldwide [Citation9]. Various studies have found that all of these policies influenced fecal-to-oral disease transmission, including norovirus gastroenteritis [Citation18–20]. There have been few studies reporting the prevalence of norovirus gastroenteritis during the COVID-19 outbreak [Citation21,Citation22], and there have to date been no studies reporting the trends of this infection related to the COVID-19 period and related factors. This study aimed to estimate the trends of detection rates of norovirus gastroenteritis and explore the associated factors before and during COVID-19 pandemic in 2020 in Guangxi Zhuang Autonomous Region, Southern China.
Methods
Study design and setting
A secondary analysis of data from the Foodborne Disease Surveillance System of Guangxi Zhuang Autonomous Region, Southern China, during 1 January 2015 to 31 December 2020 was conducted. Guangxi Zhuang Autonomous Region is the permanent venue for the ASEAN Expo Exhibition which is related to Nanning, Guilin, Liuzhou, Yulin, and Guigang cities. Guilin is an international tourism city in China which held the China-ASEAN Expo Tourism Exhibition in 2020 and 2021. The National Foodborne Disease Surveillance System and the Foodborne Disease Surveillance System of Guangxi Zhuang Autonomous Region have been in operation since 2010 and 2012, respectively, with the aim to monitor the magnitude of gastroenteritis within the province. For the surveillance of noroviral gastroenteritis, there are 11 sentinel hospitals across Guangxi Region in the surveillance system, and we used all of these hospitals.
According to the National Surveillance Protocol for Foodborne Disease, gastroenteritis is suspected if a patient visits one of the sentinel hospitals presenting with liquid, watery, mucous, or bloody purulent stool at least three times per day or diarrhea with vomiting [Citation23]. Bacterial or food poisoning gastroenteritis is suspected if there is a history of consuming contaminated food and confirmed by a stool examination. After providing consent, the gastroenteritis patients are interviewed using a standard national questionnaire, and they are then asked for a stool sample, anal swab, or gastric fluid from vomit, all of the patients including children finished the data collection with 24 h. The samples are stored at −80°C, and norovirus is then tested using a one-step real-time reverse-transcription polymerase chain reaction (RT-PCR) according to the National Surveillance Protocol for Foodborne Disease Surveillance Workbook by trained health personnel, and the patient information and norovirus test results are recorded in the electronic surveillance system. The Guangxi CDC staff check the data from the surveillance on a daily basis.
Study samples and sample size
For this secondary analysis, we included patients who were diagnosed with gastroenteritis and suspected of food poisoning from contaminated food or water recorded in the Foodborne Disease Surveillance System of Guangxi Zhuang Autonomous Region from 2015 to 2020. Those who were confirmed with Salmonella infection or having functional bowel disorders were excluded. According to a previous report, the prevalence of norovirus gastroenteritis in Guangxi Zhuang Autonomous Region in 2013–2015 was 26.3% [Citation24]. The sample size was calculated using a precision formula with a 95% confidence interval, acceptable error of 4%, and design effect of 2 using the Epicalc package [Citation25], resulting in at least 924 patients with suspected gastroenteritis each year being required. In each of the study years, the number of gastroenteritis patients in the surveillance system ranged from 1200 to 1500 cases, and thus all cases in the system were used in the study analysis.
Variables and variable definitions
The main outcome measure of this study was positive norovirus detection by RT-PCR and the main periods were ‘before COVID-19’, from 2015 to 2019, and ‘during COVID-19 year’, in 2020, the period during which the country implemented the COVID-19 prevention and control measures related to oral and hand hygiene behaviors. Other independent variables included age, gender, disease history, food types, location of food intake, antibiotic use before visiting a doctor, types of samples, time from symptom onset to diagnosis, season when symptoms appeared, hospitalization, location of hospitals, and level of hospitals.
Age was categorized into four age groups (≤5, 6–20, 21–45, and >45) and gender (male and female). Disease history referred to related diseases the patient already had prior to visiting the doctor, which was categorized by ‘yes’ and ‘no’. Food types referred to suspected foods the patient consumed before the onset of gastroenteritis. According to the National Surveillance Protocol, food types are categorized into ‘grains and grain products’, ‘fruits or vegetables’, ‘aquatic products’, ‘meat/dairy products’, and ‘other foods’. Location of food intake referred to the place where the patient bought the suspected food. Following the National Surveillance Protocol, location of food intake was categorized by ‘home’ or ‘outside’. Antibiotic-use history referred to a history of antibiotic use before visiting the doctor and was categorized by ‘yes’ or ‘no’. Specimens collected from stool, anal swab, or gastric fluid were tested for norovirus. The time from symptom onset to diagnosis referred to the time interval between the onset of symptoms and the time the patient went to the hospital to see the doctor (days), which was calculated from the hospital record system. The season when the symptoms appeared was categorized as ‘Spring’ (February–April), ‘Summer’ (May–July), ‘Autumn’ (August–October), and ‘Winter’ (November–January). The location of the sentinel hospitals was classified into ‘Yulin’, ‘Guigang’, ‘Guilin’, ‘Liuzhou’, or ‘Nanning’ cities, and the level of hospitals referred to the sentinel hospitals’ administrative level, which was categorized by ‘city level’ and ‘provincial level’.
Data management and statistical analysis
The data from the Foodborne Disease Surveillance System were retrieved and analyzed using R software version 4.1.2 (R Foundation for Statistical Computing 2020, Vienna, Austria). The detection rates were calculated by dividing the number of norovirus gastroenteritis cases by the total number of gastroenteritis patients for each year and tested across the years using chi-square test for trend.
The predicted detection rates during COVID-19 year were computed using the autoregressive integrated moving average model (ARIMA model) through the Tseries and Forecast package, based on the assumption that past values have some residual effect on current or future values and controlling for the seasonality over time. A seasonal-product ARIMA (p, d, q) (P, D, Q) model was used to due with the seasonal effect on norovirus infections, where p is the number of autoregressive, d is the degree of differencing, and q is the number of moving average terms. The best fit ARIMA model was determined by Akaike Information Criterion (AIC) or Bayesian Information Criteria (BIC) values.
The factors associated with norovirus gastroenteritis were initially analyzed using a univariate logistic regression model, and factors with p-value less than 0.2 were selected to put in the multiple logistic regression model using a backward stepwise approach. Crude odds ratios (cOR) and adjusted odds ratios (aOR) with 95% confidence intervals (95% CI) were calculated. The interactions of significant independent factors with the COVID-19 period were tested. The significance level was set as p ˂ 0.05.
Results
During the six-year study period, norovirus gastroenteritis was confirmed in 1,008 patients from a total of 7,903 gastroenteritis patients, an overall rate of 12.8%, divided into 14.3% before the COVID-19 outbreak and 6.1% during the COVID-19 study year (). The detection rates significantly changed over the years using the chi-square test for trend (p < 0.001). Actual and predicted detection rates of norovirus gastroenteritis during 2015–2020 are shown in . Bimodal distribution was seen in each year, with peaks from September to October. The predicted detection rates in 2020 were significantly higher than the actual rates (p < 0.001). There was a gradually decreasing trend in both actual and predicted detection rates over the study period. The characteristics of the norovirus gastroenteritis patients during 2015–2020 are shown in . In the COVID-19 period, age group, types of food, season when symptoms appeared, time from symptom onset to diagnosis, location of sentinel hospitals, and level of hospital were significantly associated with norovirus gastroenteritis.
Figure 1. Detection rates of norovirus gastroenteritis during 2015–2020 in Guangxi Region, China (actual detection rate and prediction rate using ARIMA models in 2020 showed a significant difference by paired t-test, p < 0.001).
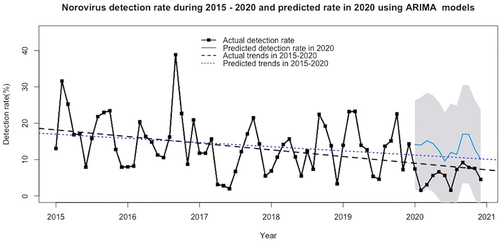
Table 1. Annual detection rates of norovirus gastroenteritis during 2015–2020.
Table 2. Characteristics of norovirus gastroenteritis patients during 2015–2020.
The final multiple logistic regression model for the factors associated with norovirus gastroenteritis is shown in . After adjusting for other associated factors, the odds of norovirus gastroenteritis detection were significantly lower during the COVID-19 year compared with the period before COVID-19 (aOR 0.11, 95% CI 0.04-0.27). Norovirus gastroenteritis was less likely to be detected when the time of symptom onset to diagnosis was 24–48 h (aOR 0.80, 95% CI 0.68-0.94) and >48 h (aOR 0.71, 95% CI 0.58-0.88) as well as the symptoms appeared in the summer season (aOR 0.66, 95% CI 0.55-0.80). Higher odds of norovirus gastroenteritis detection were found in autumn (aOR 1.38, 95% CI 1.15-1.65) and the Guilin hospital (aOR 1.72, 95% CI 1.31-2.26). The interactions between COVID-19 period and age group and location of food intake were significant. These indicate the higher odds of norovirus gastroenteritis detection during COVID-19 period in patients aged 45 years or less and in those having food intake outside their homes. There was no interaction of age group and location of food intake on noroviral gastroenteritis.
Table 3. Final logistic regression model of factors related to norovirus gastroenteritis (N = 7887).
Due to the significance of interaction, the factors associated with norovirus gastroenteritis stratified by before COVID-19 and during the COVID-19 year are presented in . Before the COVID-19, patients consuming aquatic products and meat/dairy products, who had symptoms appearing in autumn and visiting the Guilin hospital, had significantly higher odds of norovirus gastroenteritis, while the incidence of norovirus gastroenteritis was significantly lower when the symptoms appeared in summer and the time from the symptom appearance to diagnosis was longer than 24 h. The magnitude of the odds ratio for detecting norovirus gastroenteritis during COVID-19 year was approximately three to five times higher than before the COVID-19 period. During the COVID-19 year, the odds ratios of detecting norovirus gastroenteritis in those aged ≤5, 6–20, and 21–45 years were 4.80 (95% CI 1.80-12.79), 5.68 (95% CI 2.01-16.06), and 3.77 (95% CI 1.41-10.05) when compared with those aged >45 years. Having food intake outside the home before COVID-19 period was less likely to be associated with norovirus gastroenteritis (aOR 0.78, 95% CI 0.65-0.94), while it was more likely during the COVID-19 (aOR 1.90, 95% CI 1.11-3.26).
Table 4. Factors associated with detection of norovirus infection stratified by COVID-19 period.
Discussion
The rate of norovirus gastroenteritis in Guangxi Zhuang Autonomous Region, Southern China gradually decreased from 2015 to 2020. The actual detection rate during COVID-19 year in 2020 was 6.1% which was significantly lower than the predicted rate. The COVID-19 period interacted with the age group and the location of food intake, indicating the influence of younger ages and those having food outside their home on the relationship of COVID-19 period with norovirus gastroenteritis. Type of food, season when symptoms appeared, time from symptom onset to diagnosis, and location of sentinel hospitals were associated with norovirus gastroenteritis before the COVID-19 period.
The decreasing trend of norovirus gastroenteritis from 2015 to 2020 found in our study was similar to the surveillance reports of previous studies conducted in Denmark during 2011–2018 [Citation26], in Yunnan province, China during 2018–2020 [Citation10] and in Zhejiang province, China 2016–2020 [Citation27]. This consistency can be assumed to be from improvements in public food safety along with food safety monitoring and risk assessment systems. In contrast, a study in the United States of America conducted earlier during 2011–2015 showed a highly static trend of norovirus gastroenteritis [Citation28]. During the COVID-19 pandemic in 2020, studies from various countries reported dramatically lower rates of norovirus gastroenteritis compared with previous periods, namely the United States of America [Citation19], Germany [Citation29], Australia [Citation30], Korea [Citation31], Japan [Citation32], and other province in China [Citation10] This may be explained through the interventions for preventing COVID-19 infection, such as staying at home, social distancing, and hand hygiene, which can also prevent norovirus transmission [Citation10,Citation19].
The effects of COVID-19 period on norovirus gastroenteritis modified by younger age and consuming food outside the home were found in our study. To date, we cannot find any studies reporting on the interaction effect of norovirus gastroenteritis with other factors both before and during the COVID-19 periods. After stratifying by the COVID-19 period into the before COVID-19 and during COVID-19, the children in the age group of 6–20 years had highly significant odds of acquiring norovirus gastroenteritis during the COVID-19 year, but this finding was not statistically significant before the COVID-19 period. Increasing odds of noroviral gastroenteritis in school or university ages may be due to the probability of being exposed to unsafe foods, a known risk factor of norovirus infection [Citation15,Citation33].
The odds of norovirus gastroenteritis in patients consuming aquatic or meat/dairy products were significantly higher than those consuming grain/rice products only at before COVID-19 period. This could be because before the COVID-19 period, aquatic or meat products were easily contaminated by the norovirus [Citation34–36], while there were strictly enforced food safety policies related to the improvement of food-hand hygiene during the COVID-19 year in 2020 [Citation14,Citation37,Citation38]. These policies can reduce the risk of foods contaminated with norovirus. The detection rates were high in autumn both before and during the COVID-19, a finding consistent with previous studies in the same and different provinces of China [Citation10,Citation36]. The temperature, humidity, and rainfall in this season in China may be related to the norovirus infection [Citation27]. A systematic review of global prevalences for norovirus searched up to March 2014 also confirmed the theory that norovirus is a foodborne disease [Citation39].
A longer duration of time from symptom onset to diagnosis was less likely to be associated with norovirus gastroenteritis in both before and during COVID-19, but the relationship was non-significant during the COVID-19 year because of a wide confidence interval due to the small sample size. The CDC norovirus guideline suggests testing for norovirus during the acute phase within 48 h of symptom onset [Citation40]. Likewise, the tourist city, Guilin, had a significantly greater rate before, but not during, the COVID-19 year. This can be explained by the higher number of travelers before COVID-19 had high probability of transmitting norovirus from one place to another [Citation41].
Our study highlights the detection rate of norovirus-infected gastroenteritis over a six-year period, including the periods before COVID-19 and during the COVID-19 year, which was longer than previous studies which ranged from 1 to 3 years. We were unable to find publications identifying the effect of other independent factors on the relationship between COVID-19 and norovirus gastroenteritis.
There were some limitations to our study. First, we used the data from surveillance systems where the data on some possible confounders may not have been included. However, this is a national surveillance program collecting data for the most important factors related to norovirus gastroenteritis based on the local CDC office. Second, only the year 2020 was examined and used as a proxy for the COVID-19 effect of other years, because the magnitude of the covid effect was highest during this year. However, it would be better to analyze the data in the following years and observe the longitudinal patterns, which we plan to do in a future study. Third, genotype information for the norovirus was not available in this surveillance system. Fourth, the samples in this surveillance were not tested for other viruses, such as adenovirus, rotavirus, or parasites. Fifth, the sample size of gastroenteritis in 2020 considered as during COVID-19 was relatively small compared to the numbers before COVID-19, which may have affected the results of the analysis. Finally, the factors related to norovirus gastroenteritis during COVID-19 may not be generalized to other countries since the policy implementation varied across countries.
Conclusion
There was a decreasing trend of norovirus gastroenteritis over the six-year study period in Guangxi, China. In 2020, during the first year of the COVID-19 pandemic, its rate was half of the average of the five previous years. Type of food, season when symptoms appeared, time from symptom onset to diagnosis, and location of sentinel hospitals were associated with norovirus gastroenteritis. The relationship between norovirus gastroenteritis detection and COVID-19 period was modified by age group and location of food intake, showing higher detection among patients aged 45 years or less and having food intake outside the home during the COVID-19 year. These findings suggest targeting the high-risk population and foodborne transmission of norovirus infection for increased preventive measures. Further longitudinal studies of norovirus gastroenteritis and the effect from COVID-19 are required.
Author contributions
All authors participated in study conceptualization and design. The data curation was managed by YJ, HL, DT, and YP. The data were analyzed and interpreted by YZ, TL, and HL. YZ and TL prepared the manuscript, and the final version of manuscript was read and approved by all authors.
Competing interests
YZ, YJ, DT, and YP are on the staff at the Food Safety Monitoring and Evaluation Department, Guangxi Zhuang Autonomous Region Centre for Disease Control and Prevention who are responsible for disease control; however, they declare no direct involvement of data collection doctor who works on the quality of data reported from the sentinel hospitals and had no duties involving data collection. TL declares no conflicts of interest.
Consent to participate
This was a secondary data analysis using the existing data from national surveillance system with routine consent. The data were deidentified before release to YZ, the first author.
Ethics approval
The proposal for this secondary analysis was approved by the Human Research Ethics Committee of Faculty of Medicine, Prince of Songkla University, Thailand, with exempt determination (REC.65-135-18-1).
Acknowledgments
We acknowledge the great work of all the staffs from all the sentinel hospitals of Guangxi Zhuang Autonomous Region, China, who collected the surveillance data. We also gratefully thank Mr David Patterson, the International Affairs Office, Faculty of Medicine, Prince of Songkla University for the English editing.
Data availability statement
Data are available on request due to privacy or other restrictions of the Guangxi Zhuang Autonomous Region Centre for Disease Control and Prevention.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- UNICEF/World Health Organization. Ending preventable deaths from pneumonia and diarrhoea by 2025. Geneva: WHO; 2013.
- Chow C, Leung A, Hon K. Acute gastroenteritis: from guidelines to real life. Clin Exp Gastroenterol. 2010;3:97–9. doi: 10.2147/CEG.S6554
- World Health Organization. Diarrhoeal disease [Internet]. [cited 2021 Jan 30] https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease
- Webb A, Starr M. Acute gastroenteritis in children. Aust Fam Physician. 2005;34(4):227–231.
- Wang X, Wang S, Zhang C, et al. Development of a surrogate neutralization assay for norovirus vaccine evaluation at the cellular level. Viruses. 2018;10(1):27. doi: https://doi.org/10.3390/v10010027
- Marshall J, Bruggink L. The dynamics of norovirus outbreak epidemics: recent insights. Int J Environ Res Public Health. 2011;8(4):1141–1149. doi: 10.3390/ijerph8041141
- Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1
- CDC. Norovirus Worldwide [Internet]. 2020. [cited 2021 Jan 30] https://www.cdc.gov/norovirus/trends-outbreaks/worldwide.html
- Pan A, Liu L, Wang C, et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323(19):1915–1923. doi: https://doi.org/10.1001/jama.2020.6130
- Yang L, Shi S, Na C, et al. Rotavirus and norovirus infections in children under 5 years old with acute gastroenteritis in Southwestern China, 2018–2020. J Epidemiol Glob Health. 2022;12(3):292–303. doi: 10.1007/s44197-022-00050-8
- Kaushik M, Agarwal D, Gupta A. Cross-sectional study on the role of public awareness in preventing the spread of COVID-19 outbreak in India. Postgrad Med J. 2021;97(1154):777–781. doi: 10.1136/postgradmedj-2020-138349
- Zhou H, Zhen S, Wang J, et al. Burden of acute gastroenteritis caused by norovirus in China: a systematic review. J Infect. 2017;75(3):216–224. doi: 10.1016/j.jinf.2017.06.004
- Wang J, Zhou H, Mo Z, et al. Burden of viral gastroenteritis in children living in rural China: population-based surveillance. Int J Infect Dis. 2020;90:151–160. doi: 10.1016/j.ijid.2019.10.029
- de Wit M, Koopmans M, van Duynhoven Y. Risk factors for norovirus, Sapporo-like virus, and group a rotavirus gastroenteritis. Emerg Infect Dis. 2003;9(12):1563–1570. doi: 10.3201/eid0912.020076
- Thébault A, David J, Kooh P, et al. Risk factors for sporadic norovirus infection: a systematic review and meta-analysis. Microb Risk Anal. 2021;17:100135. doi: 10.1016/j.mran.2020.100135
- Gruber J, Bowman N, Becker-Dreps S, et al. Risk factors for norovirus gastroenteritis among Nicaraguan children. Am J Trop Med Hyg. 2017;97(3):937–943. doi: 10.4269/ajtmh.16-0799
- Lian Y, Wu S, Luo L, et al. Epidemiology of norovirus outbreaks reported to the public health emergency event surveillance system, China, 2014–2017. Viruses. 2019;11(4):342. doi: https://doi.org/10.3390/v11040342
- Nachamkin I, Richard-Greenblatt M, Yu M, et al. Reduction in sporadic norovirus infections following the start of the COVID-19 pandemic, 2019–2020, Philadelphia. Infect Dis Ther. 2021;10(3):1793–1798. doi: 10.1007/s40121-021-00473-z
- Lennon R, Griffin C, Miller E, et al. Norovirus infections drop 49% in the United States with strict COVID-19 public health interventions. Acta Med Acad. 2020;49(3):278–280. doi: 10.5644/ama2006-124.317
- Ondrikova N, Clough H, Douglas A, et al. Differential impact of the COVID-19 pandemic on laboratory reporting of norovirus and campylobacter in England: a modelling approach. PLoS One. 2021;16(8):e0256638. doi: 10.1371/journal.pone.0256638
- White A, Ciampa N, Chen Y, et al. Characteristics of campylobacter and salmonella infections and acute gastroenteritis in older adults in Australia, Canada, and the United States. Clin Infect Dis. 2019;69(9):1545–1552. doi: 10.1093/cid/ciy1142
- Melhem N, Zaraket H, Kreidieh K, et al. Clinical and epidemiological characteristics of norovirus gastroenteritis among hospitalized children in Lebanon. World J Gastroenterol. 2016;22(48):10557–10565. doi: 10.3748/wjg.v22.i48.10557
- Cao R, Ma X, Li W, et al. Epidemiology of norovirus gastroenteritis in hospitalized children under five years old in western China, 2015–2019. J Microbiol Immunol Infect. 2021;54(5):918–925. doi: 10.1016/j.jmii.2021.01.002
- Yanxu Z, Yuyan J, Yihong X, et al. Analysis of norovirus infection in gastroenteritis patients from foodborne disease surveillance sentinel hospitals between 2013 to 2015, Guangxi Zhuang Autonomous Region. J Applied Prev Med. 2017;23:328–330.
- Chongsuvivatwong V. Analysis of epidemiological data using R and Epicalc. 2nd ed. Bangkok: Paperback Printing; 2015. Sample size calculation; p. 253–256 .
- Korcinska M, Dalsgaard Bjerre K, Dam Rasmussen L, et al. Detection of norovirus infections in Denmark, 2011-2018. Epidemiol Infect. 2020;148:e52. doi: 10.1017/S0950268820000461
- Qi X, Alifu X, Chen J, et al. Descriptive study of foodborne disease using disease monitoring data in Zhejiang province, China, 2016–2020. BMC Public Health. 2022;22(1):1831. doi: 10.1186/s12889-022-14226-1
- Grytdal S, Browne H, Collins N, et al. Trends in incidence of norovirus-associated acute gastroenteritis in 4 Veterans Affairs Medical Center populations in the United States, 2011–2015. Clin Infect Dis. 2020;70(1):40–48. doi: 10.1093/cid/ciz165
- Eigner U, Verstraeten T, Weil J. Decrease in norovirus infections in Germany following COVID-19 containment measures. J Infect. 2021;82(6):276–316. doi: 10.1016/j.jinf.2021.02.012
- Bruggink L, Garcia-Clapes A, Tran T, et al. Decreased incidence of enterovirus and norovirus infections during the COVID-19 pandemic, Victoria, Australia, 2020. Commun Dis Intell (2018). 2021;45: doi: 10.33321/cdi.2021.45.5
- Ahn S, Park J, Lim I, et al. Changes in the occurrence of gastrointestinal infections after COVID-19 in Korea. J Korean Med Sci. 2021;36(24):e180. doi: https://doi.org/10.3346/jkms.2021.36.e180
- Fukuda Y, Tsugawa T, Nagaoka Y, et al. Surveillance in hospitalized children with infectious diseases in Japan: pre- and post-coronavirus disease 2019. J Infect Chemother. 2021 Epub 2021 Aug 4;27(11):1639–1647. doi: 10.1016/j.jiac.2021.07.024
- Queiros-Reis L, Lopes-João A, Mesquita J, et al. Norovirus gastroenteritis outbreaks in military units: a systematic review. BMJ Mil Health. 2021;167(1):59–62. doi: https://doi.org/10.1136/bmjmilitary-2019-001341
- Qin S, Chan T, Cai J, et al. Genotypic and epidemiological trends of acute gastroenteritis associated with noroviruses in China from 2006 to 2016. Int J Environ Res Public Health. 2017;14(11):1341. doi: 10.3390/ijerph14111341
- Rohayem J. Norovirus seasonality and the potential impact of climate change. Clin Microbiol Infect. 2009;15(6):524–527. doi: 10.1111/j.1469-0691.2009.02846.x
- Morgan M, Watts V, Allen D, et al. Challenges of investigating a large food-borne norovirus outbreak across all branches of a restaurant group in the United Kingdom, October 2016. Euro Surveill. 2019;24(18):1800511. doi: 10.2807/1560-7917.ES.2019.24.18.1800511
- Lu L, Quintela I, Lin C, et al. A review of epidemic investigation on cold-chain food-mediated SARS-CoV-2 transmission and food safety consideration during COVID-19 pandemic. J Food Saf. 2021;41(6):e12932. doi: 10.1111/jfs.12932
- Ma H, Zhang J, Wang J, et al. COVID-19 outbreak caused by contaminated packaging of imported cold-chain products — Liaoning province, China, July 2020. China CDC Weekly. 2021;3(21):441–447. doi: 10.46234/ccdcw2021.114
- Ahmed S, Hall A, Robinson A, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(8):725–730. doi: 10.1016/S1473-3099:70767-4
- CDC.Norovirus Specimen Collection [Internet]. 2021. [cited 2021 June 6]. https://www.cdc.gov/norovirus/lab/specimen-collection.html
- Qiao N, Ren H, Liu L. Genomic diversity and phylogeography of norovirus in China. BMC Med Genomics. 2017;10(S3):51. doi: https://doi.org/10.1186/s12920-017-0287-9

