ABSTRACT
Background: The claustrum is a brain structure found in both hemispheres beneath the inner surface of the neocortex in the region of the insula and is bordered by the external and extreme capsule. Generally, it is divided into a dorsal part (claustrum proper or insular claustrum) and ventral part (endopiriform nucleus). The claustrum possesses complex reciprocal connections with the cortex, striatum, dorsal thalamic nuclei and hippocampus. Reports of patients with unilateral lesion of the claustrum are few in number. Herein, we present a case report of an ischemic stroke restricted to the left claustrum in a 55-year-old female
Case Report: The patient presented with intense feeling of dizziness, ataxic gait, sensation of ‘vacuity’ in the head, decreased hearing and abnormal gustatory sensations. The neurological examination revealed a mixed horizontal and rotary nystagmus to the right side. Brain CT upon admission was assessed as normal without pathological findings. MRI was performed and showed an ischemic stroke restricted to the left claustrum with no other lesions. Following 4 weeks of therapy the patient recovered fully and duplex sonography showed preserved circulation in the affected areas.
Conclusion: The present case report underlines the complexity of clinical symptomatology of the claustrum.
Introduction
The claustrum is a brain structure found in the telencephalon of nearly all mammalian species (Citation1–Citation3). It is a thin, vertical, sheet-like structure with irregular shape, found in both hemispheres beneath the inner surface of the neocortex in the region of the insula (Citation4). It lies between the insular cortex and the outer surface of the putamen and is bordered by the external and extreme capsule (Citation2,Citation4). Kowianski et al. (Citation5) distinguish between five types of claustral shape based on its structure, volume and cellular differentiation: type I – in insectivores and rodents, type II – in guinea pigs and rabbits, type III – in carnivores, type IV – in non-human primates and type V – in humans, with the human cortex having the most complex structure.
Fig. 1. Coronal section of MRI performed on the day after admission, showing an ischemic stroke restricted to the left claustrum (red arrow).
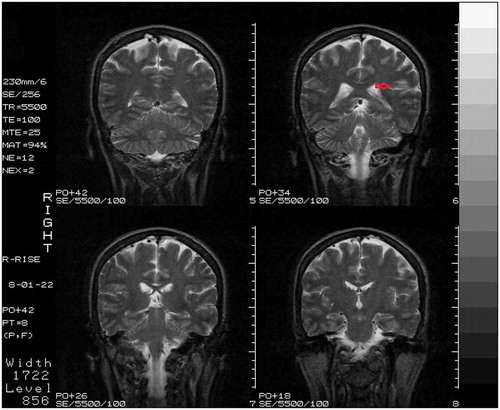
Fig. 2. Coronal section of follow-up MRI, showing an ischemic stroke restricted to the left claustrum (red arrow).
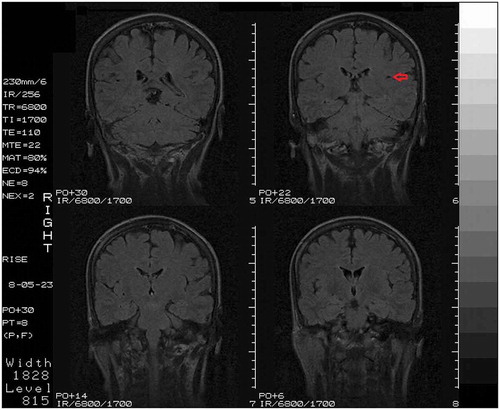
The anatomical subdivisions of the claustrum are well established (Citation6). Generally, the claustrum is divided into a dorsal part (claustrum proper or insular claustrum) and ventral part (endopiriform nucleus) (Citation1–Citation3,Citation7). These two subdivisions are embryologically distinct (Citation7). The dorsal claustrum (DC) is located deep to the insular cortex and is extensively connected with the neocortex (Citation8,Citation9). The ventral claustrum (VC), on the other hand, is located deep to the piriform cortex and its interconnections with the prepiriform and entorhinal cortex have been well documented (Citation3,Citation9,Citation10). The claustrum appears to project primarily ipsilaterally to the cortex, while a weaker contralateral projection does exist (Citation8,Citation9,Citation11). In addition to the claustrum’s complex reciprocal connections with the cortex, claustral projections to various subcortical structures have been documented, such as the striatum (Citation12), dorsal thalamic nuclei (Citation9,Citation10,Citation13) and hippocampus (Citation14). Previous studies have reported the presence of five types of neurons in the human claustrum: spiny neurons varying in size and shape (type I); large aspiny neurons (type II); large aspiny neurons without pigment deposits (type III), small pigment-laden aspiny neurons (type IV); small aspiny neurons without lipofuscin granules (type V) (Citation15). Several studies have investigated the presence and distribution of a number of substances in the claustrum of humans and other mammals, such as NADPH-diaphorase, neuronal nitric oxide synthase (nNOS), neuropeptide Y, leucine enkephalin, etc (Citation16–Citation21).
Reports of patients with unilateral lesion of the claustrum are few in number. Herein, we present a case report of an ischemic stroke restricted to the left claustrum in a 55-year-old female and comment on the clinical implications of such injury.
Case report
A 55-year-old right-handed female, non-smoker and without history of cardiac disease, diabetes or other diseases, presented with intense feeling of dizziness upon waking up. Her gait was ataxic, resembling the gait of a drunken person. Several hours later other symptoms developed such as sensation of ‘vacuity’ in the head, abnormal feeling of discomfort in the epigastric region (without nausea or vomiting), decreased hearing in the left ear, abnormal gustatory sensation (paresthesia) in the left half of the tongue and paresthesia in the left peribuccal region spreading to the left half of the face and finally affecting the whole left half of the head. Her blood pressure was measured as 110/60 mmHg. Duplex sonography of the carotid and vertebral arteries was performed and revealed mild atherosclerosis of both carotid bifurcations. The patient was admitted at the Centre for Neurology and Neurosurgery, Military Medical Academy, Sofia, Bulgaria and stayed there over a period of 5 days. The somatic examination was consistent with the physiological state at this age. Blood pressure upon admission was measured as 110/80 mmHg. The standard laboratory blood tests were normal. The neurological examination revealed a mixed horizontal and rotary nystagmus to the right side. Romberg’s test was positive for static ataxia. The patient’s gait was determined as ataxic. The mental examination was normal. Otoneurological tests revealed a central otoneurological syndrome with brain stem localization. Brain CT upon admission was assessed as normal without pathological findings. In the differential diagnosis, we considered a possible ischemic injury with localization either in the brain stem or in the cerebellum due to her ataxia and apparent central otoneurological syndrome. Sensory involvement was also discussed. The day after admission, MRI with contrast matter was performed and showed an ischemic stroke restricted to the left claustrum with no other lesions (). In the hospital the patient was treated with Piracetam (Nootropil®) 2 × 200 mg i.v. During the following months she was treated as an outpatient. Following 4 weeks of therapy with Vinpocetine (Cavinton®) 2 × 10 mg, Betahistine (Serc®) 3 × 16 mg and Piracetam (Nootropil®) 2 × 800 mg the patient recovered fully. Control colour duplex sonography of the carotid and vertebral arteries showed preserved circulation. Transcranial Doppler sonography revealed preserved circulation of the right vertebral artery and mildly increased resistance of the left vertebral artery. Electroencephalography (EEG) was normal. Follow-up MRI revealed a local ischemic stroke restricted only to the left claustrum ().
Discussion
Pure claustrum strokes are very rare in clinical practice (Citation9). The stroke of the claustrum may present with various clinical manifestations due to its anatomical location and functional connections. Reversible bilateral lesions of the claustrum and external capsule (confirmed by MRI) present with epileptic seizures, loss of vision, speech and hearing (Citation22). As the claustrum is situated in the close proximity of the insular cortex, damage of the claustrum is very likely to damage the insula and vice versa (Citation23). The angioarchitecture of the claustrum is rather complex. Both the deeper and the more superficial part of the middle cerebral artery are implicated in the pathogenesis of the stroke (Citation22). It appears that vessels permeating the insula also provide blood supply to the claustrum (Citation1). This may lead to coexisting lesions and to result in complexity of the clinical manifestations (Citation1,Citation23). Duffau et al. (Citation24) reported a series of 42 patients who were operated due to cerebral glioma involving the claustrum and found that 3 months after surgery, 39 of them recovered full neurological potential, while 3 patients experienced a permanent left hemiparesis due to a deep stroke involving the perforating arteries.
Insular strokes are rare (Citation25). An acute insular stroke may present with various clinical symptoms due to anatomical and functional complexity of the insular lobe and its wide connections with the frontal, temporal, parietal and olfactory cortex and with basal ganglia, thalamus and limbic structures (Citation23,Citation25). The insular lobe is an important somatosensory, gustatory and visceral motor and sensory processing area, a component of the vestibular and limbic cortex and is implicated in pain processing volition, swallowing, cardiovascular control and cerebrogenic sudden death (Citation25).
In the present case report, we discussed that the gustatory sensations could be provoked by the involvement of the claustrum or the insular cortex. The normal EEG was not surprising since EEG abnormalities may be absent in regions which are enfolded or otherwise poorly oriented for scalp recording of their potential fields. MRI findings were deduced to represent an ischemic injury to the claustrum. A possible differential diagnostic problem was caused by the fact that seizure-related cerebral lesions may present with T2 hyperintensity (Citation26). Since seizures can coexist with symptoms of acute stroke, it is sometimes difficult to distinguish between the two conditions in the clinical setting. In such cases, assessment of perfusion on MRI may be useful to determine whether an area is hypoperfused (which would indicate ischemic etiology) or hyperperfused (associated with seizure-related pathology) (Citation27). The patient was treated with Vinpocetine and Piracetam, which are the drugs of choice for enhancement of brain function and improvement of the brain blood supply, along with Betahistine, which was given in order to specifically resolve her otoneurological symptoms. She responded well to the therapy without any side effects, which supported our hypothesis of ischemic etiology of the symptoms.
Conclusion
The present case report underlines the complexity of clinical symptomatology of the claustrum. The clinical observations of the lesions restricted to the claustrum open a unique window on the functional organization of the brain. It is evident that the clinical symptoms of local ischemic stroke in the claustrum might create difficulties with regard to the differential diagnosis. Understanding the function of the claustrum requires still many experiments, clinical observations and studies in clinical and molecular neuroscience.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Edelstein LR, Denaro FJ. The claustrum: a historical review of its anatomy, physiology, cytochemistry and functional significance. Cell Mol Biol. 2004;50:1–4.
- Hinova-Palova DV, Edelstein L, Landzhov B, et al. Topographical distribution and morphology of NADPH-diaphorase-stained neurons in the human claustrum. Front Syst Neurosci. 2014;8:96.
- Landzhov B, Hinova-Palova D, Edelstein L, et al. Comparative investigation of neuronal nitric oxide synthase immunoreactivity in rat and human claustrum. J Chem Neuroanat. 2017;86:1–14.
- Crick FC, Koch C. What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci. 2005;360(1458):1271–1279.
- Kowiański P, Dziewiatkowski J, Kowiańska J, et al. Comparative anatomy of the claustrum in selected species: a morphometric analysis. Brain Behav Evol. 1999;53(1):44–54.
- Hinova-Palova DV, Edelstein LR, Paloff AM, et al. Parvalbumin in the cat claustrum: ultrastructure, distribution and functional implications. Acta Histochem. 2007;109(1):61–77.
- Guirado S, Real MA, Olmos JL, et al. Distinct types of nitric oxide-producing neurons in the developing and adult mouse claustrum. J Comp Neurol. 2003;465(3):431–444.
- Olson CR, Graybiel AM. Sensory maps in the claustrum of the cat. Nature. 1980;288(5790):479–481.
- Mathur BN. The claustrum in review. Fron Syst Neurosci. 2014;8:48.
- Dinopoulos A, Papadopoulos GC, Michaloudi H, et al. Claustrum in the hedgehog (Erinaceus europaeus) brain: cytoarchitecture and connections with cortical and subcortical structures. J Comp Neurol. 1992;316(2):187–205.
- Smith JB, Alloway KD. Functional specificity of claustrum connections in the rat: interhemispheric communication between specific parts of motor cortex. J Neurosci. 2010;30(50):16832–16844.
- Arikuni T, Kubota K. Claustral and amygdaloid afferents to the head of the caudate nucleus in macaque monkeys. Neurosci Res. 1985;2(4):239–254.
- Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508(2):212–237.
- Amaral DG, Cowan WM. Subcortical afferents to the hippocampal formation in the monkey. J Comp Neurol. 1980;189(4):573–591.
- Braak H, Braak E. Neuronal types in the claustrum of man. Anat Embryol (Berl). 1982;163(4):447–460.
- Hinova-Palova D, Edelstein L, Papantchev V, et al. Light and electron-microscopic study of leukine enkephalin immunoreactivity in the cat claustrum. J Mol Histol. 2012;43:641–649.
- Hinova-Palova D, Edelstein L, Landzhov B, et al. Light microscopic immunocytochemical identification of leucine enkephalin in human claustrum. Scr Sci Med. 2013;45(Suppl.1):23–28.
- Edelstein L, Hinova-Palova D, Malinova L, et al. Distribution of neuropeptide Y in the dorsal claustrum of the cat. Light and electron-microscopic identification of distinct neuronal populations. In: Society for Neuroscience, 41st Annual Meeting . Washington, DC; 2011. Abstract #817.19.
- Edelstein L, Denaro FJ, Stamm JS, et al. Distribution of CB1 receptors in the claustrum of rats undergoing acute stress: an immunohistochemical study. In Society for Neuroscience, 41st Annual Meeting Washington, DC: 2011. Abstract # 734.12.
- Edelstein L, Hinova-Palova D, Denaro F, et al. NADPH-diaphorase-positive neurons in the human claustrum. In: Society for Neuroscience, 42nd Annual Meeting. San Diego, CA; 2012. Abstract # 895.20.
- Edelstein L, Hinova-Palova D, Landzhov B, et al. Neuronal nitric oxide synthase immunoreactivity in the human claustrum: light- and electron-microscopic investigation. In: Society for Neuroscience, 42nd Annual Meeting. San Diego, CA; 2012. Abstract # 895.21.
- Sperner J, Sander B, Lau S, et al. Severe transitory encephalopathy with reversible lesions of the claustrum. Pediat Radiol. 1996;26(11):769–771.
- Cereda C, Ghika J, Maeder P, et al. Strokes restricted to the insular cortex. Neurology. 2002;59(12):1950–1955.
- Duffau H, Mandonnet E, Gatignol P, et al. Functional compensation of the claustrum: lessons from low-grade glioma surgery. J Neurooncol. 2007;81:327–329.
- Mandrioli J, Zini A, Cavazzuti M, et al. T wave inversion in pure left insular stroke associated with hyperhomocysteinaemia. J Neurol Neurosurg Psychiatry. 2004;75:1788–1789.
- Cianfoni A, Caulo M, Cerase A, et al. Seizure-induced brain lesions: a wide spectrum of variably reversible MRI abnormalities. Eur J Radiol. 2013;82:1964–1972.
- Kim NY, Baek HJ, Choi DS, et al. MR findings of seizure-related cerebral cortical lesions during periictal period. Investig Magn Reson Imaging. 2017;21:82–90.

