ABSTRACT
Chromoblastomycosis is a chronic granulomatous subcutaneous fungal disease caused mainly by Fonsecaea monophora in southern China. Melanin is an important virulence factor in wild strain (Mel+), and the strains lack of the polyketide synthase gene is a melanin-deficient mutant strain (Mel-). We investigated the effect of melanin in F. monophora on Dectin-1 receptor-mediated immune responses in macrophages. Conidia and tiny hyphae of Mel+ and Mel- were co-cultured with THP-1 macrophages expressing normal or low levels of Dectin-1. Compare the killing rate, phagocytosis rate, and expression levels of the inflammatory cytokines tumour necrosis factor-α, interleukin-1β, interleukin-6, and nitric oxide in each group. The results showed that the killing rate, phagocytosis rate, and pro-inflammatory factor levels of Mel+ infected macrophages with normal expression of Dectin-1 were lower than those of Mel-. And the knockdown of Dectin-1 inhibited the phagocytic rate, killing rate, and proinflammatory factor expression in macrophages infected with Mel+ and Mel-. And there was no significant difference in the above indexes between Mel+ and Mel- groups in Dectin-1 knockdown macrophages. In summary, the study reveals that melanin of F. monophora inhibits the immune response effect of the host by hindering its binding to Dectin-1 on the surface of macrophage, which may lead to persistent fungal infections.
1. Introduction
Chromoblastomycosis (CBM) is an infectious disease of the skin and subcutaneous tissue caused by a variety of dematiaceous fungi (Kurien et al. Citation2023). The disease typically affects the limbs, especially the lower extremities, and is characterized by slowly developing pleomorphic lesions, such as nodules, verrucous hyperplasia, plaques, scarring, and atrophy (Brito and Bittencourt Citation2018). Fonsecaea monophora was identified as a new species of Fonsecaea by De Hoog et al. (Citation2004) using the sequencing of ribosomal DNA internal transcriptional spacers. It has been proven that F. monophora is the main pathogen for CBM in southern China by morphology, rDNA sequence diversity, and random amplified polymorphic DNA typing (Fransisca et al. Citation2017; Shi et al. Citation2018; Soda et al. Citation2018).
The wild strain of F. monophora (Mel+) is characterised by a slow-growing, black, cotton-like colony (Xi et al. Citation2009). Melanin is considered to be an important virulence factor in opportunistic and pathogenic fungi. Melanin can protect fungi from oxidative damage, inhibit cell-mediated immune response, interfere with complement activation, and reduce the sensitivity of antifungal agents (Santos et al. Citation2007). In our group’s previous study, we constructed a melanin-deficient mutant strain (Mel-) by structuring a pks gene targeting disruption vector and then inserting it into F. monophora using Agrobacterium-mediated transformation. We found that the pksA gene in F. monophora is the main polyketide synthase required for melanin synthesis, and Mel- obtained by knocking out the pksA gene can reduce melanin production, growth rate, and sporulation capacity (Xiao et al. Citation2021). Furthermore, Mel- is more susceptible to oxidative stress, extreme pH environments, and antifungal agents such as itraconazole, terbinafine, and amphotericin B (Sun et al. Citation2011).
Macrophages possess various pattern recognition receptors (PRRs) on their surface, which can effectively identify the pathogen-associated molecular patterns (PAMPs) on the surface of pathogenic microorganisms such as bacteria, fungi, and parasites to activate immune response (Shiokawa et al. Citation2017). The dendritic cell-associated C-type lectin-1 (Dectin-1) receptor is one of the PRRs that has been demonstrated to induce intracellular signalling. Dectin-1 has been proven to recognise a variety of fungi, including Aspergillus, Candida, Saccharomyces, Saccharomyces, and Pneumocystis, and it can promote protective immune responses, including phagocytosis, respiratory burst, and the production of proinflammatory factors (Drummond and Brown Citation2011). Siqueira et al. (Citation2017) demonstrated that the internalisation of F. pedrosoi in vitro requires the recognition of FcγR and Dectin-1. de Castro et al. (Citation2017) demonstrated that the production of IL-1β in F. pedrosoi-infected macrophages depends on Dectin-1, −2, and −3 receptors and the Syk-NF-kB signalling pathway using inhibitors and gene knock-out cells. These results indicate that the recognition of F. monophora by macrophages may be related to Dectin-1. However, how melanin affects the immunoregulatory mechanism mediated by Dectin-1 when F. monophora infects the macrophages has not been reported yet.
In this study, we used a melanin-producing wild strain (Mel+) and a melanin-deficient mutant strain (Mel-) of F. monophora to evaluate the effect of melanin on the immune response by macrophages. We also assessed the effect of Dectin-1 receptor knockdown in macrophages on the killing effect of Mel+ and Mel-.
2. Materials and methods
2.1. F. monophora strain preparation
The wild strain (Mel+) of F. monophora (CBS269.37) was generously donated by Central Bureau Voor Schimmel cultures Fungal Biodiversity Centre (CBS). The melanin-deficient mutant strain of F. monophora (Mel-) was a mutant strain that lacked melanin production due to the knock-out of the pksA gene in the wild strain. F. monophora was cultivated on PDA medium at 26 °C for approximately 14 days, and the colonies exhibited robust growth. Mel+ colonies had a flat, greyish-green surface and were covered with fluffy hyphae resembling goose down (Figure S1a). In contrast, Mel- colonies appeared uneven with a white surface reminiscent of bean curd residue (Figure S1b). The colonies of the growing F. monophora were scraped off and transferred into a 1.5 mL Eppendorf (EP) tube with RPMI-1640 medium. The mixture of conidia and hyphae was filtered with a 40 μmol/L filter, which retains conidia and small hypha fragments of F. monophora. Upon microscopic observation, brown clumps of hyphae and smaller spores were discernible for Mel+ (Figure S1c), while antler-shaped mycelia and white conidia were visible for Mel- (Figure S1d).
2.2. Cells culture and polarisation
THP-1 cells have been widely used in studies of monocyte and macrophage-related mechanisms, signalling pathways, nutrition, and drug delivery due to their ease of culture and amplification in the laboratory. Human macrophage THP-1 cells were purchased from American Type Culture Collection (ATCC) and cultured in RPMI-1640 medium supplemented with 10% foetal bovine serum (Gibco, USA) and 1% penicillin-streptomycin solution (HyClone, USA). The cells were maintained at 37 °C in a humidified incubator containing 5% CO2. THP-1 macrophages were prepared by stimulating with 100 ng/mL phorbol 12-myristate 13-acetate (PMA, Sigma, USA) for 24 h before use.
2.3. Cell transfection
THP-1 was transfected with small interfering RNA (siRNA) and siRNA-Mate transfection reagent (GenePharma, China) according to the manufacturer’s instructions. Briefly, siRNA (100 nmol/L) and transfection reagent (8 μL) were added to serum-free RPMI-1640 medium, and incubated for 10 min to form a transfection complex. Then the transfection complex was added to cultured THP-1 cells. THP-1 cells were incubated with the transfection complexes at 37 °C for 48 h and then the expression of gene was detected by RT-qPCR and Western blot to assess silence efficiency. The targeted sequences for each gene are as follows: Dectin-1 siRNA (5’-UUCUCCGAACGUGUCACGUTT/ACGUGACACGUUCGGAGAATT-3’), Negative control (5’-GUAGUAAGGAGGACAGAAATT/UUUCUGUCCUCCUUACUACTT-3’).
2.4. Total RNA isolation and qPCR analysis
The mRNA expression of different genes was detected by real-time fluorescence quantitative PCR. Firstly, total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Secondly, cDNA was synthesised using PrimeScript RT Master Mix (TaKaRa, Japan) according to the instructions. In addition, SYBR Green qPCR Master Mix (TaKaRa, Japan) was used to detect all target genes and the control gene GAPDH, and all data were analysed using the 2−ΔΔt method. The primer sequences used in this experiment were as follows: GAPDH forward (5’-AGGTCGGTGTGAACGGATTTG-3’) and reverse (5’-TGTAGACCATGTAGTTGAGGTCA-3’); Dectin-1 forward (5’-TGCTCCCAGCTAGGTGCTCATC-3’) and reverse (5’-TCACTCTGATTGCGGGAAAGGC-3’). The results showed that the siRNA-Dectin-1 group exhibited a significant decrease in the mRNA expression of Dectin-1 as compared to the control group with only transfection reagent siRNA-Mate and the negative control group (P < 0.05; Figure S2a).
2.5. Western blotting
THP-1 macrophages were subjected to protein extraction using RIPA lysis buffer (Beyotime) supplemented with a phosphatase inhibitor cocktail (Beyotime, China) at a ratio of 100:1. After homogenisation, the whole protein extracts were separated by 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE, Beyotime, China) and transferred onto polyvinylidene difluoride membranes (PVDF, Millipore, USA). The membranes were blocked with 5% skim milk (Absin, China) for 1 h, incubated overnight at 4 °C with primary antibodies to Dectin-1 (1:1,000 Abcam, USA), and then incubated with the secondary antibodies at room temperature for 1 h. Antibodies against β-actin (1:10,000, Abcam, USA) were used as an internal control. Finally, chemiluminescence detection was carried out using an ECL Plus kit (Beyotime, China). The results showed that the siRNA-Dectin-1 group exhibited a significant decrease in the protein expression of Dectin-1 as compared to the control group with only transfection reagent siRNA-Mate and the negative control group (P < 0.05; Figure S2b).
2.6. The co-culture system of THP-1 and F. monophora
THP-1 macrophages were seeded into a 6-well plate at a concentration of 1 × 105 cells/mL and stimulated with PMA (100 ng/mL) for 48 h. Control groups were treated with siRNA-Mate transfection reagent only, while experimental groups were treated with mixed siRNA-Dectin-1 and siRNA-Mate transfection reagent. The 6-well plate was incubated in an incubator containing 5% CO2 at 37 °C for a further 48 h. Mel+ was added to THP-1 with siRNA-Mate transfection reagent only or mixed siRNA-Dectin-1 and siRNA-Mate transfection reagent at a cell-to-spore/hyphae ratio of 1:1, so as Mel- was added.
2.7. Evaluation of the macrophage killing effect
THP-1 macrophages (1 × 105 cells/well in a 6-well plate) in RPMI medium were incubated with Mel+ or Mel- conidia and small hyphae (multiplicity of infection [MOI] = 1). After 24 h of incubation at 37 °C with the conidia, intracellular F. monophora cells were harvested. Subsequently, the wells were washed twice with ice-cold PBS to remove the conidia not phagocytosed by macrophages from the culture system. All samples were washed twice with PBST, then phagocytes were lysed by incubation in 2% Triton X-100 for 10 min. The released F. monophora was diluted by 100 times and plated onto PDA and incubated for 7 days at 28 °C. The colonies were then counted to determine the number of colony-forming units (CFU). The killing rate of THP-1 macrophages against F. monophora was calculated as follows: (CFU of control group – CFU of treatment group)/CFU of control group × 100%. The test was performed in triplicate.
2.8. Evaluation of the macrophage phagocytosis
Add 100 μL of 100 μg/mL fluorescein isothiocyanate (FITC) to F. monophora suspension and stained overnight at 4 °C. The stained precipitate was obtained by centrifuging the suspension at 4 °C for 5 min at 10,000 r/min and washing it with PBS 3 times. THP-1 macrophages were co-cultured with the stained F. monophora (MOI = 1). After co-culture for 4 h, the spores and hyphae that had not been engulfed were removed by washing with PBS 3 times. The cells were fixed with 1 mL of 4 °C paraformaldehyde at 25 °C for 20 min and washed 3 times with PBS. Add 1 mL 0.5% TritonX-100 and react at 25 °C for 10 min, then wash with PBS 3 times. 1 mL of 100 μg/mL 4’,6-diamidino-2-phenylindole (DAPI) was added to each well, incubated at 37 °C for 15 min, and then washed with PBS 3 times. The staining was observed under a fluorescence microscope and the phagocytosis rate was calculated using the formula: (number of macrophages with internalised F. monophora /number of macrophages in the visual field) × 100%. The experiment was performed in triplicate and 10 different fields were counted.
2.9. Measurement of proinflammatory cytokines and nitric oxide (NO) production
Mel+ and Mel- conidia infected Dectin-1 normal and low expressing THP-1 macrophages, respectively. Untreated cells and cells pretreated with 1 μg/mL depleted zymosan (Invivogen, San Diego, CA) (which only activates Dectin-1) were added as a negative control, cells pretreated with 100 ng/mL LPS (Sigma, USA) (TLR4 ligand) were added as a positive control. The level of tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) in macrophage supernatants were assayed with ELISA kits (Boster, China) under the manufacturer’s instructions. The cytokine concentrations were calculated using standard curves for each test. Nitric oxide (NO) production was analysed using NO assay kit (nitrate reductase method) (Sangon Biotech, Shanghai, China). The optical density (OD) was measured at an absorption wavelength of 550 nm and the nitrite concentration was calculated from the nitrite standard reference curve. The test was performed in triplicate.
2.10. Statistical analysis
The GraphPad Prism software was utilised to analyse data for statistical significance. Data are presented as mean±SD. Unpaired student’s t-test or ANOVA (Analysis of Variance) was used to determine the significance between two or multiple groups, and P < 0.05 was considered to be statistically significant.
3. Results
3.1. Killing effect of macrophages on F. monophora
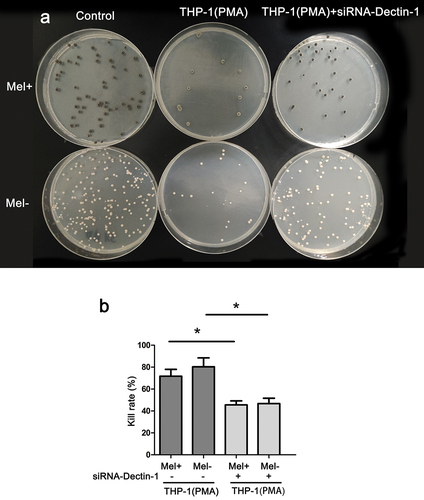
After co-culturing macrophages with F. monophora for 24 h, the fungal solution was diluted and spread onto a PDA plate for cultivation. The PDA plates were then incubated for 14 days, and subsequently, grey-green or white colonies were observed on the plates (). The killing rate of THP-1 macrophages with normal Dectin-1 expression against Mel+ was slightly lower than that of Mel-. However, when compared with the untransfected group, the transfected macrophages exhibited a significantly decreased killing rate against both Mel+ and Mel- (P < 0.05; ).
Figure 1. Killing rate of macrophages with different expressions of Dectin-1 to Fonsecaea monophora wild strain (Mel+) or melanin-deficient mutant strain (Mel-). (a) Colony image of surviving F. monophora cultured on PDA for 1 week after 24 h co-culture with macrophages. (b) Killing rate of group (*: P < 0.05).
3.2. Phagocytosis effects of macrophages on F. monophora
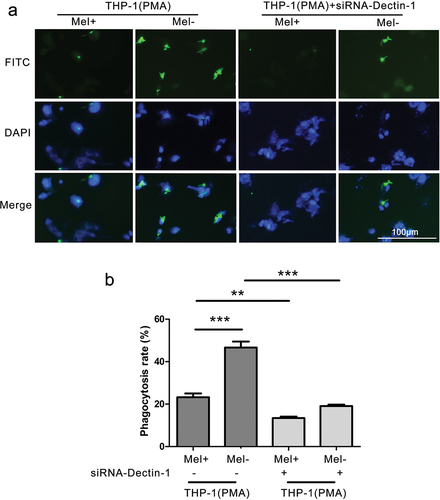
When observed under a fluorescence microscope, macrophages with normal or low expression of Dectin-1 were observed to phagocytose F. monophora (). After 4 hours of co-culture, the phagocytosis rate of macrophages with normal Dectin-1 expression against Mel+ was lower than that of Mel-. After cell transfection, the phagocytosis rate of the macrophages with low Dectin-1 expression was lower than that of the macrophages with normal Dectin-1 expression against Mel+ and Mel- (P < 0.001; ). Nevertheless, there was no significant difference in the phagocytosis rate of macrophages with low Dectin-1 expression against Mel+ and Mel-.
Figure 2. Phagocytic rate of macrophages with different expressions of Dectin-1 to Fonsecaea monophora Mel+ or Mel-. (a) After 4 hours of co-culture with macrophages and F. monophora Mel+ or Mel-, phagocytic image under inverted fluorescence microscopy at a magnification of 200; F. monophora was stained with FITC (green), and macrophage nuclei were stained with DAPI (blue). (b) Phagocytic rate of each group (**: P < 0.01; ***: P < 0.001).
3.3. Proinflammatory cytokines expression and NO production of macrophages on F. monophora
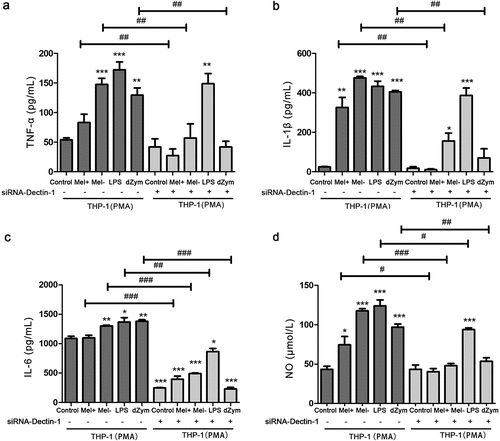
The levels of TNF-α, IL-1β, IL-6, and NO were higher in LPS (TLR4 ligand)-treated THP-1 macrophages with normal and low expression of Dectin-1 than in the blank control group (P < 0.05); while the levels of TNF-α, IL-1β, IL-6, and NO in depleted-zymosan (which only activates Dectin-1)-treated THP-1 macrophages were only higher than those in the blank control group (P < 0.05). After macrophages were co-cultured with F. monophora for 24 hours, the levels of TNF-α, IL-1β, IL-6, and NO in the supernatant of the Mel- co-culture group were significantly higher than those of the blank group and Mel+ group. After being co-cultured with either Mel+ or Mel- for 24 hours, macrophages that expressed low levels of Dectin-1 showed decreased levels of TNF-α, IL-1β, IL-6, and NO in the supernatant compared to the corresponding Dectin-1 normal expression macrophage co-culture group. However, there was no significant difference in the levels of TNF-α and IL-1β in the supernatant of macrophages with low Dectin-1 expression between the blank group, Mel+ co-culture group, and Mel- co-culture group ().
Figure 3. The production proinflammatory cytokines and NO by macrophages with different expressions of Dectin-1 infected by Mel+ and Mel-. after macrophages with different Dectin-1 expression levels were co-cultured with F. monophora Mel+ or Mel- for 24 hours, the expression levels of inflammatory cytokines TNF-α (a), IL-1β (b) and IL-6 (c) in co-cultured supernatants. (d) NO production in each group (*: P < 0.05, **: P < 0.01, ***: P < 0.001; #: P < 0.05, ##: P < 0.01, ###: P < 0.001).
4. Discussions
Innate immunity forms the first line of defence against external pathogen invasion (Brubaker et al. Citation2015). Macrophages are an important component of this system, as they are capable of recognising, phagocytising, and eliminating invading fungi. Hayakawa et al. (Citation2006) demonstrated that the macrophage-mediated killing rate varies depending on the type of pathogenic fungus, with F. pedrosoi, C. carrionii, R. aquaspersa, and P. verrucosa being highly susceptible to macrophage-mediated killing. However, the phagocytic index of macrophages towards different fungi varies significantly. F. monophora, one of the most common pathogenic fungi causing CBM in southern China, is susceptible to macrophage phagocytosis and killing (Qin et al. Citation2020). Siqueira et al. (Citation2017) demonstrated that the sclerosis bodies of F. pedrosoi require recognition by FcγR and Dectin-1 for internalisation in vivo. However, it is not clear whether the melanin of F. monophora affects phagocytosis and killing of macrophages by altering the recognition of Dectin-1. In our study, F. monophora Mel+ and Mel- were co-cultured with THP-1 macrophages for 24 hours, and the results showed that the phagocytosis and killing rate of macrophages against Mel+ was lower than that of Mel-. Macrophages recognise β-glucan on the cell wall of F. monophora through PRRs on the surface. Melanin on the cell wall of Mel+ may prevent macrophages from recognising and phagocytising F. monophora by shielding the exposure of β-glucan. Similar to our study, Keizer et al. (Citation2020) investigated the binding and internalisation of A549 epithelial II type lung cells to conidia of Aspergillus fumigatus. The dihydroxy naphthalene (DHN) melanin-deficient strain was found to expose more glucosamine and glycoprotein, making it easier for A549 cells to internalise. This indicates that the presence of DHN melanin in the spore cell wall inhibits conidia internalisation into A549 cells (Keizer et al. Citation2020). The killing rate of macrophages with normal expression of Dectin-1 against Mel+ was slightly lower than that of Mel- (71.68% ± 6.26% vs 80.29% ± 8.176%), but there was no statistical difference between them (P >0.05). This may be due to another C-type lectin receptor, the melanin-sensing C-type lectin receptor (MelLec), which can recognise the immunologically active component in the cell wall of melanised fungi-1,8-dihydroxy naphthalene (DHN)-melanin (Stappers et al. Citation2018). MelLec is expressed on myeloid cells, including various DC cell populations, monocytes, macrophages, and granulocytes. In mice, MelLec deficiency leads to reduced survival and increased fungal load in a systemic model of Aspergillus fumigatus infection through delayed neutrophil recruitment. Additionally, polymorphisms in the MelLec were associated with increased susceptibility to disseminated Aspergillus infection in stem cell transplant patients. Mel+ may be killed after the melanin on its surface is recognised by the MelLec receptor. However, the specific mechanism remains unclear, and more studies are needed to elucidate the interaction between F. monophpra melanin and MelLec receptors on the surface of macrophages. The effect of melanin on fungal killing by macrophages may be a “double-edged sword”, preventing phagocytosis by binding the membrane Dectin-1 receptor to β-glucan on the one hand, but on the other hand, it may directly bind to MelLec to promote receptor binding. We suggest that this may be a dynamic balance, with melanin inhibiting phagocytosis when Dectin-1 expression is high on the membrane surface, but promoting phagocytosis when MelLec expression is high on the membrane surface.
Previous studies have demonstrated that Dectin-1 plays a vital role in host recognition and phagocytosis of invading pathogens. For instance, the phagocytosis of macrophages treated with siRNA-Dectin-1 to Candida albicans was significantly lower than that of the siRNA-NC group, indicating that Dectin-1 can mediate phagocytosis of macrophages to Candida albicans. Additionally, the up-regulation of Dectin-1, TLR-2, and TLR-4 can enhance the phagocytosis and killing effect of macrophages against Aspergillus fumigatus (Liu et al. Citation2017). Inhibition of Dectin-1 by monoclonal antibodies has been shown to impede the binding and internalisation of Aspergillus conidia into A549 epithelial type II lung cells (Keizer et al. Citation2020). Previous studies utilised a specific monoclonal antibody, 2G8, to label the β-glucan present on the surface of Aspergillus fumigatus conidia. Immunofluorescence results indicated that pksP deficient conidia had a higher concentration of β-glucan on their surface compared to resting conidia. This increased exposed of β-glucan was recognised and phagocytised by macrophages through the Dectin-1 receptor present on their surface. To further explore the impact of F. monophora melanin on the phagocytosis and killing effect of macrophages through Dectin-1, this study treated macrophages with siRNA-Dectin-1. In this study, macrophages with normal and low Dectin-1 expression were co-cultured with F. monophora Mel+ and Mel-. The results indicated that the phagocytosis and killing rates of macrophages with low Dectin-1 expression against Mel+ and Mel- were lower than those of the corresponding normal Dectin-1 expression group. Furthermore, when Dectin-1 expression was low, there was no significant difference in the phagocytosis rate and killing rate of macrophages against Mel+ and Mel-. This is because F. monophora melanin can prevent the β-glucan on the cell wall surface from binding to Dectin-1, resulting in Mel+ evading phagocytosis and causing continuous infection. As a result, the inhibitory effect of melanin on phagocytosis by blocking β-glucan could not be reflected when Dectin-1 expression was low, and there was no significant difference in phagocytosis rate between Mel+ and Mel-.
In addition, melanin may also affect the recognition of F. monophora by other C-type lectin receptors or PRRs, such as Dectin-2, Mincle, FcγR, CR3, TLR2, TLR4 etc. Dectin-2 recognises α-mannan on the surface of F. pedrosoi conidia and induces Th17 cell differentiation. In the chronic phase of CBM, Dectin-2-/- mice have reduced Th17 cell responses and increased fungal burden (Siqueira et al. Citation2020). Simultaneously, F. monophora binds to Mincle, which blocks IL12A transcription, and IL-12 deficiency leads to impaired Th1 responses, promotes Th2 polarisation, and suppresses antifungal immunity (Wevers et al. Citation2014). Melanin may affect Dectin-2-mediated Th17 immune responses and Mincle-mediated Th2 immune responses by masking fungal surface α-mannans. If other membrane surface receptors are blocked, the phagocytosis of macrophages on F. monophora may be affected, such as FcγR, CR3, etc. A study showed that phagocytosis of F. pedrosoi muriform cells by macrophages was impaired when FcγR was blocked (Siqueira et al. Citation2017). Another study showed that CR3 plays an important role in β-glucan recognition, as blocking this receptor significantly decreased phagocytosis of β-glucan and β-glucan-induced ROS production by neutrophils (Baert et al. Citation2015). In another study, melanin inhibits phagocytosis of Sporothrix globosa and defence against macrophage attack by protecting oxygen- and nitrogen-derived free radicals, as well as inhibiting host pro-inflammatory cytokine responses (TNF-α and IL-6). Melanin is also involved in regulating the expression of TLR2 and TLR4 receptors, impairing the killing efficiency of S. globosa (Guan et al. Citation2021). Moreover, S. globosa melanin inhibits macrophage autophagy by regulating TLR2 expression and thus suppresses the immune defence of macrophages (Guan et al. Citation2023). Recognition of F. monophora (including FcγR, CR, Dectin-2, Mincle, MelLec, TLR, etc.) by other PRRs is not affected by siRNA-Dectin-1. However, the presence of melanin may affect the recognition of F. monophora by different PRRs at the same time, which still needs to be verified by further experiments.
Macrophages can secrete various cytokines and chemokines such as TNF-α, IL-1β, IL-6, IL-12, CXCL1, and CXCL2 to improve innate immunity and adaptative response (Zhang et al. Citation2013). De Castro et al. (Citation2017) have shown that the IL-1β production in macrophages after infection of F. Pedrosoi is dependent on Dectin-1, −2, and −3 receptors, as well as the Syk-NF-κB signalling pathway. In addition to its role in recognising fungal pathogens, Dectin-1 has also been shown to recognise endogenous ligands on the surface of T cells, promoting T cell activation and proliferation, and acting as a co-stimulator for antigen-presenting cells. However, it remains unclear whether F. monophora melanin affects the release of proinflammatory cytokines from macrophages, and if this effect is related to Dectin-1 recognition. The results of this study revealed that the proinflammatory cytokines TNF-α, IL-1β, IL-6, and NO released by macrophages after infection of the F. monophora Mel+ were lower compared to those released by Mel- group. This suggests that Mel+ had a weak immunogenicity possibly due to the presence of melanin on its surface that can mask PAMPs, reducing the induced cytokine response. In contrast, Mel- lacked melanin coverage on its surface, which allowed for better exposure of PAMPs to induce more proinflammatory cytokines. In a previous study, we obtained melanin mutants of F. monophora from a case of CBM, one of which was a melanised strain producing melanin, and the other was an albino strain lacking melanin. In vitro and in vivo experiments revealed that compared to the albino strain, the melanised strain was more effective in inhibiting the pro-inflammatory Th1 and Th17 reactions while enhancing the anti-inflammatory Th2 reaction. These findings suggest that melanin helps F. monophora to evade the host’s immune system and enhance its pathogenicity (Jiang et al. Citation2018). Similarly, Chai et al. (Citation2010) conducted experiments wherein human peripheral blood mononuclear cells were infected with either Aspergillus melanised conidia or albino conidia. Interestingly, the results revealed that albino conidia triggered the production of a higher amount of proinflammatory cytokines when compared to the melanised wild strain conidia. This finding indicates that the presence of a melanin layer might hinder the production of inflammatory responses by preventing the recognition of fungal PAMPs by host PRRs.
Previous studies have shown that Dectin-1 is one of the important receptors for host recognition of pathogens. Mezger et al. (Citation2008) showed that the silencing of Dectin-1 resulted in decreased expression of proinflammatory cytokines TNF-α and IL-12 in dendritic cells when exposed to Aspergillus fumigatus. Yuan et al. (Citation2017) showed that the production of IL-1β and the phosphorylation level of JNK in C57BL/6 mice pretreated with siRNA-Dectin-1 were significantly lower than those in the normal Dectin-1 expression group after corneal infection by Aspergillus fumigatus. In addition, compared with cells isolated from healthy donors, Aspergillus fumigatus has a weaker stimulatory effect on PBMCs isolated from Dectin-1-deficient individuals to produce cytokines (Chai et al. Citation2010). Our study also reveals that macrophages treated with siRNA-Dectin-1 produced lower levels of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 than the corresponding Dectin-1 normal expression group after infection with F. monophora Mel+ and Mel-. These findings suggest that F. monophora Mel+ and Mel- can activate Dectin-1 receptors on the macrophage surface and mediate cytokine production to induce an immune response. Furthermore, there was no significant difference in the release of proinflammatory factors between the siRNA-Dectin-1 treated macrophages infected with Mel- and Mel+. This is due to the decreased expression of Dectin-1 on the macrophage surface after transfection with siRNA-Dectin-1, resulting in a significant reduction in the binding of β-glucan to Dectin-1 on the surface of F. monophora Mel+ and Mel-. At this point, even though melanin has a shielding effect on β-glucan, it has no impact on the release of proinflammatory factors.
Dectin-1 is a receptor that recognises β-glucan present in the fungal cell wall, and it can trigger various immunomodulatory responses like phagocytosis and activation of oxidative bursts. When fungi are recognised by Dectin-1 receptors on macrophage surfaces, downstream signalling pathways are activated, leading to inflammation. The activation of Syk and the transcription factor NF-κB by β-glucan is dependent on Dectin-1, which then mediates the production of numerous proinflammatory factors (Jia et al. Citation2014). Duan et al. (Citation2017) showed that after macrophage infected by Candida tropicalis, the expression of Dectin-1 increased, the NF-κB, Syk, p38, and ERK1/MAPK signalling pathways are activated and the secretion of TNF-α and IL-6 enhanced in a time and dose-dependent manner. However, this study has only confirmed the impact of melanin on the inflammatory response through Dectin-1 in vitro. To explore its mechanism further, further studies can be carried out in the next step: 1) To conduct more experiments on the determination of macrophage activation indicators (including ROS, iNOS2, CD14, CD68, etc.). 2) To investigate the expression difference of downstream signalling pathway proteins such as Syk, p65, NF-κB, and CARD9. 3) To construct the Dectin-1 knock-out macrophage and evaluate the role of Dectin-1 in the immune recognition of F. monophora Mel+ and Mel-. 4) To conduct functional acquisition experiments through the overexpression of Dectin-1 by lentiviral plasmids. 5) To construct the wild strain and Dectin-1-/- mouse foot pad models of F. monophora infection, and compare the fungal load, cytokines levels, and histological characteristics of the skin infected by Mel+ and Mel-.
5. Conclusions
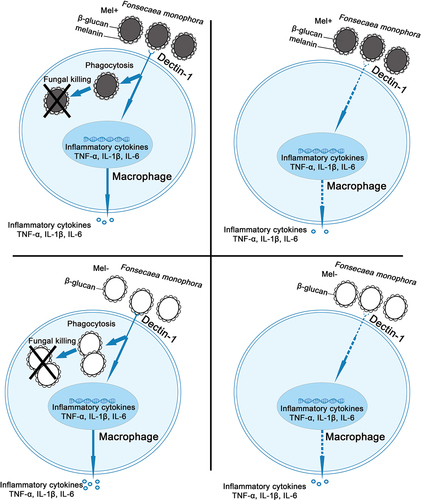
Our findings demonstrate that when Dectin-1 is expressed normally, macrophages exhibit lower killing rate, phagocytosis rate, and proinflammatory factor level against Mel+ than Mel-. However, when Dectin-1 was lowly expressed, the killing rate, phagocytosis rate, and proinflammatory factor level decreased significantly against both Mel+ and Mel-. Moreover, with low Dectin-1 expression, there was no significant difference in the killing rate, phagocytosis rate, and proinflammatory factor level between Mel+ and Mel-. These results demonstrate that melanin can prevent the recognition of F. monophora by the Dectin-1 receptor on the surface of macrophages, thereby inhibiting the inflammatory response and leading to persistent fungal infection ().
Figure 4. Role of Dectin-1 in immune response of macrophages induced by Fonsecaea monophora Mel+ and Mel-.
Author contributions
Jiaojiao Zhong, Junmin Zhang, and Jing Zhang designed the experiment; Jiaojiao Zhong and Wenying Cai conducted the experiment; Junmin Zhang, Xiqing Li, and Jianchi Ma revised and reviewed the article.
Supplemental Material
Download Zip (1 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21501203.2023.2249010.
Additional information
Funding
References
- Baert K, Sonck E, Goddeeris BM, Devriendt B, Cox E. 2015. Cell type-specific differences in beta-glucan recognition and signalling in porcine innate immune cells. Dev Comp Immunol. 48(1):192–203. doi: 10.1016/j.dci.2014.10.005.
- Brito AC, Bittencourt MJS. 2018. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 93(4):495–506. doi: 10.1590/abd1806-4841.20187321.
- Brubaker SW, Bonham KS, Zanoni I, Kagan JC. 2015. Innate immune pattern recognition: A cell biological perspective. Annu Rev Immunol. 33(1):257–290. doi: 10.1146/annurev-immunol-032414-112240.
- Chai LY, Netea MG, Sugui J, Vonk AG, van de Sande WW, Warris A, Kwon-Chung KJ, Kullberg BJ, van de Sande WWJ. 2010. Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology. 215(11):915–920. doi: 10.1016/j.imbio.2009.10.002.
- de Castro, RJA, Siqueira IM, Jeronimo MS, Basso AMM, Veloso Junior PHH, Magalhaes KG, Leonhardt LC, de Oliveira, SAM, Burgel PH, Tavares AH, et al. 2017. The major chromoblastomycosis etiologic agent Fonsecaea pedrosoi activates the NLRP3 inflammasome. Front Immunol. 8:1572. doi: 10.3389/fimmu.2017.01572.
- De Hoog GS, Attili-Angelis D, Vicente VA, Den Ende AH V, Queiroz-Telles F. 2004. Molecular ecology and pathogenic potential of Fonsecaea species. Med Mycol. 42(5):405–416. doi: 10.1080/13693780410001661464.
- Drummond RA, Brown GD. 2011. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol. 14(4):392–399. doi: 10.1016/j.mib.2011.07.001.
- Duan Z, Chen X, Du L, Liu C, Zeng R, Chen Q, Li M. 2017. Inflammation induced by Candida parapsilosis in THP-1 cells and human peripheral blood mononuclear cells (PBMCs). Mycopathologia. 182(11–12):1015–1023. doi: 10.1007/s11046-017-0187-8.
- Fransisca C, He Y, Chen Z, Liu H, Xi L. 2017. Molecular identification of chromoblastomycosis clinical isolates in Guangdong. Med Mycol. 55(8):896. doi: 10.1093/mmy/myx093.
- Guan M, Yao L, Zhen Y, Song Y, Liu X, Liu Y, Chen R, Cui Y, Li S, Nosanchuk J. 2023. Sporothrix globosa melanin regulates autophagy via the TLR2 signaling pathway in THP-1 macrophages. PLoS Negl Trop Dis. 17(5):e0011281. doi: 10.1371/journal.pntd.0011281.
- Guan MQ, Yao L, Zhen Y, Song Y, Cui Y, Li SS. 2021. Melanin of Sporothrix globosa affects the function of THP-1 macrophages and modulates the expression of TLR2 and TLR4. Microb Pathog. 159:105158. doi: 10.1016/j.micpath.2021.105158.
- Hayakawa M, Ghosn EE, da Gloria Teixeria de Sousa M, Ferreira KS, Almeida SR. 2006. Phagocytosis, production of nitric oxide and pro-inflammatory cytokines by macrophages in the presence of dematiaceous [correction of dematiaceus] fungi that cause chromoblastomycosis. Scand J Immunol. 64(4):382–387. doi: 10.1111/j.1365-3083.2006.01804.x.
- Jia XM, Tang B, Zhu LL, Liu YH, Zhao XQ, Gorjestani S, Hsu YM, Yang L, Guan JH, Xu GT, et al. 2014. CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity. J Exp Med. 211(11):2307–2321. doi:10.1084/jem.20132349.
- Jiang M, Cai W, Zhang J, Xie T, Xi L, Li X, Sun J. 2018. Melanization of a meristematic mutant of Fonsecaea monophora increase the pathogenesis in a BALB/c mice infection model. Med Mycol. 56(8):979–986. doi: 10.1093/mmy/myx148.
- Keizer EM, Wosten HAB, de Cock H. 2020. EphA2-dependent internalization of A. fumigatus conidia in A549 lung cells is modulated by DHN-Melanin. Front Microbiol. 11:534118. doi:10.3389/fmicb.2020.534118.
- Kurien G, Sugumar K, Chandran V. 2023. Chromoblastomycosis. USA: StatPearls. Treasure Island (FL). [accessed 2023 Mar 8]. https://www.ncbi.nlm.nih.gov/books/NBK470253/.
- Liu C, Wang M, Sun W, Cai F, Geng S, Su X, Shi Y. 2017. PU.1 serves a critical role in the innate defense against Aspergillus fumigatus via dendritic cell-associated C-type lectin receptor-1 and toll-like receptors-2 and 4 in THP-1-derived macrophages. Mol Med Rep. 15(6):4084–4092. doi: 10.3892/mmr.2017.6504.
- Mezger M, Kneitz S, Wozniok I, Kurzai O, Einsele H, Loeffler J. 2008. Proinflammatory response of immature human dendritic cells is mediated by dectin-1 after exposure to Aspergillus fumigatus germ tubes. J Infect Dis. 197(6):924–931. doi: 10.1086/528694.
- Qin J, Zhang J, Shi M, Xi L, Zhang J. 2020. Effect of Fonsecaea monophora on the polarization of THP–1 cells to macrophages. Mycopathologia. 185(3):467–476. doi: 10.1007/s11046-020-00444-x.
- Santos AL, Palmeira VF, Rozental S, Kneipp LF, Nimrichter L, Alviano DS, Rodrigues ML, Alviano CS. 2007. Biology and pathogenesis of Fonsecaea pedrosoi, the major etiologic agent of chromoblastomycosis. FEMS Microbiol Rev. 31(5):570–591. doi: 10.1111/j.1574-6976.2007.00077.x.
- Shi M, Li X, Feng J, Jia S, Xiao X, Chen C, Fransisca C, Xi L, Zhang J. 2018. High-resolution melting analysis assay for identification of Fonsecaea species. J Clin Lab Anal. 32(2):e22257. doi: 10.1002/jcla.22257.
- Shiokawa M, Yamasaki S, Saijo S. 2017. C-type lectin receptors in anti-fungal immunity. Curr Opin Microbiol. 40:123–130. doi: 10.1016/j.mib.2017.11.004.
- Siqueira IM, de Castro RJA, Leonhardt LCM, Jeronimo MS, Soares AC, Raiol T, Nishibe C, Almeida N, Tavares AH, Hoffmann C, et al. 2017. Modulation of the immune response by Fonsecaea pedrosoi morphotypes in the course of experimental chromoblastomycosis and their role on inflammatory response chronicity. PLoS Negl Trop Dis. 11(3):e0005461. doi: 10.1371/journal.pntd.0005461.
- Siqueira IM, Wuthrich M, Li M, Wang H, Las-Casas LO, de Castro, RJA, Klein B, Bocca AL. 2020. Early immune response against Fonsecaea pedrosoi requires Dectin-2-mediated Th17 activity, whereas Th1 response, aided by Treg cells, is crucial for fungal clearance in later stage of experimental chromoblastomycosis. PLoS Negl Trop Dis. 14(6):e0008386. doi: 10.1371/journal.pntd.0008386.
- Soda M, Ito S, Matsumaru N, Nakamura S, Nagase I, Takahashi H, Ohno Y, Yasuda M, Yamamoto M, Tsukamoto K, et al. 2018. Evaluation of the microbiological efficacy of a Single 2-gram dose of extended-release azithromycin by population pharmacokinetics and simulation in Japanese patients with gonococcal urethritis. Antimicrob Agents Chemother. 62(1):e01409–17. doi: 10.1128/AAC.01409-17.
- Stappers MHT, Clark AE, Aimanianda V, Bidula S, Reid DM, Asamaphan P, Hardison SE, Dambuza IM, Valsecchi I, Kerscher B, et al. 2018. Recognition of DHN-melanin by a C-type lectin receptor is required for immunity to Aspergillus. Nature. 555(7696):382–386. doi: 10.1038/nature25974.
- Sun J, Zhang J, Najafzadeh MJ, Badali H, Li X, Xi L, de Hoog GS. 2011. Melanization of a meristematic mutant of Fonsecaea monophora increases tolerance to stress factors while no effects on antifungal susceptibility. Mycopathologia. 172(5):373–380. doi: 10.1007/s11046-011-9439-1.
- Wevers BA, Kaptein TM, Zijlstra-Willems EM, Theelen B, Boekhout T, Geijtenbeek TB, Gringhuis SI. 2014. Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host & Microbe. 15(4):494–505. doi: 10.1016/j.chom.2014.03.008.
- Xi L, Lu C, Sun J, Li X, Liu H, Zhang J, Xie Z, De Hoog GS. 2009. Chromoblastomycosis caused by a meristematic mutant of Fonsecaea monophora. Med Mycol. 47(1):77–80. doi: 10.1080/13693780802322588.
- Xiao X, Li Y, Lan Y, Zhang J, He Y, Cai W, Chen Z, Xi L, Zhang J. 2021. Deletion of pksA attenuates the melanogenesis, growth and sporulation ability and causes increased sensitivity to stress response and antifungal drugs in the human pathogenic fungus Fonsecaea monophora. Microbiol Res. 244:126668. doi: 10.1016/j.micres.2020.126668.
- Yuan K, Zhao G, Che C, Li C, Lin J, Zhu G, He K. 2017. Dectin-1 is essential for IL-1beta production through JNK activation and apoptosis in Aspergillus fumigatus keratitis. Int Immunopharmacol. 52:168–175. doi: 10.1016/j.intimp.2017.09.008.
- Zhang J, Wang L, Xi L, Huang H, Hu Y, Li X, Huang X, Lu S, Sun J. 2013. Melanin in a meristematic mutant of Fonsecaea monophora inhibits the production of nitric oxide and Th1 cytokines of murine macrophages. Mycopathologia. 175(5–6):515–522. doi: 10.1007/s11046-012-9588-x.

