ABSTRACT
The application of interleukin-17 (IL-17) inhibitors, including secukinumab, ixekizumab, brodalumab, and bimekizumab, are associated with elevated risk of candidiasis. These medications interfere with the IL-17 pathway, which is essential for maintaining mucosal barriers and coordinating the immune response against Candida species. The observational data and clinical trials demonstrate the increased incidence of candidiasis in individuals treated with IL-17 inhibitors. Brodalumab and bimekizumab pose a greater risk than secukinumab in eliciting candidiasis, whereas the data regarding ixekizumab are equivocal. Higher doses and prolonged treatment duration of IL-17 inhibitors increase the risk of candidiasis by compromising the immune response against Candida species. Prior to prescribing IL-17 inhibitors, healthcare professionals should comprehensively evaluate patients’ medical histories and assess their risk factors. Patients should be educated on the signs and symptoms of candidiasis to facilitate early detection and intervention. Future research should focus on identifying the risk factors associated with candidiasis in patients receiving IL-17 inhibitors. Prospective studies and long-term surveillance are required to explore the impact of specific inhibitors on the incidence and severity of candidiasis and to evaluate the effectiveness of combination therapies, such as concurrent use of IL-17 inhibitors and prophylactic antifungal agents.
1. Introduction
Candidiasis is a common fungal infection caused by the Candida species that can affect several parts of the body, including the skin, mouth, throat, and even genital areas (Lopes and Lionakis Citation2022). The Candida species are commensal organisms that typically reside innocuously on healthy humans’ epidermis and mucous membranes (Shao et al. Citation2022). However, Candida can overgrow and lead to infection under specific circumstances, such as immunosuppression, antibiotic use, or diabetes (Peroumal et al. Citation2022).
Interleukin-17 (IL-17) is a cytokine that performs a vital role in the immune response against candidiasis (Wilharm et al. Citation2022). IL-17 is produced by Th17 immune cells and is involved in the defence against extracellular bacterial and fungal pathogens (Azadeh et al. Citation2022). IL-17 promotes the activation and recruitment of neutrophils and other immune cells at the site of infection, thereby aiding in eliminating the pathogen. Additionally, IL-17 stimulates the production of immune mediators and antimicrobial peptides that help defend the host against fungal infections (Berry et al. Citation2022).
IL-17 inhibitors are biologics medications that block the activity of IL-17 and are used to treat psoriasis, rheumatoid arthritis, and ankylosing spondylitis (Perrotta et al. Citation2022). These drugs can reduce inflammation and tissue injury associated with autoimmune diseases by targeting IL-17 or its receptors. Nonetheless, IL-17 inhibitors may have undesirable side effects, such as a higher risk of infections, allergies, or metabolic disorders (Wang et al. Citation2023).
Several studies have found that using IL-17 inhibitors may increase the incidence of candidiasis. This is because these medications suppress the immune response, which is essential in fighting candidiasis (Davidson et al. Citation2022). In a randomised, controlled trial of psoriasis patients treated with secukinumab (an IL-17 inhibitor), the incidence of oral candidiasis was higher in the secukinumab group compared to the placebo group (Feng et al. Citation2022). Another research on individuals with rheumatoid arthritis receiving ixekizumab, another IL-17 inhibitor, revealed that the incidence of candidiasis was more significant in the ixekizumab group than in the placebo group (Ruggiero et al. Citation2022).
The link between candidiasis and IL-17 inhibitors has prompted concerns among physicians and patients regarding possible adverse reactions to these drugs (Saeki et al. Citation2023). However, it is noteworthy that not all IL-17 inhibitors treated individuals are susceptible to candidiasis, and the risk of infection may differ based on the patient’s underlying condition, the dose and duration of therapy, and the type of IL-17 inhibitors (Pettas et al. Citation2021).
Clinicians may employ several strategies to reduce the hazards of candidiasis linked with IL-17 inhibitors, including observing patients for signs of candidiasis, using antifungal prophylaxis in patients at high risk, and modifying the treatment dose and duration (Yeung et al. Citation2022). In addition, patients also need to be informed about the signs of candidiasis and advised to get medical assistance if they encounter any of these symptoms (Bruno et al. Citation2022).
This article provides an in-depth analysis of IL-17 and its inhibitors and discusses IL-17’s involvement in the immunological response to candidiasis. In addition, the current evidence regarding the risk of candidiasis related to IL-17 inhibitors and the medical implications of these findings will be discussed.
2. Brief overview of interleukin 17 (IL-17)
IL-17 is a family of cytokines consisting of six members (IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F) that play an essential role in immune responses and inflammation (Matsuzaki and Umemura Citation2018). IL-17A is the most thoroughly researched member of the IL-17 family and is predominantly produced by Th17 cells, a subset of CD4+ T cells (Brevi et al. Citation2020). Th17 cells differentiate and produce IL-17A in response to cytokines, such as TGF-β, IL-6, and IL-23, in the presence of IL-21 and IL-1 (Saini et al. Citation2022).
In various cell types, including epithelial cells, fibroblasts, endothelial cells, and immune cells, IL-17A stimulates the production of antimicrobial peptides (such as defensins), chemokines (i-e., CXCL1, CCL20), proinflammatory cytokines (i-e., IL-8, IL-17C), and S100 proteins (e.g. S100A7) (Rioux et al. Citation2021). These cytokines and chemokines recruit and activate other immune cells, including neutrophils, macrophages, and T cells, resulting in an inflammatory response (Mills Citation2023). The neutrophils are recruited to the site of infection through the induction of CXCL1 and CXCL2 production. Neutrophils are the first immune cells to react against infection or damaged tissue and play an essential role in eradicating pathogens (Nie et al. Citation2022). IL-17A amplifies the immune response by producing other proinflammatory cytokines, including IL-6, TNF-α, and IL-1β. In addition, IL-17A plays a critical role in mucosal immunity by promoting the proliferation of B cells and the production of immunoglobulin A (IgA) (Zhang et al. Citation2021).
Other IL-17 family members have distinct functions and are produced by distinct cell types (González-Fernández et al. Citation2020). IL-17B is generated by neutrophils, which stimulate the production of proinflammatory cytokines like TNF-α and IL-6 (Bastid et al. Citation2020). IL-17C is produced by epithelial cells, which stimulates the production of chemokines and antimicrobial peptides (Miura et al. Citation2023). IL-17D is produced by various cells, including dendritic cells, tumour cells, and others, which activate natural killer cells and T cells (Li et al. Citation2022). Th2 cells produce IL-17E, commonly known as IL-25, essential to allergic inflammation (Yuan et al. Citation2023). In last, the IL-17F is produced by Th17 cells, which is implicated in inflammation and mucosal immunity (Jiang et al. Citation2023).
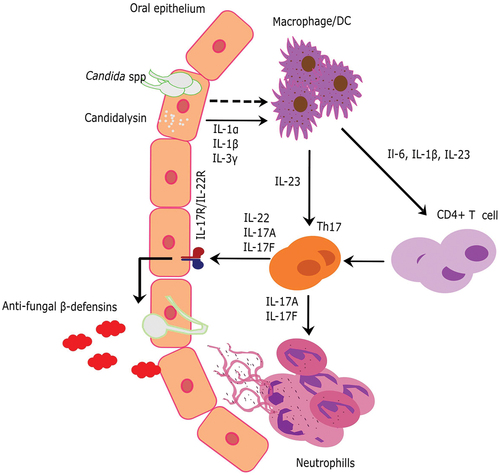
During candidiasis, T-helper 17 (Th17) cells produce IL-17, which activates immune cells, including macrophages and neutrophils, to eliminate the pathogen. IL-17 stimulates the production of various chemokines that recruit immune cells to the infection site, thus boosting their ability to engulf and eliminate the attacking Candida species (Li et al. Citation2019). IL-17 also stimulates the production of antimicrobial peptides, such as cathelicidins and defensins, which have potent fungicidal activity against Candida (: Model) (Finkina et al. Citation2022). Thus, the absence of IL-17 or its signalling pathways can result in fatal and chronic Candida infections (Tangye and Puel Citation2023).
Figure 1. Model of oral candidiasis and the immune system’s response for clearing Candida species. In response to Candida species, macrophages are activated, which recruit CD4+T cells or TH17 cells through various cytokines, in order to activate neutrophils or release β-defensin to clear Candida infection.
On the other hand, excessive IL-17 production has been linked to the pathogenesis of several autoimmune diseases, such as psoriasis, rheumatoid arthritis, and multiple sclerosis (Mills Citation2023). Several studies have shown that blocking IL-17 activity has therapeutic benefits (Bianchi and Rogge Citation2019; Chang Citation2019; Rafael-Vidal et al. Citation2020). For treating these autoimmune disorders, there are currently four biologics that target the IL-17 pathway; however, their adverse effect on the prognosis of candidiasis is a matter of concern (Yeung et al. Citation2022).
3. IL-17 inhibitors and risk of candidiasis
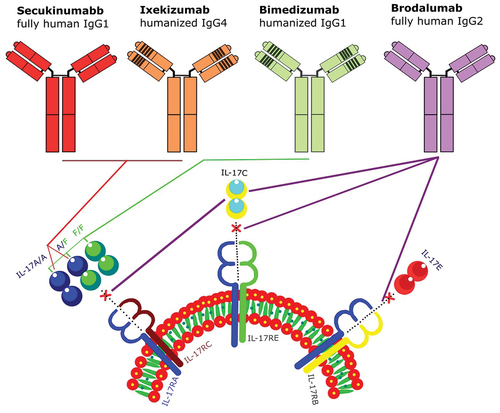
Until now, four biologics blocking the IL-17 pathways are available (). Among these, secukinumab, brodalumab, and ixekizumab, are widely available, although bimekizumab was officially licenced in Canada and Europe and is still waiting for authorisation in the United States (Sbidian et al. Citation2020).
Figure 2. IL-17 inhibitors, attachment sites, and mechanism of action. Secukinumab and ixekizumab interact with IL-17A/A, and A/F, bimekizumab attached to IL-17A/A, A/F, and F/F, and brodalumab inhibit IL-17A, IL-17C, and IL-17E.
3.1. Secukinumab
Secukinumab is a monoclonal antibody (IgG1 kappa) that suppresses the pro-inflammatory cytokine IL-17A, which is involved in the onset of several autoimmune diseases, such as psoriasis, ankylosing spondylitis, and psoriatic arthritis (Toussirot Citation2012). The molecular weight of secukinumab is about 151 kilodaltons, composed of two identical heavy chains and two identical light chains (Cao et al. Citation2022). They prevent IL-17A from binding to its receptor on immune cells through selective binding to its attachment site, lowering inflammation and its associated effects (Patrikiou et al. Citation2020). Depending on the specific condition being treated, secukinumab is administered either subcutaneously or intravenously (Frieder et al. Citation2018). For psoriasis and psoriatic arthritis, it is typically administered 300 mg subcutaneously every week up to five doses, whereas, for ankylosing spondylitis, it may be administered 150 to 300 mg intravenously every week up to five doses (Sanford and McKeage Citation2015).
Various clinical trials were conducted to assess the efficacy of secukinumab and the likelihood of candidiasis. FIXTURE and ERASURE were placebo-controlled, parallel-group, double-blind, phase III studies in adult patients with moderate to severe psoriasis. In the ERASURE trial, candidiasis was recorded in 11 out of 738 participants; one instance was from the placebo group, three cases were people who had been given 150 mg of secukinumab, and seven additional cases were involved those who had been given 300 mg of secukinumab. During the FIXUTRE trial, 11 (2.3%) and 22 (4.7%) patients receiving secukinumab 150 mg and 300 mg, respectively, developed candidiasis (Langley et al. Citation2014). SCULPTURE was a double-blind, randomised, parallel-group phase III trial involving 1,306 patients having persistent plaque psoriasis. Thirteen patients experienced candidiasis during the entire treatment period (Bissonnette et al. Citation2017). JUNCTURE and FEATURE were double-blind, randomised, placebo-controlled phase III trials that evaluated the efficiency and reliability of subcutaneous injections of secukinumab with autoinjectors and prefilled syringes, respectively. The rate of candidiasis was similar to that reported for other trials (Blauvelt et al. Citation2015; Paul et al. Citation2015; Gottlieb et al. Citation2016; Lacour et al. Citation2017). In all clinical trials, candida infections were localised, ranged from mild to moderately severe, and did not result in therapeutic cessation (Yeung et al. Citation2022).
3.2. Ixekizumab
Ixekizumab is a humanised monoclonal antibody (IgG4 kappa) that inhibits IL-17A, which is associated with the pathogenesis of autoimmune diseases such as psoriasis and psoriatic arthritis (Liu et al. Citation2016). Ixekizumab has an approximate molecular weight of 146 kilodaltons and comprises of two identical heavy chains (445 amino acids) and two identical light chains (219 amino acids) (Ixekizumab Citation2006). Ixekizumab minimises inflammation and associated symptoms by inhibiting IL-17A attachment to its receptor on immune cells (Craig and Warren Citation2020). Ixekizumab is administered subcutaneously, with dosage frequency varying depending on the specific condition being treated (van der Heijde et al. Citation2018). It is typically administered as a priming dose of two injections (160 mg) for psoriatic arthritis and ankylosing spondylitis, followed by one injection (80 mg) every four weeks (Fujita et al. Citation2018). Two injections (160 mg) are administered as an initial dose for moderate-to-severe plaque psoriasis, followed by one injection (80 mg) at week 4, 6, 8, 10, and 12 (Mease et al. Citation2020).
UNCOVER-1, −2, and 3 were placebo-controlled, double-blind, randomised, phase III studies for psoriasis. There were 16 cases of candidiasis reported from the combined data of all trials. The number of cases within weeks 0 and 60 was identical to within weeks 0 and 12. In all trials, most Candida infections were not much more severe, except for UNCOVER-2, where one patient experienced severe otitis externa and two others had severe oral candidiasis (Griffiths et al. Citation2015; Gordon et al. Citation2016; Blauvelt et al. Citation2021).
3.3. Brodalumab
Brodalumab is a human IgG2 monoclonal antibody that inhibits interleukin-17 receptor A (IL-17RA), a protein implicated in the signalling pathway of multiple IL-17 cytokines (Gaspari and Tyring Citation2015). Brodalumab has an approximate molecular weight of 144 kilodaltons and consists of a pair of heavy and a pair of light chains (Brodalumab Citation2006). Brodalumab reduces the inflammatory response associated with autoimmune diseases such as psoriasis by inhibiting IL-17RA (Foulkes and Warren Citation2019). Brodalumab is administered subcutaneously, with the frequency of administration varying according to the disorder being treated (Nakagawa et al. Citation2016). Typically, two injections are used to deal with moderate-to-severe plaque psoriasis, followed by a maintenance dose of one injection every two weeks (Pinter et al. Citation2019). The suggested dosage is 210 mg given subcutaneously at weeks 0, 1, and 2, then 210 mg every two weeks after that (Fujita et al. Citation2018).
AMAGINE 1, 2, and 3 were randomised, controlled, phase III clinical investigations in adult patients with substantial psoriasis. Depending on the trial, patients were randomly assigned to either brodalumab 210 mg or brodalumab 140 mg treatment. Candidiasis was more common in all three trials with brodalumab, particularly with a high dose of 210 mg. None of the infections were systemic, and none resulted in treatment discontinuation (Lebwohl et al. Citation2015; Papp et al. Citation2016, Citation2020; Puig et al. Citation2020).
3.4. Bimekizumab
Bimekizumab is a humanised IgG1 monoclonal antibody that inhibits IL-17A and IL-17F, two pro-inflammatory cytokines implicated in developing autoimmune diseases (Adams et al. Citation2020). It consists of a pair of identical heavy chains and a pair of identical light chains and has an approximate molecular weight of 147 kilodaltons (Adams et al. Citation2020). Bimekizumab is administered subcutaneously, with dosage frequency varying depending on the specific condition being treated (Papp et al. Citation2018). It is typically administered as a preparing dose of two injections for psoriasis, followed by one injection every four weeks. Adults with plaque psoriasis should take 320 mg (given as two 160 mg subcutaneous injections) every eight weeks starting at weeks 0 through 16 (Ritchlin et al. Citation2020).
BE READY was a placebo-controlled, double-blind, randomised, phase III clinical trial in adults with moderate to severe psoriasis. Oral Candida infection was observed in 21 (6%) individuals receiving bimekizumab treatment during the initial treatment phase (Gordon et al. Citation2021). BE SURE, BE RADIANT, and BE VIVID were active-comparator controlled, randomised phase III clinical studies in adult patients with moderate-to-severe psoriasis. In these investigations, active comparators were adalimumab, secukinumab, or ustekinumab, respectively. Candidiasis was significantly more developed in the bimekizumab group (9% to 21%) than in active-comparator group (0% to 5%) (Griffith et al. Citation2021; Reich et al. Citation2021, Citation2021). BE BRIGHT is a continuing clinical intervention planned as an open-label extension (OLE) for individuals who attained Psoriasis Area Severity Index 90 (PASI 90) after 52 weeks of BE VIVID or 56 weeks of BE SURE (Leonardi et al. Citation2022). All the cases observed in the trials mentioned above were mild or moderate oral candidiasis, most of which were resolved by antifungal treatment during the trial periods (Yeung et al. Citation2022).
4. Mechanisms of IL-17 inhibitors and the provocation of candidiasis
In treating autoimmune diseases, IL-17 inhibitors have become a crucial class of therapeutic drugs, modifying the immune system and providing significant relief to patients suffering from various autoimmune diseases. Similarly, IL-17 plays a vital role in the host’s defence against Candida species. It stimulates numerous immune cells, such as neutrophils and macrophages, that are essential in eliminating the infection (Bojang et al. Citation2021). Neutrophils are crucial in the early phases of an infection, as they promptly migrate to the infection site, engulf the Candida species, and kill it through mechanisms, including the production of reactive oxygen species (ROS) and the release of antimicrobial peptides (Pathakumari et al. Citation2020). Macrophages are also crucial in clearing the Candida cells, as they engulf and digest them (Pountain et al. Citation2021). Additionally, IL-17 stimulates the production of antimicrobial peptides, such as defensins and cathelicidins, which eliminate Candida species directly. These peptides are small, cationic molecules that may adhere to and rupture the membrane of Candida cells, causing cell lysis and death (Sawada et al. Citation2021).
The immune response against Candida species is compromised when IL-17 is inhibited either through biologics or genetic knockout (Gaffen and Moutsopoulos Citation2020). Neutrophil migration and activation are inhibited, as well as the production of lymphoid and myeloid chemo-attractants, and antimicrobial peptides, particularly β defensin-3, are inhibited (Davidson et al. Citation2022). This permits Candida cells to proliferate excessively and cause infections. Additionally, IL-17 contributes to the maintenance of the mucosal barrier’s integrity. It induces the synthesis of mucus and tight junction proteins, inhibiting Candida species’ mucosal surface penetration (Wang et al. Citation2019). Upon IL-17 inhibitions, the mucosal barrier becomes compromised, allowing Candida species such as C. albicans to penetrate and cause infection more readily (Gaffen and Moutsopoulos Citation2020).
The Candida infections are more common on the body’s mucosal surfaces, including the oral cavity, gastrointestinal tract, and female genital tract (Talapko et al. Citation2021). This is due to the fact that the Candida cells colonise these areas and are additionally subjected to various behavioural and environmental factors, such as antimicrobial agents, hormonal fluctuations, and sexual activity, which may compromise the mucosal barrier and change the microbial community, making it more susceptible to Candida species’ expansion and the onset of infection (Basmaciyan et al. Citation2019; d’Enfert et al. Citation2021). In addition, it has been demonstrated that candidiasis caused by IL-17 inhibitors is typically mild to moderate and not associated with an increased risk of systemic or severe infections (Yeung et al. Citation2022).
5. Real-world data on the risk of candidiasis during IL-17 inhibitors
Several observational studies were conducted to determine the risk of candidiasis during IL-17 inhibitor therapy. In 2019 a study from Spain found that candidiasis risk during 52 weeks of secukinumab therapy. A total of 5 (3.7%) patients out of 136 developed candidiasis, and all reported cases were oral candidiasis (Notario et al. Citation2019). In 2020, a two years study from Sweden reported 1.7% of vaginal and oral candidiasis out of 848 patients during secukinumab treatment (Srinivas et al. Citation2020). Another secukinumab therapy study from Italy reported 4% oral candidiasis cases out of 151 patients, in which in three cases, the treatment was discontinued due to candida infection (Galluzzo et al. Citation2020). In 2021 the US pharmacovigilance report stated that two patients developed oral candidiasis out of 2,677 brodalumab treated patients during the two years study duration, in which, the treatment was discontinued in one case due to the Candida infection (Rodríguez-Cerdeira et al. Citation2021).
In 2022, Davidson et al. conducted a study based on WHO VigiBase, a population-based drug prescription registry (PHARMO), and the Netherlands psoriasis Cohort to determine the risk of candidiasis during secukinumab, ixekizumab, and brodalumab therapy (Davidson et al. Citation2022). According to the WHO global database 15,768 cases were treated with secukinumab. Among these, 12 cases of onychomycosis, 28 vulvovaginal, 44 cutaneous, 135 oropharyngeal, 37 oesophageal, and 5 deep-seated candidiasis were reported. Similarly, 1,633 patients received ixekizumab, among which vulvovaginal candidiasis was reported in 2 cases, cutaneous candidiasis in 13, oropharyngeal candidiasis in 30, and oesophageal candidiasis in 2 patients (Davidson et al. Citation2022). According to European public assessment reports (EPAR), 692 patients received secukinumab (150 mg), among which three patients developed candidiasis during 12 weeks induction period, while 21 patients developed it during 52 weeks induction period. Similarly, 690 patients received secukinumab (300 mg), in which eight patients developed candidiasis during 12 weeks induction period and 41 developed candidiasis during 52 weeks induction period. Moreover, 4,204 patients received ixekizumab, in which candidiasis was developed in 128 patients (Davidson et al. Citation2022). The same study reported that 66 psoriasis patients from the Netherlands received secukinumab, ixekizumab, and brodalumab, among which 38 (58%) developed candidiasis. Bimekizumab is a comparatively new biologic for treating autoimmune diseases, especially psoriasis. A recent meta-analysis, including five studies, reported 2%–19.3% of oral candidiasis cases during the treatment (Davidson et al. Citation2022).
6. Comparison of the risk of candidiasis between different IL-17 inhibitors
The patients treated with various IL-17 inhibitors reported different proportions of candidiasis. A clinical trial reported 4.0%, 3.3%, and 1.7% candidiasis for brodalumab, ixekizumab, and secukinumab, respectively (Rodríguez-Cerdeira et al. Citation2021). Similarly, a systematic review reported 1.9%–21.2% candidiasis cases for bimekizumab, 1.4%–13.5% for secukinumab, 0.3%–7% for brodalumab (0.3%–7%), and 0–3.5% for ixekizumab (Yamanaka-Takaichi et al. Citation2022). Studies reported that candidiasis risk is high for brodalumab and bimekizumab. In patients with plaque psoriasis, dual inhibition of IL-17A and IL-17F has demonstrated superior cutaneous clearance compared to targeting IL-17A alone with secukinumab (Reich et al. Citation2021). Due to the functions of both cytokines in antifungal host defence, double neutralisation was also linked with a higher incidence of mild to moderate oral candidiasis compared to secukinumab and ixekizumab (Puel et al. Citation2011; Ling and Puel Citation2014; Gordon et al. Citation2021). Another possible explanation is that, as brodalumab inhibits IL-17 R, it prevents the activity of all other IL-17 cytokines, even IL-17E, which may indirectly inhibit Th17 responses (Iwakura et al. Citation2011). In addition, if IL-17 R is not entirely prevented after the dosing cycle, residual IL-17 May likely confer defence against Candida (Armstrong et al. Citation2022). However, further studies are required on this topic for better understanding.
7. Risk of candidiasis based on the dose of IL-17 inhibitors
Studies reported that high doses of IL-17 inhibitors maximise the risk of candidiasis (Yamanaka-Takaichi et al. Citation2022). Secukinumab is available in three dosage forms; 75 mg, 150 mg, and 300 mg. For 75 mg of secukinumab, the candidiasis was reported at 1%–2%, while for 150 mg and 300 mg, 1%–7% and 2.5%–8% of candidiasis were reported, respectively (Langley et al. Citation2014; Blauvelt et al. Citation2015; Mrowietz et al. Citation2015; Reich et al. Citation2021). The bimekizumab is provided in two dosage forms: 160 mg and 320 mg, in which the 320 mg dose is most commonly used and the risk of candidiasis for it was reported at 6.4% to 21.2% (Papp et al. Citation2018; Blauvelt et al. Citation2020; Ritchlin et al. Citation2020; van der Heijde et al. Citation2020; Griffith et al. Citation2021; Reich et al. Citation2021). Another study showed that for the 160 mg dose, only one out of 43 treated patients developed candidiasis, while for the 320 mg dose, two out of 41 treated patients developed candidiasis. The brodalumab is given in two dosages; 140 mg and 210 mg. High rates of candidiasis were reported in 210 mg dosage, which was 1.3% to 2.3%, while for 140 mg, it was 0.5% to 1.3% (Lebwohl et al. Citation2015; Papp et al. Citation2016). For ixekizumab 80 mg, insufficient evidence regarding the impact of dose on the risk of candidiasis is available (Combe et al. Citation2020; Yamanaka-Takaichi et al. Citation2022). Further studies are required to determine the effect of various doses of ixekizumab and the risk of candidiasis.
8. Prevention and management of candidiasis in patients on IL-17 inhibitors
The immunosuppressive effects of IL-17 inhibitors may make patients more vulnerable to candidiasis (Huppler et al. Citation2012). A comprehensive strategy is recommended to prevent and treat candidiasis in these individuals. This strategy consists of educating patients about hazards and signs, ongoing surveillance, adopting good hygiene, using topical antifungal drugs for mild infections, and contemplating systemic antifungal treatment for recurrent and severe infections. Follow-up visits with medical professionals are vital for monitoring the ongoing treatment progress and refining the care plan (Boehncke and Brembilla Citation2022).
8.1. Screening and monitoring for candidiasis in patients receiving IL-17 inhibitors
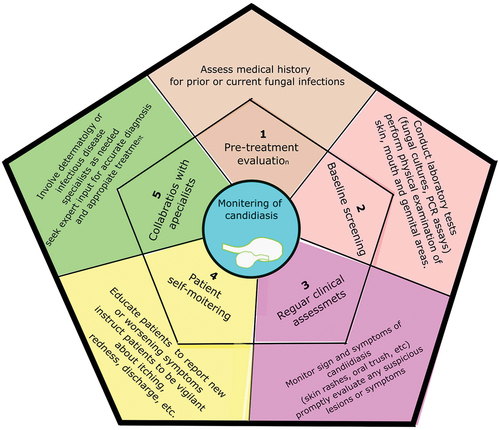
Screening and monitoring for candidiasis in IL-17 inhibitor-treated patients are necessary for the early detection and management of fungal infections (Silfvast-Kaiser et al. Citation2019). The subsequent measures may be taken:
Before initiating an IL-17 inhibitor treatment, a thorough evaluation of the patient’s medical history, such as any earlier or present fungal infections, should be performed. This information can assist in identifying individuals who may be at a higher risk for candidiasis (Kearns et al. Citation2021).
Before commencing IL-17 inhibitors, a candidiasis baseline assessment should be considered. This might entail performing laboratory tests such as microbial cultures or polymerase chain reaction (PCR) analyses. In addition, a physical examination of the epidermis, mouth, and genital areas can aid in identifying any existing candidiasis (Davidson et al. Citation2022).
People receiving IL-17 inhibitors ought to have routine clinical evaluations to ensure the effectiveness of IL-17 inhibitors in managing autoimmune diseases and to promptly address any potential concerns in the form of candidiasis symptoms. This includes examining the patient for oral thrush, skin rashes, and vaginal infections. Any suspicious lesions or symptoms should be evaluated as soon as possible by a medical professional (Busse et al. Citation2013; Yin et al. Citation2020).
Patients need to be advised on self-monitoring for candidiasis. They should be educated to report any emerging or worsening symptoms in the afflicted areas, such as redness, itching, discomfort, or discharge. Aiding patient participation in their own care may help in the early identification and treatment of candidiasis. In addition, encouraging open and honest communication between the patient and their healthcare practitioner is crucial since it makes it possible to quickly address any potential symptoms or concerns that may emerge during the course of IL-17 inhibitor medication (Pappas et al. Citation2016).
In some instances, it may be advantageous to involve specialists, such as infectious disease specialists or dermatologists, in the surveillance and therapy of candidiasis in IL-17 inhibitor-receiving patients. Such experts can offer additional guidance for accurate diagnosis and treatment (Ruiz de Morales et al. Citation2020).
By adopting these screening and monitoring approaches, healthcare professionals can proactively identify candidiasis in IL-17 inhibitor-treated patients and instigate prompt actions to prevent complications and secure optimal patient care ().
8.2. Treatment options for candidiasis in patients on IL-17 inhibitors
The candidiasis associated with IL-17 inhibitors is rarely systematic and mainly causes topical infection, among which oral candidiasis is prominent (Okada et al. Citation2016). However, the severity of candidiasis depends on the patient’s underlying conditions, which might increase the severity of the infection (Vincent et al. Citation1998). The severity of the condition and whether the patient is pregnant often influence the initial therapy option. According to current recommendations, the azoles like isavuconazole, itraconazole, fluconazole, posaconazole, and should be evaded during pregnancy, particularly during the first trimester, owing to the risk of congenital anomalies. Additional attention may contain; expected adherence, cost, ease of use, medications interactions, and stomach acidity (Pappas et al. Citation2016; Yeung et al. Citation2022). The guidelines for candidiasis treatment are available, which are summarised as follows ().
Figure 4. Clinical presentation, diagnosis and treatment of candidiasis associated with IL-17 inhibitors.
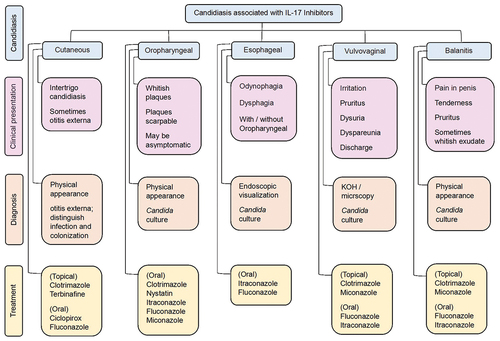
For cutaneous candidiasis, clotrimazole, terbinafine, ciclopirox, and fluconazole are available options. Clotrimazole is recommended as a 1% cream used twice a day for up to four weeks (Yeung et al. Citation2022). Terbinafine is available as a 1% cream use once a day for one to two weeks (Yeung et al. Citation2022). The ciclopirox is also a 1% topical cream, used twice a day for up to four weeks (Yeung et al. Citation2022). The fluconazole is only orally available drug for cutaneous candidiasis, which is recommended as 150 mg in a single dose (Yeung et al. Citation2022).
For oropharyngeal candidiasis, the drugs used are clotrimazole, miconazole, nystatin, fluconazole, and itraconazole. The recommended dosage of clotrimazole is a 10 mg lozenge, taken 5 times a day for 7 to 14 days (Armstrong et al. Citation2016; Pappas et al. Citation2016). Miconazole is typically administered as a buccal tablet containing 50 mg once daily for 7 to 14 days (Pappas et al. Citation2016). The recommended dosage of nystatin is 5 ml (500,000 units) four times daily (swabbed, retained, and swallowed) for 7 to 14 days (Pappas et al. Citation2016). Fluconazole is recommended as 200 mg taken oral tablet on day 1, subsequently 100 mg every day for 7–14 days (Yeung et al. Citation2022). Itraconazole is administered in daily dosing of 200 mg for 7–14 days (Pappas et al. Citation2016).
For vulvovaginal candidiasis, clotrimazole, miconazole, fluconazole and itraconazole are available. The clotrimazole is recommended 10%, 2%, or 1% cream used intra-vaginally as a sole dose, for 3 or 7 days respectively (Armstrong et al. Citation2016; Pappas et al. Citation2016). Similarly, vaginal tablets containing 500 mg, 200 mg, or 100 mg clotrimazole can be administered as a single dose for 3 or 7 days, respectively (Armstrong et al. Citation2016; Pappas et al. Citation2016; Workowski et al. Citation2021). Miconazole can be prescribed as a 4% or 2% cream used intra-vaginally for 3 or 7 days, respectively (Armstrong et al. Citation2016; Workowski et al. Citation2021). Similarly, 1,200 mg, 200 mg, or 100 mg vaginal tablets can be used as a single dose, for 3 or 7 days respectively (Armstrong et al. Citation2016; Workowski et al. Citation2021). Fluconazole can be prescribed as a single oral dose of 150 mg, however, in severe cases, three doses for three days may be contemplated (Yeung et al. Citation2022). Itraconazole can be administered orally once daily at 200 mg for 3 to 7 days.
For oesophageal candidiasis, it is recommended to administer fluconazole 400 mg orally on day one, followed by 200 mg for 14 to 21 days, and itraconazole 200 mg, one a day orally for 14 to 28 days (Pappas et al. Citation2016). For Candida balanitis no therapeutic guidelines are available from the Infectious Diseases Society of America (IDSA), however; a study stated topically clotrimazole and miconazole and orally, fluconazole and itraconazole as recommended prescription (Pappas et al. Citation2016).
9. Conclusions
In conclusion, the use of IL-17 inhibitors, including secukinumab, ixekizumab, brodalumab, and bimekizumab, has been associated with an increased risk of candidiasis, a fungal infection caused by Candida species. These medications target the interleukin-17 pathway, which is vital in maintaining mucosal barriers and coordinating immune responses to combat fungal pathogens. However, by inhibiting IL-17, these inhibitors disrupt the delicate immune balance, rendering individuals more susceptible to fungal overgrowth and subsequent candidiasis.
Numerous clinical trials and real-world studies have consistently demonstrated a higher incidence of candidiasis in individuals subjected to IL-17 inhibitors. The difference in the incidence of candidiasis is being reported during the treatment of IL-17 inhibitors. Studies found that brodalumab and bimekizumab treated patients are associated with a comparatively higher vulnerability than secukinumab. While the observational data about ixekizumab is currently insufficient to draw a clear-cut conclusion. The risk of candidiasis appears to be dose-dependent, with higher doses of IL-17 inhibitors correlating with an increased likelihood of developing fungal infections. Prolonged treatment duration can also contribute to the risk, as continuous inhibition of IL-17 May further compromise the immune response against Candida species.
Considering the risk of fungal infections is crucial in prescribing IL-17 inhibitors. Healthcare professionals must thoroughly evaluate patients’ medical histories, paying particular attention to risk factors such as previous fungal infections, immunosuppression, or comorbidities that increase susceptibility to fungal overgrowth. Close monitoring of patients receiving IL-17 inhibitors is essential, and any signs or symptoms indicative of candidiasis, such as oral thrush, genital infections, or skin rashes, should be promptly evaluated and addressed.
Patient education regarding the risk of candidiasis is paramount for early detection and intervention. Patients should be informed about the signs and symptoms of fungal infections and encouraged to report any changes or concerns to their healthcare providers. Timely recognition of candidiasis allows for the early initiation of appropriate antifungal treatment, minimising the risk of complications and ensuring optimal patient management.
Furthermore, future research should focus on understanding the risk factors contributing to candidiasis in patients receiving IL-17 inhibitors. Studies should explore the impact of specific IL-17 inhibitors, such as secukinumab, ixekizumab, brodalumab, and bimekizumab, on the incidence and severity of fungal infections. Investigating the effectiveness of combination therapies, such as concurrent use of IL-17 inhibitors and prophylactic antifungal agents, could potentially mitigate the risk of candidiasis while preserving the therapeutic benefits of IL-17 inhibition.
Prospective studies and long-term surveillance are necessary to assess the true extent of the risk of candidiasis and identify any potential variations across different patient populations and IL-17 inhibitor agents. These research efforts will contribute to evidence-based guidelines for the safe and effective use of IL-17 inhibitors, enabling healthcare providers to optimise patient care and minimise the risk of fungal infections.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Adams R, Maroof A, Baker T, Lawson ADG, Oliver R, Paveley R, Rapecki S, Shaw S, Vajjah P, West S, et al. 2020. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 11:1894. doi: 10.3389/fimmu.2020.01894.
- Armstrong AW, Blauvelt A, Mrowietz U, Strober B, Gisondi P, Merola JF, Langley RG, Ståhle M, Lebwohl M, Netea MG, et al. 2022. A practical guide to the management of oral candidiasis in patients with plaque psoriasis receiving treatments that target interleukin-17. Dermatol Ther (Heidelb). 12(3):787–800. doi:10.1007/s13555-022-00687-0.
- Armstrong AW, Bukhalo M, Blauvelt A. 2016. A clinician’s guide to the diagnosis and treatment of candidiasis in patients with psoriasis. Am J Clin Dermatol. 17(4):329–336. doi: 10.1007/s40257-016-0206-4.
- Azadeh H, Alizadeh-Navaei R, Rezaiemanesh A, Rajabinejad M. 2022. Immune-related adverse events (irAes) in ankylosing spondylitis (AS) patients treated with interleukin (IL)-17 inhibitors: A systematic review and meta-analysis. Inflammopharmacol. 30(2):435–451. doi: 10.1007/s10787-022-00933-z.
- Basmaciyan L, Bon F, Paradis T, Lapaquette P, Dalle F. 2019. Candida albicans interactions with the host: Crossing the intestinal epithelial barrier. Tissue Barriers. 7(2):1612661. doi: 10.1080/21688370.2019.1612661.
- Bastid J, Dejou C, Docquier A, Bonnefoy N. 2020. The emerging role of the IL-17B/IL-17RB pathway in cancer. Front Immunol. 11:718. doi: 10.3389/fimmu.2020.00718.
- Berry SPD, Dossou C, Kashif A, Sharifinejad N, Azizi G, Hamedifar H, Sabzvari A, Zian Z. 2022. The role of IL-17 and anti-IL-17 agents in the immunopathogenesis and management of autoimmune and inflammatory diseases. Int Immunopharmacol. 102:108402. doi: 10.1016/j.intimp.2021.108402.
- Bianchi E, Rogge L. 2019. The IL-23/IL-17 pathway in human chronic inflammatory diseases-new insight from genetics and targeted therapies. Genes Immun. 20(5):415–425. doi: 10.1038/s41435-019-0067-y.
- Bissonnette R, Luger T, Thaçi D, Toth D, Messina I, You R, Guana A, Fox T, Papavassilis C, Gilloteau I, et al. 2017. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 177(4):1033–1042. doi: 10.1111/bjd.15706.
- Blauvelt A, Lebwohl MG, Mabuchi T, Leung A, Garrelts A, Crane H, ElMaraghy H, Patel H, Ridenour T, See K, et al. 2021. Long-term efficacy and safety of ixekizumab: A 5-year analysis of the UNCOVER-3 randomized controlled trial. J Am Acad Dermatol. 85(2):360–368. doi:10.1016/j.jaad.2020.11.022.
- Blauvelt A, Papp KA, Merola JF, Gottlieb AB, Cross N, Madden C, Wang M, Cioffi C, Griffiths CEM. 2020. Bimekizumab for patients with moderate to severe plaque psoriasis: 60-week results from BE ABLE 2, a randomized, double-blinded, placebo-controlled, phase 2b extension study. J Am Acad Dermatol. 83(5):1367–1374. doi: 10.1016/j.jaad.2020.05.105.
- Blauvelt A, Prinz JC, Gottlieb AB, Kingo K, Sofen H, Ruer-Mulard M, Singh V, Pathan R, Papavassilis C, Cooper S. 2015. Secukinumab administration by pre-filled syringe: Efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 172(2):484–493. doi: 10.1111/bjd.13348.
- Boehncke WH, Brembilla NC. 2022. Pathogenesis-oriented therapy of psoriasis using biologics. Expert Opin Biol Ther. 22(12):1463–1473. doi: 10.1080/14712598.2022.2100219.
- Bojang E, Ghuman H, Kumwenda P, Hall RA. 2021. Immune sensing of Candida albicans. J Fungi. 7(2):10.3390/jof7020119. doi: 10.3390/jof7020119.
- Brevi A, Cogrossi LL, Grazia G, Masciovecchio D, Impellizzieri D, Lacanfora L, Grioni M, Bellone M. 2020. Much more than IL-17A: Cytokines of the IL-17 family between microbiota and cancer. Front Immunol. 11:565470. doi: 10.3389/fimmu.2020.565470.
- Brodalumab. 2006. Drugs and lactation database (LactMed®). Bethesda (MD): National Institute of Child Health and Human Development.
- Bruno M, Davidson L, Koenen H, van den Reek J, van Cranenbroek B, de Jong E, van de Veerdonk FL, Kullberg BJ, Netea MG. 2022. Immunological effects of anti‒IL-17/12/23 therapy in patients with psoriasis complicated by Candida infections. J Invest Dermatol. 142(11):2929–2939.e2928. doi: 10.1016/j.jid.2022.05.1083.
- Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, Lin SL. 2013. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 188(11):1294–1302. doi: 10.1164/rccm.201212-2318OC.
- Cao Z, Liu Z, Zhu X, Yang Q, Xu Q, Zhang C. 2022. Successful secukinumab treatment in focal segmental glomerulosclerosis associated with plaque psoriasis. Ren Fail. 44(1):826–830. doi: 10.1080/0886022x.2022.2073893.
- Chang SH. 2019. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch Pharm Res. 42(7):549–559. doi: 10.1007/s12272-019-01146-9.
- Combe B, Rahman P, Kameda H, Cañete JD, Gallo G, Agada N, Xu W, Genovese MC. 2020. Safety results of ixekizumab with 1822.2 patient-years of exposure: an integrated analysis of 3 clinical trials in adult patients with psoriatic arthritis. Arthritis Res Ther. 22(1):14. doi: 10.1186/s13075-020-2099-0.
- Craig S, Warren RB. 2020. Ixekizumab for the treatment of psoriasis: up-to-date. Expert Opin Biol Ther. 20(6):549–557. doi: 10.1080/14712598.2020.1729736.
- Davidson L, van den Reek J, Bruno M, van Hunsel F, Herings RMC, Matzaraki V, Boahen CK, Kumar V, Groenewoud HMM, van de Veerdonk FL, et al. 2022. Risk of candidiasis associated with interleukin-17 inhibitors: A real-world observational study of multiple independent sources. Lancet Reg Health Eur. 13:100266. doi: 10.1016/j.lanepe.2021.100266.
- d’Enfert C, Kaune AK, Alaban LR, Chakraborty S, Cole N, Delavy M, Kosmala D, Marsaux B, Fróis-Martins R, Morelli M, et al. 2021. The impact of the fungus-host-microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol Rev. 45(3): doi:10.1093/femsre/fuaa060
- Feng Y, Zhou B, Wang Z, Xu G, Wang L, Zhang T, Zhang Y, Poddighe D. 2022. Risk of Candida infection and serious infections in patients with moderate-to-severe psoriasis receiving biologics: A systematic review and meta-analysis of randomized controlled trials. Int J Clin Pract. 2022:1–11. doi: 10.1155/2022/2442603.
- Finkina EI, IV B, AA I, Kanushkina MD, Egorova EA, Voropaev AD, Stukacheva EA, Ovchinnikova TV. 2022. Antifungal activity, structural stability, and immunomodulatory effects on human immune cells of defensin from the lentil lens culinaris. Membranes. 12(9):855. doi: 10.3390/membranes12090855.
- Foulkes AC, Warren RB. 2019. Brodalumab in psoriasis: Evidence to date and clinical potential. Drugs Context. 8:212570. doi: 10.7573/dic.212570.
- Frieder J, Kivelevitch D, Menter A. 2018. Secukinumab: A review of the anti-IL-17A biologic for the treatment of psoriasis. Ther Adv Chronic Dis. 9(1):5–21. doi: 10.1177/2040622317738910.
- Fujita H, Terui T, Hayama K, Akiyama M, Ikeda S, Mabuchi T, Ozawa A, Kanekura T, Kurosawa M, Komine M, et al. 2018. Japanese guidelines for the management and treatment of generalized pustular psoriasis: the new pathogenesis and treatment of GPP. J Dermatol. 45(11):1235–1270. doi:10.1111/1346-8138.14523.
- Gaffen SL, Moutsopoulos NM. 2020. Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity. Sci Immunol. 5(43):eaau4594. doi: 10.1126/sciimmunol.aau4594.
- Galluzzo M, D’Adamio S, Silvaggio D, Lombardo P, Bianchi L, Talamonti M. 2020. In which patients the best efficacy of secukinumab? Update of a real-life analysis after 136 weeks of treatment with secukinumab in moderate-to-severe plaque psoriasis. Expert Opin Biol Ther. 20(2):173–182. doi: 10.1080/14712598.2020.1708897.
- Gaspari AA, Tyring S. 2015. New and emerging biologic therapies for moderate-to-severe plaque psoriasis: Mechanistic rationales and recent clinical data for IL-17 and IL-23 inhibitors. Dermatol Ther. 28(4):179–193. doi: 10.1111/dth.12251.
- González-Fernández C, Chaves-Pozo E, Cuesta A. 2020. Identification and regulation of interleukin-17 (IL-17) family ligands in the teleost fish European sea bass. Int J Mol Sci. 21(7):2439. doi: 10.3390/ijms21072439.
- Gordon KB, Colombel JF, Hardin DS, Langley RG, Luger T, Ohtsuki M, Reich K, Amato D, Ball SG, Braun DK, et al. 2016. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 375(4):345–356. doi: 10.1056/NEJMc1610828.
- Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, Vanvoorden V, Madden C, White K, Cioffi C, et al. 2021. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): A multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 397(10273):475–486. doi:10.1016/S0140-6736(21)00126-4.
- Gottlieb AB, Blauvelt A, Prinz JC, Papanastasiou P, Pathan R, Nyirady J, Fox T, Papavassilis C. 2016. Secukinumab self-administration by prefilled syringe maintains reduction of plaque psoriasis severity over 52 weeks: results of the FEATURE trial. J Drugs Dermatol JDD. 15(10):1226–1234.
- Griffith SK, Ahn GS, Wu JJ. 2021. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 385(12):1149–1150. doi: 10.1056/NEJMc2113092.
- Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, Cameron GS, Erickson J, Zhang L, Secrest RJ, et al. 2015. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): Results from two phase 3 randomised trials. Lancet. 386(9993):541–551. doi:10.1016/s0140-6736(15)60125-8.
- Huppler AR, Bishu S, Gaffen SL. 2012. Mucocutaneous candidiasis: The IL-17 pathway and implications for targeted immunotherapy. Arthritis Res Ther. 14(4):217. doi: 10.1186/ar3893.
- Iwakura Y, Ishigame H, Saijo S, Nakae S. 2011. Functional specialization of interleukin-17 family members. Immunity. 34(2):149–162. doi: 10.1016/j.immuni.2011.02.012.
- Ixekizumab. 2006. Drugs and lactation database (LactMed®). Bethesda (MD): National Institute of Child Health and Human Development.
- Jiang P, Zheng C, Xiang Y, Malik S, Su D, Xu G, Zhang M. 2023. The involvement of TH17 cells in the pathogenesis of IBD. Cytokine Growth Factor Rev. 69:28–42. doi: 10.1016/j.cytogfr.2022.07.005.
- Kearns DG, Uppal S, Chat VS, Wu JJ. 2021. Comparison of guidelines for the use of interleukin-17 inhibitors for psoriasis in the United States, Britain, and Europe: A critical appraisal and comprehensive review. J Clin Aesthet Dermatol. 14(6):55–59.
- Lacour JP, Paul C, Jazayeri S, Papanastasiou P, Xu C, Nyirady J, Fox T, Papavassilis C. 2017. Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: Results of the randomized controlled JUNCTURE trial. J Eur Acad Dermatol Venereol. 31(5):847–856. doi: 10.1111/jdv.14073.
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, et al. 2014. Secukinumab in plaque psoriasis — Results of two phase 3 trials. N Engl J Med. 371(4):326–338. doi:10.1056/NEJMoa1314258.
- Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, Papp K, Spelman L, Toth D, Kerdel F, et al. 2015. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 373(14):1318–1328. doi: 10.1056/NEJMoa1503824.
- Leonardi C, Sator P, Morita A, Kokolakis G, Blauvelt A, Warren R, De Cuyper D, Madden C, Vanvoorden V, Wang M. 2022. Bimekizumab efficacy and safety up to two years in patients with moderate to severe plaque psoriasis switching from ustekinumab: Results from the interim BE BRIGHT open-label extension trial. Australas J Dermatol. 63:13–14.
- Li T, Liu Y, Yu X, Wang P, Sun S, Liu D. 2022. IL-17D affects the chemokines and chemokine receptors of intestinal epithelial cells under hyperoxia. Int Immunopharmacol. 113(Pt A):109386. doi: 10.1016/j.intimp.2022.109386.
- Li Z, Lu G, Meng G. 2019. Pathogenic fungal infection in the lung. Front Immunol. 10:1524. doi: 10.3389/fimmu.2019.01524.
- Ling Y, Puel A. 2014. IL-17 and infections. Actas Dermosifiliogr. 105(1):34–40. doi: 10.1016/S0001-7310(14)70016-X.
- Liu L, Lu J, Allan BW, Tang Y, Tetreault J, Chow CK, Barmettler B, Nelson J, Bina H, Huang L, et al. 2016. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. JIR. 9:39–50. doi: 10.2147/jir.s100940.
- Lopes JP, Lionakis MS. 2022. Pathogenesis and virulence of Candida albicans. Virulence. 13(1):89–121. doi: 10.1080/21505594.2021.2019950.
- Matsuzaki G, Umemura M. 2018. Interleukin-17 family cytokines in protective immunity against infections: Role of hematopoietic cell-derived and non-hematopoietic cell-derived interleukin-17s. Microbiol Immunol. 62(1):1–13. doi: 10.1111/1348-0421.12560.
- Mease PJ, Smolen JS, Behrens F, Nash P, Liu Leage S, Li L, Tahir H, Gooderham M, Krishnan E, Liu-Seifert H, et al. 2020. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 79(1):123–131. doi: 10.1136/annrheumdis-2019-215386.
- Mills KHG. 2023. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. 23(1):38–54. doi: 10.1038/s41577-022-00746-9.
- Miura S, Garcet S, Li X, Cueto I, Salud-Gnilo C, Kunjravia N, Yamamura K, Gonzalez J, Murai-Yamamura M, Rambhia D, et al. 2023. Cathelicidin antimicrobial peptide LL37 induces toll-like receptor 8 and amplifies IL-36γ and IL-17C in human keratinocytes. Journal Of Investigative Dermatology. 143(5):832–841.e834. doi:10.1016/j.jid.2022.10.017.
- Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, Szepietowski JC, Regnault P, Thurston H, Papavassilis C. 2015. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 73(1):27–36.e21. doi: 10.1016/j.jaad.2015.04.011.
- Nakagawa H, Niiro H, Ootaki K. 2016. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 81(1):44–52. doi: 10.1016/j.jdermsci.2015.10.009.
- Nie YJ, Wu SH, Xuan YH, Yan G. 2022. Role of IL-17 family cytokines in the progression of IPF from inflammation to fibrosis. Mil Med Res. 9(1):21. doi: 10.1186/s40779-022-00382-3.
- Notario J, Deza G, Vilarrasa E, Valentí F, Muñoz C, Mollet J, Rocamora V, Carrascosa JM, Del Alcázar E, Alsina M, et al. 2019. Treatment of patients with plaque psoriasis with secukinumab in a real-life setting: A 52-week, multicenter, retrospective study in Spain. J Dermatol Treat. 30(5):424–429. doi:10.1080/09546634.2018.1528000.
- Okada S, Puel A, Casanova JL, Kobayashi M. 2016. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clin Transl Immunol. 5(12):e114. doi: 10.1038/cti.2016.71.
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, et al. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 62(4):e1–50. doi: 10.1093/cid/civ933.
- Papp K, Menter A, Leonardi C, Soung J, Weiss S, Pillai R, Jacobson A. 2020. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: Subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 183(6):1037–1048. doi: 10.1111/bjd.19132.
- Papp KA, Merola JF, Gottlieb AB, Griffiths CEM, Cross N, Peterson L, Cioffi C, Blauvelt A. 2018. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: Results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 79(2):277–286.e210. doi: 10.1016/j.jaad.2018.03.037.
- Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, Toth D, Langley RG, Cather J, Gottlieb AB, et al. 2016. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 175(2):273–286. doi: 10.1111/bjd.14493.
- Pathakumari B, Liang G, Liu W. 2020. Immune defence to invasive fungal infections: A comprehensive review. Biomed Pharmacother. 130:110550. doi: 10.1016/j.biopha.2020.110550.
- Patrikiou E, Liaskos C, Mavropoulos A, Ntavari N, Gkoutzourelas A, Simopoulou T, Fechner K, Scheper T, Meyer W, Katsiari CG, et al. 2020. Autoantibodies against specific nuclear antigens are present in psoriatic disease and are diminished by secukinumab. Clin Chim Acta. 510:400–407. doi: 10.1016/j.cca.2020.07.037.
- Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, Guindon C, You R, Papavassilis C. 2015. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: A randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 29(6):1082–1090. doi: 10.1111/jdv.12751.
- Peroumal D, Sahu SR, Kumari P, Utkalaja BG, Acharya N, Alanio A. 2022. Commensal fungus Candida albicans maintains a long-term mutualistic relationship with the host to modulate gut microbiota and metabolism. Microbiol Spectr. 10(5):e0246222. doi: 10.1128/spectrum.02462-22.
- Perrotta FM, Scriffignano S, Ciccia F, Lubrano E. 2022. Therapeutic targets for ankylosing spondylitis - Recent insights and future prospects. Open Access Rheumatol Res Rev. 14:57–66. doi: 10.2147/oarrr.s295033.
- Pettas E, Savva V, Theofilou VI, Georgaki M, Nikitakis NG. 2021. Oral Candida infection in psoriatic patients treated with IL17A inhibitors: Report of 3 cases and a comprehensive review of the literature. Diagnostics. 12(1). doi: 10.3390/diagnostics12010003.
- Pinter A, Bonnekoh B, Hadshiew IM, Zimmer S. 2019. Brodalumab for the treatment of moderate-to-severe psoriasis: Case series and literature review. Clin Cosmet Investig Dermatol. 12:509–517. doi: 10.2147/ccid.s211938.
- Pountain AW, Collette JR, Farrell WM, Lorenz MC, Goldman GH. 2021. Interactions of both pathogenic and nonpathogenic CUG clade Candida species with macrophages share a conserved transcriptional landscape. mBio. 12(6):e0331721. doi: 10.1128/mbio.03317-21.
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, et al. 2011. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Sci. 332(6025):65–68. doi: 10.1126/science.1200439.
- Puig L, Lebwohl M, Bachelez H, Sobell J, Jacobson AA. 2020. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial. J Am Acad Dermatol. 82(2):352–359. doi: 10.1016/j.jaad.2019.05.095.
- Rafael-Vidal C, Pérez N, Altabás I, Garcia S, Pego-Reigosa JM. 2020. Blocking IL-17: A promising strategy in the treatment of systemic rheumatic diseases. Int J Mol Sci. 21(19). doi: 10.3390/ijms21197100.
- Reich K, Papp KA, Blauvelt A, Langley RG, Armstrong A, Warren RB, Gordon KB, Merola JF, Okubo Y, Madden C, et al. 2021. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): Efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 397(10273):487–498. doi:10.1016/S0140-6736(21)00125-2.
- Reich K, Warren RB, Lebwohl M, Gooderham M, Strober B, Langley RG, Paul C, De Cuyper D, Vanvoorden V, Madden C, et al. 2021. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 385(2):142–152. doi: 10.1056/NEJMoa2102383.
- Rioux G, Simard M, Morin S, Lorthois I, Guérin SL, Pouliot R. 2021. Development of a 3D psoriatic skin model optimized for infiltration of IL-17A producing T cells: Focus on the crosstalk between T cells and psoriatic keratinocytes. Acta Biomater. 136:210–222. doi: 10.1016/j.actbio.2021.09.018.
- Ritchlin CT, Kavanaugh A, Merola JF, Schett G, Scher JU, Warren RB, Gottlieb AB, Assudani D, Bedford-Rice K, Coarse J, et al. 2020. Bimekizumab in patients with active psoriatic arthritis: Results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet. 395(10222):427–440. doi:10.1016/S0140-6736(19)33161-7.
- Rodríguez-Cerdeira C, González-Cespón JL, Martínez-Herrera E, Carnero-Gregorio M, López-Barcenas A, Sergeev A, Saunte DM. 2021. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Ital J Dermatol Venereol. 156(5):545–557. doi: 10.23736/S2784-8671.20.06580-3.
- Ruggiero A, Megna M, Fabbrocini G, Fornaro L, Villani A. 2022. Drug safety evaluation of ixekizumab for psoriasis: A review of the current knowledge. Expert Opin Drug Saf. 21(10):1249–1257. doi: 10.1080/14740338.2022.2134855.
- Ruiz de Morales JMG, Puig L, Daudén E, Cañete JD, Pablos JL, Martín AO, Juanatey CG, Adán A, Montalbán X, Borruel N, et al. 2020. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun Rev. 19(1):102429. doi: 10.1016/j.autrev.2019.102429.
- Saeki H, Mabuchi T, Asahina A, Abe M, Igarashi A, Imafuku S, Okubo Y, Komine M, Sano S, Torii H, et al. 2023. English version of Japanese guidance for use of biologics for psoriasis (the 2022 version). J Dermatol. 50(2):e41–e68. doi:10.1111/1346-8138.16691.
- Saini C, Sapra L, Bhardwaj A, Tarique M, Sharma A, Khanna N, Ramesh V, Puri P, Srivastava RK. 2022. IL-21 plays an important role in modulating “Th17-treg” cell axis in leprosy type 1 reactions. Cytokine. 152:155821. doi: 10.1016/j.cyto.2022.155821.
- Sanford M, McKeage K. 2015. Secukinumab: First global approval. Drugs. 75(3):329–338. doi: 10.1007/s40265-015-0359-0.
- Sawada Y, Setoyama A, Sakuragi Y, Saito-Sasaki N, Yoshioka H, Nakamura M. 2021. The role of IL-17-producing cells in cutaneous fungal infections. Int J Mol Sci. 22(11). doi: 10.3390/ijms22115794.
- Sbidian E, Chaimani A, Afach S, Doney L, Dressler C, Hua C, Mazaud C, Phan C, Hughes C, Riddle D, et al. 2020. Systemic pharmacological treatments for chronic plaque psoriasis: A network meta-analysis. Cochrane Database Syst Re. 1(1):Cd011535. doi: 10.1002/14651858.CD011535.pub3.
- Shao TY, Haslam DB, Bennett RJ, Way SS. 2022. Friendly fungi: Symbiosis with commensal Candida albicans. Trends Immunol. 43(9):706–717. doi: 10.1016/j.it.2022.07.003.
- Silfvast-Kaiser A, Paek SY, Menter A. 2019. Anti-IL17 therapies for psoriasis. Expert Opin Biol Ther. 19(1):45–54. doi: 10.1080/14712598.2019.1555235.
- Srinivas C, Odsbu I, Linder M. 2020. Risk of common infections among individuals with psoriasis in Sweden: A nationwide cohort study comparing secukinumab to ustekinumab. Pharmacoepidemiol Drug Saf. 29(12):1562–1569. doi: 10.1002/pds.5132.
- Talapko J, Juzbašić M, Matijević T, Pustijanac E, Bekić S, Kotris I, Škrlec I. 2021. Candida albicans—The virulence factors and clinical manifestations of infection. J Fungi. 7(2):79. doi: 10.3390/jof7020079.
- Tangye SG, Puel A. 2023. The Th17/IL-17 axis and host defense against fungal infections. J Allergy Clin Immunol Pract. 11(6):1624–1634. doi: 10.1016/j.jaip.2023.04.015.
- Toussirot E. 2012. The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm Allergy Drug Targets. 11(2):159–168. doi: 10.2174/187152812800392805.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, Mease P, Deodhar A, Maksymowych WP, Van den Bosch F, Sieper J, Tomita T, Landewé R, et al. 2018. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 392(10163):2441–2451. doi: 10.1016/s0140-6736(18)31946-9.
- van der Heijde D, Gensler LS, Deodhar A, Baraliakos X, Poddubnyy D, Kivitz A, Farmer MK, Baeten D, Goldammer N, Coarse J, et al. 2020. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: Results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis. 79(5):595–604. doi: 10.1136/annrheumdis-2020-216980.
- Vincent JL, Anaissie E, Bruining H, Demajo W, el-Ebiary M, Haber J, Hiramatsu Y, Nitenberg G, Nyström PO, Pittet D, et al. 1998. Epidemiology, diagnosis and treatment of systemic Candida infection in surgical patients under intensive care. Intensive Care Med. 24(3):206–216. doi: 10.1007/s001340050552.
- Wang SS, Tang YL, Pang X, Zheng M, Tang YJ, Liang XH. 2019. The maintenance of an oral epithelial barrier. Life Sci. 227:129–136. doi: 10.1016/j.lfs.2019.04.029.
- Wang J, Wang C, Liu L, Hong S, Ru Y, Sun X, Chen J, Zhang M, Lin N, Li B, et al. 2023. Adverse events associated with anti-IL-17 agents for psoriasis and psoriatic arthritis: A systematic scoping review. Front Immunol. 14:993057. doi: 10.3389/fimmu.2023.993057.
- Wilharm A, Binz C, Sandrock I, Rampoldi F, Lienenklaus S, Blank E, Winkel A, Demera A, Hovav AH, Stiesch M, et al. 2022. Interleukin-17 is disease promoting in early stages and protective in late stages of experimental periodontitis. PloS One. 17(3):e0265486. doi:10.1371/journal.pone.0265486.
- Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. 2021. Sexually transmitted infections treatment guidelines, 2021. MMWR Recommend Reports: Morbidity And Mortality Weekly Report Recommend Rep. 70(4):1–187. doi: 10.15585/mmwr.rr7004a1.
- Yamanaka-Takaichi M, Ghanian S, Katzka DA, Torgerson RR, Alavi A. 2022. Candida infection associated with anti-IL-17 medication: A systematic analysis and review of the literature. Am J Clin Dermatol. 23(4):469–480. doi: 10.1007/s40257-022-00686-z.
- Yeung J, Bunce PE, Lynde CW, Turchin I, Vender RB. 2022. Review and practical guidance on managing fungal infections in patients with psoriasis receiving anti-IL-17 therapies. J Cutan Med Surg. 26(1_suppl):3s–23s. doi: 10.1177/12034754221111111.
- Yin Y, Wang M, Liu M, Zhou E, Ren T, Chang X, He M, Zeng K, Guo Y, Wu J. 2020. Efficacy and safety of IL-17 inhibitors for the treatment of ankylosing spondylitis: A systematic review and meta-analysis. Arthritis Res Ther. 22(1):111. doi: 10.1186/s13075-020-02208-w.
- Yuan Q, Peng N, Xiao F, Shi X, Zhu B, Rui K, Tian J, Lu L. 2023. New insights into the function of interleukin-25 in disease pathogenesis. Biomark Res. 11(1):36. doi: 10.1186/s40364-023-00474-9.
- Zhang Q, Liao Y, Liu Z, Dai Y, Li Y, Li Y, Tang Y. 2021. Interleukin-17 and ischaemic stroke. Immunology. 162(2):179–193. doi: 10.1111/imm.13265.