ABSTRACT
This study aimed to estimate the prevalence of poultry aspergillosis and evaluate the accuracy of histopathology (test under evaluation) and mycological culture (an imperfect reference test). Farms raising layer and breeder or broiler birds, with suspected aspergillosis cases, clinical or subclinical, were eligible and visited for sampling. After necropsy, histopathology and mycological culture examinations were conducted by two evaluators. A Bayesian latent class model was used to estimate the accuracy of histopathology when compared to the imperfect reference test, mycological culture. A total of 142 chicken farms, 96 laying and breeding hen farms, and 46 broiler farms were used for the study. True aspergillosis median prevalence was estimated at 63.7% (95% credibility intervals, CrI: 53.8%, 73.0%) in layers and breeders and at 65.2% (95% CrI: 50.2%, 78.3%) in the broiler farms’ population. The median diagnostic sensitivity of histopathology and culture were estimated at, respectively, 98.8% (95% CrI: 94.6%, 100.0%) and 90.4% (95% CrI: 83.6%, 95.3%). Tests’ diagnostic specificity was estimated at, respectively, 97.3% (95% CrI: 87.7%, 99.9%) and 95.7% (95% CrI: 91.8%, 98.2%). Both tests had very high and comparable positive predictive values, but, in a population where disease prevalence was 25%, histopathology had a higher negative predictive value than culture.
1. Introduction
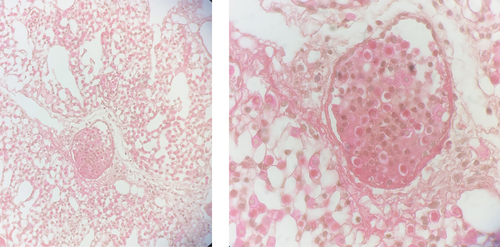
Poultry farming is an essential link in the animal production system. However, this sector faces many problems that disrupt its development. These include the quality of feed costs, and problems related to the marketing of chickens, but especially pathological constraints leading to morbidities and mortalities (Brou et al. Citation2018). Among these insidious pathologies with major health and economic impact, aspergillosis occupies a prominent place worldwide (Dahlhausen et al. Citation2004; Fischer et al. Citation2018; Thompson et al. Citation2021). Aspergillosis is an infectious and non-contagious fungal disease caused mainly by the complexes Aspergillus fumigatus, A. niger, A. flavus, and A. terreus, acquired following the inhalation of their conidia, or through dermal colonisation (Montazeri et al. Citation2020). Aspergillosis manifests itself in two forms, namely severe acute in young subjects with high morbidity and mortality rates, and chronic in adults (Martin et al. Citation2007). Aspergillosis symptoms are not specific, making diagnosis difficult (Dahlhausen et al. Citation2004). Moreover, diseases such as mycoplasmosis or infectious bronchitis, associated or not with aspergillosis, can lead to similar symptoms (Ratemo and Denning Citation2023). Diagnosis is generally based on an accumulation of evidence from history, clinical presentation, haematology, biochemistry, serology, radiographic changes, and culture of the fungus (Beernaert et al. Citation2010). Poultry sinusal Aspergilloma to Aspergillus flavus in a context of allergic mucin (mucinous material mixed with eosinophils and hetrophils) background.
Diagnostic methods such as mycological culture and histopathology are often used (Beernaert et al. Citation2010; Erin et al. Citation2017) to diagnose aspergillosis. However, few studies on the diagnostic accuracy of these tests in humans or animals can be found. Admittedly, aspergillosis-specific PCR tests were recently developed but are only available in a few reference laboratories. In many diagnostic laboratories, this latter test is not offered due to the important incurred costs for farmers. PCR tests also have the disadvantage of being very sensitive to contamination or to fungal DNA sequences of poor quality due to the presence of microbiota in certain types of samples, mainly lung or skin samples (Thompson et al. Citation2021).
The present study aimed at estimating the prevalence of avian aspergillosis in Côte d’Ivoire and at validating a diagnostic test, histopathological examination for the diagnosis of avian aspergillosis, using Bayesian Latent Class Model (BLCM), allowing for comparison with an imperfect reference test, mycological culture.
2. Material and methods
2.1. Study design
The study was a prospective study for diagnostic test validation. The assessment of mycological culture and histopathological examinations were conducted following double-blinded procedures: The index test, histopathological examination, was interpreted without knowledge not only of the second pathologist diagnosis but also of the mycological examination. Throughout the paper, we followed recommendations of the Standards for the Reporting of Diagnostic accuracy studies that use Bayesian Latent Class Models (STARD-BLCM) for reporting (Kostoulas et al. Citation2017).
2.2. Study area and data collection
This study was conducted from 1 January 2015 to 31 December 2021 in the farms of Bingerville, Abidjan, Dabou, Bouaflé, Yamoussoukro, Azaguié, Agnibelikrou, Grand-Bassam, and Modeste. These areas accounted for more than 90% of national poultry production. The project was approved by our institutional board ethical agreement (#2015–2022/DiagnosAspCIV). The monitoring of harmful effects following these two diagnostic tests study was not applicable, except for the fact that diseased poultry were humanely euthanised to perform the necropsies.
2.3. Inclusion criteria
All types of production systems of domestic hens (Gallus gallus domesticus) were considered for this study, including broilers, or layers and breeders. Chicks less than three weeks old were excluded. All suspected cases of aspergillosis, acute, subacute, or chronic, whether initially infected or complicating a pre-existing condition, were eligible. As examples, but not exhaustive, these complications could be colibacillosis, mycoplasmosis, coryza, fowl typhoid, malnutrition, long antibiotic treatments, or immunosuppression. For the purposes of the investigation, for each poultry farm, samples were collected from five birds, more specifically two to three birds that had already died the same day and two to three still diseased birds who had been euthanised. Euthanasia was achieved by cervical dislocation.
2.4. Definition of clinical suspect case of poultry aspergillosis
Birds presented with dyspnoea including hyperpnoea, drowsiness, nervous signs, inappetence, wasting, and/or increased thirst were considered as potential cases of poultry aspergillosis and retained for necropsy. At necropsy, pulmonary lesions were characterised by the constitution of cream-coloured plaques from several millimetres to several centimetres in diameter. These plaques and mycelial masses could be found in the air sacs, liver, brain, intestines, and eyes and consisted of whitish-creamy, cheese-like to gelatinous coatings, nodular and confluent-diphtheroid lesions, and/or fluffy, greenish pigmented fungal mycelium (Fischer et al. Citation2018).
2.5. Sampling procedures
In each farm included in the study, the five birds tested were considered, together, as a sampling unit representing the farm status. For a given diagnostic test, the farm was considered test-positive whenever ≥one bird tested positive. Thus, the combined enrolment of all farms included in the study represents the sample size of the study. To estimate the required number of farms, we used approximative methods. Briefly, the number of farms required for estimating a given accuracy parameter (diagnostic sensitivity, DSe or specificity, DSp of a test) with a given precision was computed using the equation for estimating sample size for simple proportions. Using these simple methods, we estimated that a sample of 140 (70 diseased and 70 non-diseased) farms would be sufficient to estimate the DSe or DSp with a precision of ±8.5% points, given that these accuracy parameters were ≥85%. These approximative calculations, of course, did not consider the imperfect nature of the reference test nor the use of informative priors for estimating these unknown parameters.
2.6. Laboratory assays
Before performing the necropsy, the sick birds were euthanised by cervical dislocation. At the end of the necropsy, three samples were collected on each case by dividing the organs removed into three equal parts so that one sample could be used for mycological culture, the second for histopathological examination, and the last for microbiological examination. This latter step was included to identify potential contamination of the samples. The sample prepared for mycological culture or microbiological culture was packaged in a sterile hard plastic vial of 25–30 mL, while the one for histopathological examination was packaged in the same type of vial containing formaldehyde buffered to 10%.
2.7. Mycological cultures
All types of organs such as kidneys, trachea, lungs, sinuses, air sacs, liver, heart, brain, spleen, cloaca, and bursa of Fabricius, and showing lesions such as masses, nodules, or granulomas were placed on plates containing malt agar (containing maltose as an energy source, dextrin, a polysaccharide derived from high-quality starch, glycerol as carbon source, and peptone as a nitrogen source) or Sabouraud glucose agar (containing gentamicin, chloramphenicol, and infusion of brain and heart) and incubated at 37 °C for 72 h. As a second step, a fungus-selective medium (Chromagar and Mycosel; Becton Dickinson AG) was used at 25 °C for 14 d. The preparation was regularly observed to characterise the genus and species of the fungus based on macroscopic and microscopic criteria (De Hoog et al. Citation2011). Two independent double-blinded mycological culture examinations were carried out for each sample. If the results differed, a third culture was made to reach a definitive diagnosis.
2.8. Histopathological examination
This examination was carried out on the samples obtained from the necropsy. All histopathological slides of each patient were examined because not all lesions and parts of Aspergillus spp. are necessarily on the same microscopic field, or on the same slide. Samples previously fixed in 10% formaldehyde were included in paraffin before undergoing tissue sections 4–5 μm thick. Haematoxylin eosin safran (HES) staining was applied to all these sections. Special staining of Grocott with silver methenamine was used for a second confirmation of the diagnosis made by HES staining. This special staining made it possible to characterise more specifically the hyphae, heads, and conidia typical of Aspergillus spp. in histological sections. Using an optical microscope, the diagnosis of Aspergillus spp. infection was made. Considering the complexity of this exam, two examiners, blinded to the results of the other examiner, read the histopathological sections, and, when they disagreed with the results, they reviewed the case together to obtain a consensus. When consensus could not be obtained, they referred to a third independent observer for a final decision.
2.9. Contamination
In the histopathological slide with haematoxylin staining eosin safran, contamination was defined as the presence of yeast-like or bacteria organisms without pathological reaction, the presence of yeast-like or bacteria organisms in serial adjacent sections, or the presence of yeast-like or bacteria organisms in one staining method and not another. Concerning mycological exams, contamination was defined as cultures that yielded known contaminant bacteria or yeast such as non-pathogenic E. coli serotypes, Micrococcus spp., Pseudomonas spp., or Malassezia spp. In case of contamination, the correspondent mycological or histopathological results were excluded.
2.10. Bayesian priors
External studies were used to inform priors for the DSe and DSp of mycological culture. The sensitivity of this test in human populations was reported in a few studies. Recent studies reported DSe estimates of 72% (95% confidence interval, CI: 59% to 85%) and 59% (95% CI: 41% to 77%; Montesinos et al. Citation2017; Wagner et al. Citation2018). Given that this prior information was obtained from a different population than the target population, this information was transformed into a rather diffuse prior with a median DSe of 65% and a 2.5% of 40%. This statistical distribution was represented as a beta (10.895, 6.328) distribution (). For mycological culture DSp, these human studies reported DSp estimates of 100% (95% CI: 100% to 100%) (Wagner et al. Citation2018) and 95% (95% CI: 89% to 100%) (Montesinos et al. Citation2017). Given that we also expected a high specificity for culture in birds, a reasonably precise prior with a median of 95% and a 2.5% of 90% was therefore used for this parameter. This prior was presented as a beta (100.000, 6.210) distribution ().
Table 1. Priors used in Bayesian latent class models developed to estimate the accuracy of histopathological examination and mycological culture using data from two populations (96 farms of breeders and layers birds vs. 46 broilers farms), along with the posterior distributions obtained.
2.11. Bayesian latent class model
For this study, we considered a two-population (broilers vs. layers and breeders), and two-diagnostic tests (histopathological examination vs. mycological culture) BLCM. In this model six unknown parameters had to be estimated: DSe and DSp of histopathological examination, DSe and DSp of mycological culture, and the actual prevalence of aspergillosis in each population. Cross-tabulation of the data generated six degrees of freedom; therefore, the model was deemed to be identifiable (Cheung et al. Citation2021). Nevertheless, since informative priors were available for the DSe and DSp of mycological culture, we did consider them in our initial model (). Conversely, flat priors [beta (1.0, 1.0); ] were initially used for disease prevalence and, most importantly, for accuracy parameters of the histopathological examination (i.e. the test under investigation). Within this BLCM, positive (PPV) and negative predictive values (NPV) were computed for each test, and for two theoretical prevalence of aspergillosis, 25% and 65%. A sensitivity analysis was then conducted to evaluate the robustness of our results to the chosen informative priors. For this sensitivity analysis, we ran again the BLCM, but using flat priors for all parameters ().
2.12. Model estimation
For all Bayesian analyses, we used the R2JAGS package (version 0.7-1) within the open-source R software 4.2.1 using the RStudio platform and JAGS version 4.3.1 (Plummer Citation2003). For each analysis, three Markov chains were run independently for 20,000 iterations after an initial burn-in of 1,000 iterations. Posterior distributions were analysed using the CODA package (version 0.19-4; Plummer et al. Citation2006). Briefly, the trace plot of each Markov chain was inspected to ensure convergence, and the effective sample size was computed to ensure that it was >10,000 for all parameters. To improve reproducibility, the R script used is available at https://doi.org/10.5683/SP3/4UW62J.
3. Results
3.1. Demographics of the population included in the study
Initially, 155 farms were included (). After conducting the necropsies, 10 cases were rejected because one or more organs were at least partially autolysed. Then, 145 farms were tested using the two approaches: mycological culture and histopathologic examination. Moreover, bacteriological culture was used on all samples to assess potential contamination of the sample. Following bacteriological culture, three additional cases were discarded due to contamination. Finally, 142 farms with complete data were retained for the study purposes (). In total, there were 96 laying or breeding hen farms and 46 broiler farms.
3.2. Tests results
Among the 87 histopathologic positive cases, there was disagreement on two cases. Finally, a consensus was reached for these two cases so that they were included in the 87 histopathologic positive cases. The first case was diagnosed by the first author as severe fibrino-heterophilic, lymphocytic, fibroplasia of Mycoplasma pneumonia with invasive aspergillosis substantiated by mild angioinvasion. It was diagnosed as invasive aspergillosis for the second reader. The final diagnosis was Mycoplasma pneumonia infection with invasive aspergillosis. Concerning the second case, a trachea specimen, it was classified as infectious laryngotracheitis, substantiated by undifferentiated and regenerating cells replacing necrotic epithelium, with a fibrino-heterophilic deposit containing syncytial cells displaying eosinophilic intranuclear inclusion bodies, associated with Aspergillus tracheitis. The second pathologist diagnosed Aspergillus and viral fibrino-heterophilic tracheitis. The consensual diagnosis was Aspergillus tracheitis and infectious laryngotracheitis.
Regarding mycological culture examination, perfect between-readers agreement was obtained for all samples. In samples from layer and breeder farms, among the 62 culture-positive cases, there were 42 Aspergillus fumigatus, 13 Aspergillus flavus, and 6 Aspergillus niger cases. In samples from broiler farms, of the 31 mycological culture-positive cases, 19 were Aspergillus fumigatus, 6 were Aspergillus flavus and 6 were Aspergillus niger. In both groups (laying hen-breeder and broiler), it is important to note that infraorbital or nasal chamber sinusitis involved only Aspergillus flavus. In both the broiler and layer-breeder groups, there was perfect agreement between mycological culture examination and histopathological examination on the presence of Aspergillus fumigatus invasive or non-invasive infections. There were no doubtful or undetermined diagnoses for mycological standard culture examination or histopathology. Cross-classified results from the two tests in the two populations are presented in .
Table 2. Cross-classified results of histopathological evaluation (hist) and mycological culture (myc) on samples from layer and breeder farms vs. broiler farms.
3.3. Estimates of diagnostic test characteristics
Based on BLCM () the median disease prevalence estimate was 63.7% (95% credibility interval, CrI: 53.8%, 73.0%) in layers and breeders and 65.2% (95% CrI: 50.2%, 78.3%) in the broiler farms. The median relative DSe and DSp of histopathological examination were 98.8% (95% CrI: 94.6%, 100.0%) and 97.3% (95% CrI: 87.7%, 99.9%) and for the mycological culture were 90.4% (95% CrI: 83.6%, 95.3%) and 95.7% (95% CrI: 91.8%, 98.2%), respectively. The posterior distributions for the DSe and DSp are illustrated in .
Figure 2. Histopathological examination and mycological culture diagnostic sensitivity and specificity posterior distributions were obtained using Bayesian latent class models with data from two populations (96 farms of breeders and layers vs. 46 broilers farms).

The tests’ PPV and NPV are reported in and illustrated in . Briefly, when applied in a population with a prevalence of disease of 65%, the median PPV of histopathological examination and of mycological culture were, respectively, 98.5% (95% CrI: 93.8%, 99.9%) and 97.5% (95% CrI: 95.3%, 99.0%). Moreover, the median NPV for a histopathological exam was also very high (97.8%; 95% CrI: 90.5%, 99.9%), but the median mycology NPV was slightly lower (84.4%; 95% CrI: 75.9%, 91.7%). In a population with a lower prevalence of disease (25%), again, relatively high PPV and NPV were obtained for both tests. Moreover, in this situation, relatively large overlaps between the tests’ PPV and NPV posterior distributions were observed, thus indicating that the predictive values of the two tests were relatively similar.
Figure 3. Positive (PPV) and negative predictive values (NPV) for diagnosing avian aspergillosis for two tests and for two hypothetical disease prevalence.

When flat priors were used for all parameters, posterior distributions remained mostly unchanged (). The model, therefore, seemed quite robust to the choice of informative priors. The only noticeable differences were a slightly lower histopathology DSp median estimate (93.7% vs. 97.3%) and a slightly higher mycology DSe median estimate (96.9% vs. 90.4%) in the model making use of flat priors on all parameters. Thus, the disagreements between the two tests (which were only in the form of histology positive and mycology negative results) were more evenly distributed as misclassification resulting from each test in the model with flat priors, while these were considered to be more likely a false-negative result of the mycology test in the model with informative priors. Consequently, we observed relatively small differences between the PPV and NPV posterior distributions generated using the BLCM with informative vs. flat priors.
4. Discussions
Our methodology, mainly the use of BLCM, was reported as one of the most robust approaches to estimating the real prevalence of pathology, and the diagnostic performance of tests, especially in the absence of a perfect or gold-standard examination. To our knowledge, this is the first time that a study reports on the prevalence of avian aspergillosis in Africa, but also the performance of diagnostic tests for this condition. The prevalence observed was very revealing of the importance of this mycosis in poultry farms suspected of the disease in Côte d’Ivoire.
There is a scarcity of studies evaluating the performance of confirmatory examinations (e.g. mycological culture, histopathology, or others) for human or animal aspergillosis (Herbrecht et al. Citation2015). In a retrospective study, with clinical and microbiological data as the gold standard, the DSe and DSp of mycological culture were estimated, respectively, at 72.3% and 100% (Wagner et al. Citation2018). In our study, mycological culture DSe seemed superior to this estimate. This could be due to the low DSp of the reference test used in Wagner et al. (Citation2018). With the modeling approach used in that latter study (i.e. considering the reference test as a gold standard), all samples with a negative result on culture and a positive result on the reference test would be considered as a failure (i.e. a lack of DSe) of culture. The difference in DSe for mycology between our study and the Wagner et al. (Citation2018) study could also be due, however, to different distributions of Aspergillus species between studies, or the different types of samples collected. Indeed, Jeffrey et al. (Citation2003) isolated more frequently fungus species from bronchoalveolar lavage and bronchial wash specimens than other types of specimens.
Regarding the accuracy of the histopathologic exam, there is currently no study assessing its DSe and DSp for diagnosing aspergillosis in birds or other animals. Nevertheless, in human patients, one study evaluated the agreement between histopathology and mycological culture for the diagnosis of invasive septate mould infections (Jeffrey et al. Citation2003). In this latter study, among 1,706 tissue specimens (obtained from autopsy or biopsy, or during surgery), only 30% of the positive results (21/70) were positive for both tests. Thus, substantial disagreement between tests was observed in this type of sample and for that condition. Consequently, likely, the DSe and DSp of these tests for that latter condition are, at best, moderate, which seems strikingly different from the results obtained in our study, where these tests were used for the diagnostic of avian aspergillosis, and for which both tests appeared to perform well. Beyond its high accuracy, histopathological examination has other advantages. It is simpler to perform, less time-consuming, and less expensive than culture. This is especially crucial in low-income countries where financial resources, materials, and laboratory facilities are often limited.
The better DSe observed for histopathological examination compared to culture could be explained by factors inhibiting the growth of Aspergillus spp. For instance, Aspergillus spp. are obligate aerobic organisms that do not grow under anaerobic conditions (Jeffrey et al. Citation2005). Necrotic and anoxic tissues, such as abscesses, could likely affect the survival of Aspergillus spp. to the point where they cannot be recovered anymore using culture methods. Moreover, a minimal load of Aspergillus spp. per gram of tissue would be needed for culture. On the other hand, the hyphae, heads, and conidia typical of Aspergillus spp. would still be observable in histological sections.
Finally, the PPV and NPV of the two tests in our study suggested that practitioners could interpret the results of histopathological or culture examination of a given farm with a high level of confidence and that both in cases where the prevalence of aspergillosis is expected to be low (25%) or high (65%). Regarding the PPV, we observed large overlaps between the PPV posterior distributions of histopathological and culture examination, and this, for the two prevalences under investigation. We cannot, therefore, conclude on the superiority of one of the testing approaches for that latter parameter. Regarding the NPV, however, a histopathological-examination-negative result seemed to indicate with greater certainty the true farm’s status, as compared to culture, mainly in a high (65%) prevalence situation. In populations where a low (25%) prevalence of the disease is expected, then this latter advantage would be negligible (i.e. median NPV of 99.6% and 96.8% for histopathology and culture, respectively). Histopathological examination could, therefore, be suggested as the best testing approach in the population where a high prevalence of aspergillosis is expected, and when greater confidence in a negative test result is needed. In other situations, both tests could be excellent options.
5. Conclusions
The current study suggested, with a strong level of evidence, that histopathological examination and mycological culture could be valid tests for clinical, research, and surveillance purposes, with DSe and DSp median estimates of 98.8% and 97.3%, for histopathological examination, and 90.4% and 95.7%, for mycological culture. In situations where a high (e.g. 65%) aspergillosis prevalence is suspected, both tests would yield very high PPV, but a histopathological exam would be slightly superior in terms of NPV. In a population where a lower disease prevalence is suspected, both tests would behave equally well.
Author contributions
The study was conceived by Alassane TOURE who was also responsible for data curation. Material preparation and data collection were done by Alassane TOURE, Josepha KOFFI, Olivier Assoi ETCHIAN, André Offianan TOURE, and Brahima DOUKOURE. Laboratory analyses were conducted by Alassane TOURE, Ruth Josepha KOFFI, Brahima DOUKOURE, and André Offianan TOURE. Statistical analyses were performed by Simon DUFOUR and reviewed by Alassane TOURE. The first draft of the manuscript was written by Alassane TOURE and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics approval
The institutional board ethical agreement number is 2015–2022/DiagnosAspCIV. The authors adhere to the Basel Declaration, ethical guidelines of the Association for the Study of Animal Behaviour, and those of the International Association of Veterinary Editors.
Consent to participate
Owners signed a written informed consent including in the registry of the laboratory in case of using their animal data for research purposes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Beernaert LA, Pasmans F, Waeyenberghe VL, Haesebrouck A, Martel F. 2010. Aspergillus infections in birds: A review. Avian Pathol. 39(5):325–331. doi: 10.1080/03079457.2010.506210.
- Brou GKG, Diaby M, Silue N, Soro YR. 2018. État des lieux des mesures de prophylaxie sanitaire dans les élevages de poulets de chairs, souche COBB 500, dans le département de Korhogo (Côte d’Ivoire). J App Bioscience. 126(1):12717–12723. doi: 10.4314/jab.v126i1.9.
- Cheung A, Dufour S, Jones G, Kostoulas P, Stevenson MA, Singanallur NB, Firestone SM. 2021. Bayesian latent class analysis when the reference test is imperfect. Rev Sci Tech. 40:271–286. doi: 10.20506/rst.40.1.3224.
- Dahlhausen B, Abbott R, VanOverloop P. 2004. Rapid detection of pathogenic Aspergillus species in avian samples by real-time PCR assay: A preliminary report. Proceedings of the 25th Annual Conference & Expo of the Association of Avian Veterinarians; August 17–19; New Orleans LA. p. 37.
- De Hoog GS, Guarro J, Gene J, Figueras MJ. 2011. Atlas of clinical fungi, electronic version 3.1. Utrecht: Centraal bureau voor Schimmel cultures; [accessed 2023 July 31]. http://www.cbs.knaw.nl/2011.
- Erin EMS, Older ECE, Branco M, Bryan LK, Lawhon SD, Suchodolski JS, Gomez G, Manse J, Hoffmann AR, Hoffmann AR. 2017. Panfungal polymerase chain reaction for identification of fungal pathogens in formalin-fixed animal tissues. Vet Pathol. 54(4):640–648. doi: 10.1177/0300985817698207.
- Fischer D, van Waeyenberghe L, Failing K, Martel A, Lierz M. 2018. Single tracheal inoculation of Aspergillus fumigatus conidia induced aspergillosis in juvenile falcons (Falco spp.). Avian Pathol. 47(1):33–46. doi: 10.1080/03079457.2017.1360470.
- Herbrecht R, Thomas F, Patterson SMA, Marchetti O, Maertens J, Johnson EM, Schlamm HT, Donnelly PJ, Pappas PG. 2015. Application of the 2008 definitions for invasive fungal diseases to the trial comparing voriconazole versus amphotericin B for therapy of invasive aspergillosis: A collaborative study of the mycoses study group (MSG 05) and the European Organization for Research and Treatment of Cancer Infectious Diseases group. Clin Infect Dis. 60(5):713–720. doi: 10.1093/cid/ciu911.
- Jeffrey JT, Han XY, Kontoyiannis DP, May GS. 2005. Aspergillus hyphae in infected tissue: Evidence of physiologic adaptation and effect on culture recovery. J Clin Microbiol. 43(1):382–386. doi: 10.1128/JCM.43.1.382-386.2005.
- Jeffrey JM, Irfan T, Mario L, Luna W, Han M, XY MG, Kontoyiannis DP. 2003. Diagnosis of invasive septate mold infections: A correlation of microbiological culture and histologic or cytologic examination. Am J Clin Pathol. 119(6):854–858. doi: 10.1309/EXBVYAUPENBM285Y.
- Kostoulas P, Nielsen SS, Branscum AJ, Johnson WO, Dendukuri N, Dhand NK, Toft N, Gardner IA. 2017. STARD-BLCM: Standards for the reporting of diagnostic accuracy studies that use Bayesian latent class models. Prev Vet Med. 138:37–47. doi: 10.1016/j.prevetmed.2017.01.006.
- Martin MP, Bouck KP, Helm J, Dykstra MJ, Wages DP, Barnes HJ. 2007. Disseminated Aspergillus flavus infection in broiler breeder pullets. Avian Dis. 51(2):626–631. doi: 10.1637/0005-2086(2007)51[626:DAFIIB]2.0.CO;2 2007 200751.
- Montazeri A, Zandi H, Teymouri F, Soltanianzadeh Z, Jambarsang S, Mokhtari M. 2020. Microbiological analysis of bacterial and fungal bioaerosols from burn hospital of Yazd (Iran) in 2019. J Environ Health Sci Eng. 18(2):1121–1130. doi: 10.1007/s40201-020-00531-7.
- Montesinos I, Argudín MA, Hites M, Ahajjam F, Dodemont M, Dagyaran C, Bakkali M, Etienne I, Jacobs F, Knoop C, et al. 2017. Culture-based methods and molecular tools for azole-resistant Aspergillus fumigatus detection in a Belgian University hospital. J Clin Microbiol. 55(8):2391–2399. doi:10.1128/JCM.00520-17.
- Plummer M. 2003. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. Proceedings of the 3rd International Workshop on Distributed Statistical Computing (DSC 2003); March 20–22, Vienna, Austria. p. 1–10.
- Plummer M, Best N, Cowles K, Karen V. 2006. CODA: Convergence diagnosis and output analysis for MCMC. R News. 6(1):7–11.
- Ratemo SN, Denning DW. 2023. Burden of fungal infections in Kenya. Mycology. 14(2):142–154. doi: 10.1080/21501203.2023.2204112.
- Thompson GR 3rd, Young JH, Longo DL. 2021. Aspergillus infections. N Engl J Med. 385(16):1496–1509. doi: 10.1056/NEJMra2027424.
- Wagner K, Springer B, Pires VP, Keller PM. 2018. Molecular detection of fungal pathogens in clinical specimens by 18S rDNA high-throughput screening in comparison to ITS PCR and culture. Sci Rep. 8:6964. doi: 10.1038/s41598-018-25129-w.