ABSTRACT
Species of Micropsalliota generally grow in the tropics and are characterised by small, slender basidiomes, brown basidiospores, and cheilocystidia that vary in shape with capitate or subcapitate apex, and pigmented pileipellis. Based on morphological characters and molecular evidence, here we describe seven new species from southern China, viz. Micropsalliota ferruginea, M. fimbriata, M. gigaspora, M. longicystis, M. nana, M. squarrosa, and M. umbonata. Micropsalliota appendiculata, a species recently described from Vietnam, was first recorded in China. The Maximum likelihood and Bayesian analyses based on multi-locus sequence datasets (the nuc rDNA internal transcribed spacer region ITS1-5.8S-ITS2, nrITS; the D1–D2 domains of nuc 28S rDNA, LSU; partial sequences of the most variable region of the second-largest subunit of RNA polymerase II, rpb2, and a portion of the translation-elongation factor 1-α, tef1) shows that the genus is separated into 11 major clades and subclades. To aid in diagnosis, a key to 32 species of Micropsalliota in China is provided.
1. Introduction
The genus Micropsalliota Höhn. was established by Höhnel (Citation1914) to accommodate species within Agaricus L. [syn. Psalliota (Fr.) P. Kumm. 1871:23] that form small and slender basidiomes. The generic concept was subsequently amended by Heinemann (Citation1956), Pegler and Rayner (Citation1969), and adopted by subsequent mycologists such as Singer (Citation1975). In previous molecular systematics studies, Zhao et al. (Citation2010) provided the first molecular phylogenetic study of the genus based on nrITS and nrLSU sequences. Based on combined nrITS and nrLSU sequences, or rpb2, the genera Leucoagaricus Locq. ex Singer, Leucocoprinus Pat., and Micropsalliota together form a monophyletic clade (BS = 100%, PP = 1) (Ge et al. Citation2015; Ma et al. Citation2022). Yan et al. (Citation2022) reconstructed its phylogeny based on the three-gene dataset (nrITS, nrLSU, and rpb2) and separated the genus into 18 weakly to strongly supported clades and subclades.
Up to now, about 80 species of Micropsalliota are recorded worldwide (https://www.speciesfungorum.org/) (Al-Kharousi et al. Citation2022; Patil et al. Citation2022; Yan et al. Citation2022; Ji and He Citation2023), most of which are distributed in tropical and subtropical areas of Africa, India, America, Indonesia, and Malaysia (Heinemann Citation1980, Citation1983, Citation1988, Citation1989; Heinemann and Flower Citation1983; Guzmán-Dávalos Citation1992; Guzmán-Dávalos and Heinemann Citation1994), and few reports were from China (Wei et al. Citation2015; Li et al. Citation2021; Yan et al. Citation2022; Ji and He Citation2023). Since Micropsalliota pseudoglobocystis Li Wei & R.L. Zhao, the first new species from China, was reported (Wei et al. Citation2015), 11 new species, and 13 new records for China have been published recently (Wei et al. Citation2015; Xu et al. Citation2016; Wang et al. Citation2017; Chen et al. Citation2019; Sun et al. Citation2020; Li et al. Citation2021; Liu et al. Citation2022; Yan et al. Citation2022; Ji and He Citation2023).
In this paper, we explore the species diversity of Micropsalliota and describe seven new species and a new record from southern China based on morphological observations and molecular phylogenetic analyses.
2. Materials and methods
2.1. Morphological study
Macroscopic characters and habitat details of fresh basidiomes were photographed and recorded at the time of collecting. Odor and colour changes after bruising were recorded at the same time. GPS coordinates were documented for each collection site. To avoid mixing or crushing, aluminium foil was used for wrapping. Chemical reactions of fresh specimens were recorded soon after returning from the field. Colour terms and notations follow the Methuen Handbook of Colour (Kornerup and Wanscher Citation1978). Specimens were dried completely with a dehydrator at 50 Celsius degrees, then sealed in plastic bags, and deposited in the Herbarium of Cryptogams, Kunming Institute of Botany, Chinese Academy of Sciences (KUN, with HKAS accession numbers) (). Microscopic observations were performed on dried specimens. Tissues to be observed were mounted in 3% KOH, and then stained with 1% Congo Red reagent. When necessary, Melzer’s reagent was used to test the amyloidity of basidiospores, and 10% NH4OH was used to test the type of pigments. A minimum of 20 basidiospores, 10 basidia, and 10 cystidia per specimen were randomly measured using a LEICA DM2500 microscope (Leica, Bensheim, Germany). The notations [n/m/p] indicate that the measurements were taken on n basidiospores, from m basidiomes and p collections. Dimensions for basidiospores are given using (a) b – c (d). The b – c range contains at least 90% of the measured values, and (a) and (d) represent the extreme values whenever present. “av.” stands for the average size of basidiospores, “Q” stands for the ratio of the length and width of a spore, and Qm stands for the mean value of all basidiospores ± standard deviation of the samples (Largent Citation1986).
Table 1. Taxa, vouchers, and GenBank accession numbers used in the molecular analyses for ITS, LSU, EF1, and RPB2 datasets.
2.2. DNA extraction, PCR, and sequencing
DNA was extracted from the dried specimens using an Ezup Column Fungi Genomic DNA Purification Kit (Sangon Biotech, Shanghai, China) following the manufacturer’s protocol. Polymerase chain reaction (PCR) was implemented on an ABI 2720 thermal cycler (Applied Biosystems, Foster City, CA, USA). PCR amplification and sequencing procedures followed procedures described by Ge et al. (Citation2021). Specifically, each PCR reaction mixture contained 1 μL (10 mmol/L) of each primer, 1 μL DNA template, 0.3 μL MgCl2 (Sangon Biotech, Shanghai, China), 12.5 μL 2 × GS Taq PCR Mix (Genesand Biotech, Beijing, China) and ddH2O up to 25 μL. Primers used to amplify the internal transcribed spacer (ITS) region, the large subunit (LSU) of the ribosomal DNA, the second largest RNA polymerase subunit (rpb2), and translation elongation factor 1-α (tef1) were ITS1F/ITS4 (White et al. Citation1990; Gardes and Bruns Citation1993), LR0R/LR5 (Vilgalys and Hester Citation1990; Cubeta et al. Citation1991), bRPB2-6F/bRPB2-7 R (Matheny Citation2005) and EF1-983F/EF1-1567 R (Rehner and Buckley Citation2005), respectively. The PCR program was set as follows: Pre-denaturation at 95 °C for 3 min; 35 cycles of denaturation at 95 °C for 25 s, annealing at appropriate temperature and time (ITS and LSU: 53 °C for 25 s, rpb2 and tef1: 58 °C for 25 s) and extension at 72 °C for 15 s, followed by a final extension at 72 °C for 5 min. PCR products were sent to Biomed Biotechnology commercial company for sequencing.
2.3. Molecular phylogenetic study
Reference sequences were selected based on previous studies (Zhao et al. Citation2010; Wei et al. Citation2015; Xu et al. Citation2016; Parra et al. Citation2016; Wang et al. Citation2017; Chen et al. Citation2019; He et al. Citation2020; Sun et al. Citation2020; Li et al. Citation2021; Liu et al. Citation2022; Al-Kharousi et al. Citation2022; Patil et al. Citation2022; Yan et al. Citation2022). Sequences (ITS, LSU, rpb2, and tef1) of Micropsalliota in NCBI GenBank were downloaded and 185 out of the 359 sequences were retained after excluding redundant sequences from the same location and the sequences with low quality. Then additional 166 sequences generated from our newly collected specimens were incorporated into the data set. Leucoagaricus barssii (Zeller) Vellinga and L. centricastaneus Y.R. Ma, Z.W. Ge & T.Z. Liu were chosen as the outgroup taxon for rooting purposes based on data published by Ma et al. (Citation2022). A total of 359 sequences including 145 ITS, 107 LSU, 67 rpb2, and 40 tef1 sequences were used in subsequent analyses. Details of all sequences are listed in .
The sequences were aligned using MAFFT v7.453 (Katoh and Standley Citation2013) and then inspected and manually corrected using BioEdit v.7.0.9 (Hall Citation1999). TrimAl v.1.4.rev15 (Capella-Gutiérrez et al. Citation2009) was used to remove sites that were vaguely aligned. The resulting alignments were examined and optimised manually in AliView 1.27 (Larsson Citation2014). Phylogenetic analysis was then performed based on the concatenated ITS-LSU-rpb2-tef1 dataset using PhyloSuite v1.2.2 (Zhang et al. Citation2020). Maximum likelihood (ML) analyses were performed in RAxML 8.2.12 (Stamatakis Citation2006) with the GTRGAMMA model as the best-fit likelihood model with 1,000 replicates (Silvestro and Michalak Citation2012). The BI phylogenies were tested in MrBayes v. 3.2.6 (Ronquist et al. Citation2012) and the best-fit model (GTR+F+I+G4) was selected using ModelFinder (Kalyaanamoorthy et al. Citation2017). 10,000,000 generations were run for four chains and sampled every 1,000 generations. The first 25% of generated trees were discarded as the burn-in, and the Bayesian posterior probabilities (BPPs) were calculated from the posterior distribution of the remaining phylogenetic trees. For phylogenetic tree visualisation, FigTree 1.4.4 (Rambaut Citation2018) was used and the tree was annotated using Adobe Illustrator CC2018.
3 Results
3.1. Phylogenetic results
The combined ITS-LSU-rpb2-tef1 dataset comprised 147 sequences, representing 41 described species, two varieties, and seven undescribed species of Micropsalliota, with two sequences from Leucoagaricus as outgroups. A total of 166 new sequences were generated in this study, including 45 ITS, 44 LSU, 41 rpb2, and 38 tef1 sequences (). The final dataset included 2,649 characters with 616 bp from ITS, 857 bp from LSU, 625 bp from rpb2, and 551 bp from tef1.
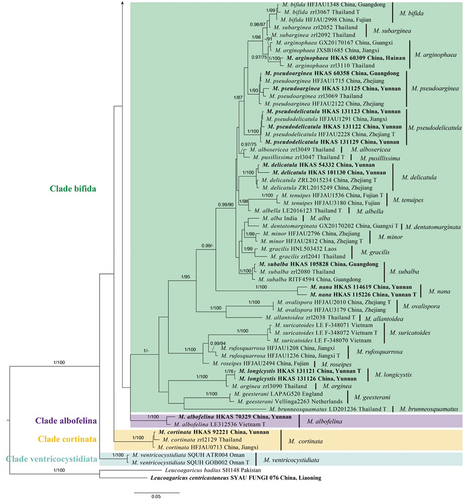
The results from RAxML and Bayesian analyses generated almost similar topologies, clades with a Bayesian posterior probability (BI-PP) ≥ 0.95 and ML bootstrap support (ML-BP) ≥ 75% were considered to be well-supported (Yan et al. Citation2022). Based on the phylogenetic analyses of the combined dataset, all taxa of Micropsalliota form a monophyletic clade (BI-PP = 1; MLBP = 100%).
As shown in the phylogenetic tree in , all taxa of Micropsalliota formed a well-supported monophyletic clade (BI-PP = 1; MLBP = 100%). Based on the phylogenetic tree and previous studies (Zhao et al. Citation2010; Wei et al. Citation2015; Li et al. Citation2021; Al-Kharousi et al. Citation2022; Patil et al. Citation2022; Yan et al. Citation2022), 11 major Clades within the genus were identified: Clade albofelina, Clade bifida, Clade cortinata, Clade ferruginea, furfuracea, Clade globocystis, Clade jiangxiensis, Clade lateritia, Clade megaspora, Clade pleurocystidiata, and Clade ventricocystidiata. These major clades are described further below.
Figure 1. Phylogram of Micropsalliota generated by Bayesian inference (BI) analysis based on sequences of a concatenated data set from four nuclear genes (ITS, LSU, rpb2, and tef1). Leucoagaricus centricastaneus and L. badius are selected as outgroups. Posterior probabilities (BI-PP) ≥ 0.95 and ML bootstrap (ML-BP) ≥ 75% are shown as PP/BP. The scale bar represents the substitutions per nucleotide site.
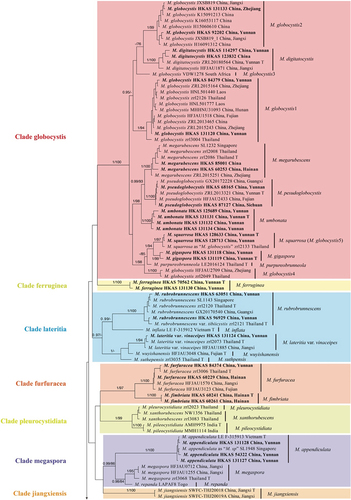
Clade globocystis was strongly supported (BI-PP = 1; MLBP = 100%) and included five known species (Micropsalliota digitatocystis R.L. Zhao, J.X. Li & M.Q. He, Micropsalliota globocystis Heinem., Micropsalliota megarubescens R.L. Zhao, Desjardin, Soytong & K.D. Hyde, M. pseudoglobocystis, Micropsalliota purpureobrunneola M.Q. He & R.L. Zhao) and three new species (M. gigaspora, M. squarrosa, and M. umbonata). The sequences in GenBank labelled as M. globocystis from China, South Africa, and Thailand formed five distinct lineages (). One of the lineages, represented by “Micropsalliota globocystis voucher SFSU zrl 2133” in GenBank (HM436632), and referred to as “globocystis 5” in Yan et al. (Citation2022), is clustered with M. squarrosa, a new species described in the present study, with strong support (BI-PP = 1; MLBP = 97%).
Clade lateritia (BI-PP = 0.97; MLBP < 75%) contained six species (varieties): Micropsalliota inflata D.D. Ivanova & O.V. Morozova, Micropsalliota lateritia var. vinaceipes R.L. Zhao, Desjardin, Soytong & K.D. Hyde, Micropsalliota rubrobrunnescens R.L. Zhao, Desjardin, Soytong & K.D. Hyde, Micropsalliota rubrobrunnescens var. tibiicystis R.L. Zhao, Desjardin, Soytong & K.D. Hyde, Micropsalliota suthepensis R.L. Zhao, Desjardin, Soytong & K.D. Hyde, and Micropsalliota wuyishanensis J.Q. Yan.
Clade furfuracea (BI-PP = 1; MLBP = 97%) contained two species, M. furfuracea and the new species Micropsalliota fimbriata described in the present study.
Clade pleurocystidiata (BI-PP = 1; MLBP = 99%) consisted of Micropsalliota pileocystidiata P.B. Patil & S.A. Vaidya, Micropsalliota pleurocystidiata Heinem. & Little Flower, and Micropsalliota xanthorubescens Heinem.
Clade megaspora was strongly supported (BI-PP = 0.99; ML-BP = 86%) and consisted of a newly recorded taxon of China (Micropsalliota appendiculata D.D. Ivanova & O.V. Morozova) and two known species: Micropsalliota megaspora R.L. Zhao, Desjardin, Soytong & K.D. Hyde and Micropsalliota repanda Heinem.
Clade bifida (BI-PP = 1; MLBP < 75%) consisted of 24 species. Among these, Micropsalliota nana and M. longicystis were new taxa.
Clade albofelina, Clade cortinata, Clade ferruginea, Clade jiangxiensis, and Clade ventricocystidiata consisted of a single species respectively.
3.2. Taxonomy
Micropsalliota appendiculata D.D. Ivanova & O.V. Morozova. Phytotaxa. 626(4):247–258.
Figure 2. Basidiomata of Micropsalliota species. (a – c) M. appendiculata; (a) KUN-HKAS 54322; (b, c) KUN-HKAS 131127. (d, e) M. ferruginea; (d) KUN-HKAS 70562 (Holotype); (e) KUN-HKAS 131130. (f) M. fimbriata; KUN-HKAS 60241 (holotype). (g – i) M. gigaspora; (g, h) KUN-HKAS 131119 (Holotype); (i) KUN-HKAS 131118. (j, k) M. longicystis; KUN-HKAS 131126. (l, m) M. nana; (l) KUN-HKAS 114619; (m) KUN-HKAS 115226 (holotype). (n, o) M. squarrosa; (n) KUN-HKAS 128633 (Holotype); (o) KUN-HKAS 128713. (p, q) M. umbonata; (p) KUN-HKAS 125689; (q) KUN-HKAS 131134. Bars = 10 mm.

Figure 3. Microscopic features of Micropsalliota appendiculata (KUN-HKAS 54322). (a) Basidiospores. (b) Basidia. (c) Cheilocystidia. (d) Pleurocystidia. (e) Pileus squamules. Bars: a – d = 10 μm; e = 20 μm.
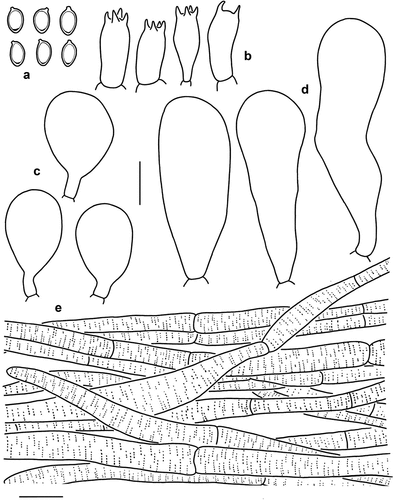
Macroscopic description: Pileus 3–11 mm in diam., convex or hemispherical in early stage, expanding to plano-convex with age, sometimes with apparent umbo, yellowish white (1A2); surface dry, densely covered with squamules, light yellow (5A4) or yellowish brown (5D8) when young, dark brown (6E6) when mature, margin with light yellow (5A4) or yellowish brown (5E6) appendiculate. Context thickened at the disc and thin towards the margin, white (1A1). Lamellae free, close, with 3–5 series of lamellulae, 1–1.5 mm broad, cream (5A2) to brown (6E6). Stipe 13–27 × 0.5–1 mm, cylindrical, straight, surface with tiny fibrils, cream (5A2) when young, with a light brown (5C4) tone with age. Annulus membranous, single, superior, up to 1–2 mm broad, cream (5A2) to dark brown (5F4), fragile, and disappears in age. No colour changes were observed when bruised or cut.
Microscopic description: Basidiospores [40/2/2] (5) 5.5–7 × 3.5–4 μm, av. = 6.15 × 3.85 μm, Q = (1.43) 1.50–1.75, Qm = 1.60 ± 0.11, amygdaliform to ellipsoid, with apical thickening, without germ pore, light brown, inamyloid. Basidia (13) 14–16 × 6–7 μm, clavate, hyaline, 4-spored, sometimes 2-spored. Pleurocystidia 35–52 (61) × 9–16 μm, broadly clavate to clavate-capitate, hyaline, smooth. Cheilocystidia 16–25 × 10–13 (14) μm, pyriform to subglobose, hyaline, smooth. Pileus squamules composed of hyphae 6–15 μm in diam., hyaline, smooth, cylindrical, slightly constricted at septa, with light brown membranous pigments.
Distribution: Vietnam and southwestern China (Yunnan).
Habit and habitat: Gregarious on soil in broad-leaved forest.
Known distribution: Vietnam and the tropical region of Yunnan Province, China.
Specimens examined: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Jinghong City, Dadugang Township, 100.918056°E, 22.611111°N, alt. 1,370 m, 29 June 2008, Z.W. Ge 2112 (KUN-HKAS 54322); ibid. Mengla County, Menglun Township, Xishuangbanna Tropical Botanical Garden, Greenstone Forest Park, on soil under broad-leaved forest, 101.281291°E, 21.9102435°N, alt. 590 m, 8 July 2021, H. Qu 422 (KUN-HKAS 131127); ibid., 101.28188°E, 21.9120206°N, alt. 520 m, 8 July 2021, H. Qu 429 (KUN-HKAS 131128).
Notes: Micropsalliota appendiculata, which was originally described from Vietnam, is characterised by yellowish brown, squamulose pileus with apparent umbo, and pyriform to subglobose cheilocystidia. The morphology of three Chinese materials overall matches the description of the type specimen of M. appendiculata (Ivanova et al. Citation2023). The similarity of ITS sequences between Chinese collections and type specimens is higher than 99%. However, all the specimens collected from China possess broadly clavate to clavate-capitate pleurocystidia, which is described as absent in the protologue. Despite the differences, considering the similarity of other morphologies and ITS sequences, we identified the three specimens of Yunnan, China as M. appendiculata.
Considering the overall appearance, Micropsalliota appendiculata is similar to Micropsalliota albosericea Heinem. & Leelav., M. allantoidea, Micropsalliota albella M.Q. He & R.L. Zhao, and Micropsalliota delicatula R.L. Zhao, J.X. Li & M.Q. He. However, M. alboserice differs in forming pure white pileus and smaller basidiospores (4.5–6 × 3–4 μm) (Heinemann and Leelavathy Citation1991); M. allantoidea differs in forming sausage-shaped pileipellis elements (Zhao et al. Citation2010); M. albella differs in forming smaller basidiomes (pilei 2–5 mm in diam., stipe 5–13 × 0.3–0.6 mm) with glabrous pileus (He et al. Citation2020). M. delicatula R.L. Zhao, J.X. Li & M.Q. He differs in forming pure white squamules, and capitate cheilocystidia 26–36 μm long with capitulum 3–5 μm in diam. (Li et al. Citation2021).
In the phylogenetic tree (), Micropsalliota appendiculata groups together with M. megaspora and M. repanda. However, M. megaspora differs in forming brown (7E6) to dark brown (8E4) pileus, bigger spores (6–8 × 3.8–4.5 μm) and the smaller basidia (11–15 × 6–7.5 μm) (Zhao et al. Citation2010); M. repanda differs in forming larger basidiomes (pilei 15–45 mm in diam., stipe 25–40 × 2–5 mm), purplish-red squamules and narrower cheilocystidia (broad 7–10 μm) (Heinemann Citation1980).
Micropsalliota ferruginea T. Gao & Z.W. Ge, sp. nov.
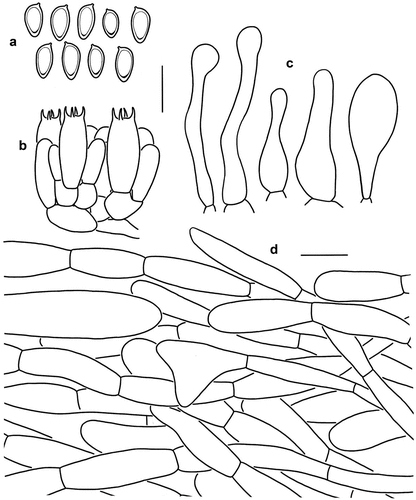
Figure 4. Microscopic features of Micropsalliota ferruginea (holotype, KUN-HKAS 70562). (a) Basidiospores. (b) Basidia. (c) Cheilocystidia. (d) Pileus squamules. Bars: a – c = 10 μm; d = 20 μm.
Fungal names: FN571731.
Etymology: Referring to its reddish-brown pileus.
Types: China, Yunnan Province, Honghe Hani and Yi Autonomous Prefecture, Gejiu City, on the trail to Shazhudi from Manhao in broad-leaved forest, 103.434444°E, 23.025556°N, alt. 860 m, 22 September 2011, Z.W. Ge 3058 (KUN-HKAS 70562, holotype). GenBank: ITS = OR799884, LSU = OR799929, rpb2 = OR962225, tef1 = OR962181.
Diagnosis: Micropsalliota ferruginea is distinguished from closely related species by the relatively large basidiomes with more or less recurved squamules that are brown to dark brown, and has larger recurved squamules on the margin, lamellae staining slightly blue when bruised or cut.
Macroscopic description: Pileus 35–50 mm in diam., convex in early stage, plano-convex to applanate with age; squamules small, pointed, up to 1 mm high, more or less recurved, dense on the disc and scattered near the margin, reddish brown (7D7), brown (5E8) to dark brown (8F8) on the disc, turning white (1A1) to cream (4A3) towards the margin; larger squamules on margin, recurved, concolorous with the disc. Context firm, up to 1 mm thick, cream (4A2). Lamellae free, crowded, with 3 series of lamellulae, 3–4 mm broad, light yellow (3A3) to greyish brown (3C2). Lamellae staining blue (23A4) when bruised or cut. Stipe 40–60 × 2–4 mm, cylindrical, hollow, with fibrillose to tiny reddish brown squamules, white or with reddish brown tone. Annulus pendent, single, superior, edge entire, persistent, membranous, white (1A1) in early stages, light brown (5B3) when mature.
Microscopic description: Basidiospores [20/1/1] (6) 6.5–7.5 × 3.5–4 μm, av. = 6.95 × 3.93 μm, Q = (1.50) 1.63–2.00 (2.14), Qm = 1.78 ± 0.14, amygdaliform to cymbiform, with apical thickening, without germ pore, light brown, inamyloid. Basidia 14.5–15.5 × 6–7 μm, clavate, hyaline, 4-spored. Pleurocystidia absent. Cheilocystidia 15–33 × 3–10 μm, tibiiform, cylindrical or slightly swollen near base and middle, with a narrower neck and apical capitulum (3) 4–6 μm in diam., hyaline, smooth. Pileus squamules composed of hyphae 11–20 μm in diam., hyaline, yellowish brown, smooth, cylindrical, constricted at the septa on some hyphae.
Distribution: Southwestern China (Yunnan).
Habit and habitat: Solitary or scattered on soils in broad-leaved forest.
Known distribution: Tropical region of Yunnan Province, China.
Additional specimens examined: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Mengla County, Menglun Township, Xishuangbanna Tropical Botanical Garden, Greenstone Forest Park, on soil under evergreen broad-leaved forest, 101.28117°E, 21.910726°N, alt. 650 m, 25 June 2020, G.S. Wang 1017 (KUN-HKAS 131130).
Notes: Micropsalliota ferruginea is characterised by its relatively large basidiomes with more or less recurved, brown to dark brown pileus squamules, and lamellae staining a slightly blue when bruised or cut.
Micropsalliota ferruginea differs from Micropsalliota albonuda (Beeli) Heinem. in that the latter forms a blue-white and glabrous pileus, and smaller basidiospores (5.1–5.8 × 3.1–3.8 μm) (Heinemann Citation1956). Micropsalliota brunneosquamata Linda J. Chen, R.L. Zhao & K.D. Hyde is similar to M. ferruginea by having reddish brown squamules. However, M. brunneosquamata differs in forming bigger squamules, the stipe below the annulus heavily covered by brown fibrils, and having smaller basidiospores measuring 5–6 × 3–4 μm (Chen et al. Citation2016). Micropsalliota furfuracea R.L. Zhao, Desjardin, Soytong & K.D. Hyde, and M. globocystis have similar-sized pileus to that of M. ferruginea. However, M. furfuracea and M. globocystis stain strong reddish brown when bruised (Zhao et al. Citation2010). In addition, M. ferruginea forms a well-supported monophyletic clade (BI-PP = 1; MLBP = 100%) in the four-locus phylogeny ().
Micropsalliota fimbriata T. Gao & Z.W. Ge, sp. nov.
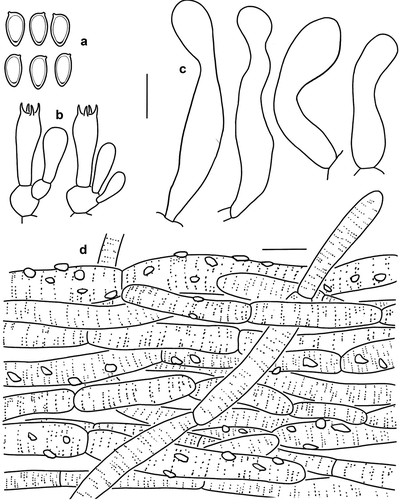
Figure 5. Microscopic features of Micropsalliota fimbriata (KUN-HKAS 60261). (a) Basidiospores. (b) Basidia. (c) Cheilocystidia. (d) Pileus squamules. Bars: a – c = 10 μm; d = 20 μm.
Fungal Names: FN571732.
Etymology: Referring to fimbriated fibrils of its pileus.
Types: China, Hainan Province, Wuzhishan National Nature Reserve, in broad-leaved forest, 109.579°E, 18.865°N, alt. 650 m, 31 July 2010, Z.W. Ge 2565 (KUN-HKAS 60241, holotype). GenBank: ITS = OR799886, LSU = OR799931, rpb2 = OR962227, tef1 = OR962198.
Diagnosis: Micropsalliota fimbriata is characterised by its reddish brown or greyish brown pileus with villous fibrils and appendiculate margin; whitish stipe with fibrillose surface, and clavate to capitate or subcapitate cheilocystidia.
Macroscopic description: Pileus 5–20 mm in diam., convex in early stage, plano-convex to applanate with age, surface dry, white (1A1) to whitish (1B1), covered with reddish brown (7D5) or greyish brown (6D3) villous fibrils, dense on the disc, margin appendiculate, fimbriated. Context less than 0.5 mm thick, white (1A1). Lamellae free, moderately distant, with 3–4 series of lamellulae, 1–2 mm broad, white (1A1). Stipe 20–35 × 1–2 mm, cylindrical, hollow, surface fibrillose, whitish (5A2). Annulus pendent, single, superior, persistent, edge entire, membranous, white (1A1). No colour change was observed when bruised or cut.
Microscopic description: Basidiospores [40/2/2] 6.5–7.5 × (3) 3.5–4 μm, av. = 6.88 × 3.78 μm, Q = 1.63–2.00 (2.17), Qm = 1.83 ± 0.15, amygdaliform, with apical thickening, without germ pore, light brown, inamyloid. Basidia (12) 13–17 × 6–9 μm, clavate, hyaline, 4-spored. Pleurocystidia absent. Cheilocystidia 25–44 × 5–9 μm, clavate to irregularly tibiiform, capitate or subcapitate with long narrow neck, capitulum 7–8.5 (9) μm in diam., hyaline, smooth. Pileus squamules composed of hyphae 5–10 μm in diam., cylindrical, slightly constricted at septa, with reddish brown, distinctly incrusting, and membranous pigments ().
Distribution: Tropical regions of Southern China (Hainan).
Habit and habitat: Solitary on soil in broad-leaved forest.
Known distribution: Hainan Province, China.
Additional specimens examined: China, Hainan Province, Hainan Island, Wuzhishan National Nature Reserve, in broad-leaved forest, 109.579°E, 18.865389°N, alt. 650 m, 1 August 2010, Z.W. Ge Citation2585 (KUN-HKAS 60261).
Notes: Micropsalliota fimbriata is characterised by its pileus covered with reddish brown or greyish brown villous fibrils, appendiculate pileus margin, and clavate to capitate or subcapitate cheilocystidia.
Micropsalliota fimbriata is similar to M. megaspora. However, the latter forms a brown pileus, finely squamulose stipe, and ventricose-rostrate to pyriform cheilocystidia (Zhao et al. Citation2010). Micropsalliota malabarensis Heinem. & Little Flower and Micropsalliota subalpina Guzm.-Dáv. & Heinem. differ in forming different spores: the former forms cymbiform spores, measuring 5–6 × 3–4 μm (Heinemann and Flower Citation1983), and the spores of the latter are ellipsoid to amygdaliform (5.1–6.4 × 3.2–3.7 μm) (Guzmán-Dávalos and Heinemann Citation1994), in contrast to the amygdaliform spores with larger measurements (6.5–7.5 × 3.5–4 μm) formed by M. fimbriata. Micropsalliota cortinata (Heinem.) Heinem. differs in forming cortinate partial veil that leaves remnants only on the pileus margin, cheilocystidia clavate to ventricose with a large base (Heinemann Citation1980).
Micropsalliota fimbriata, forming an independent clade (BI-PP = 1; MLBP = 100%) in Clade furfuracea, can be confused with M. furfuracea in overall appearance. However, M. furfuracea differs in forming relatively bigger basidiomes, flake-like squamules, and flesh staining red when bruised or cut (Zhao et al. Citation2010).
Micropsalliota gigaspora T. Gao & Z.W. Ge, sp. nov.
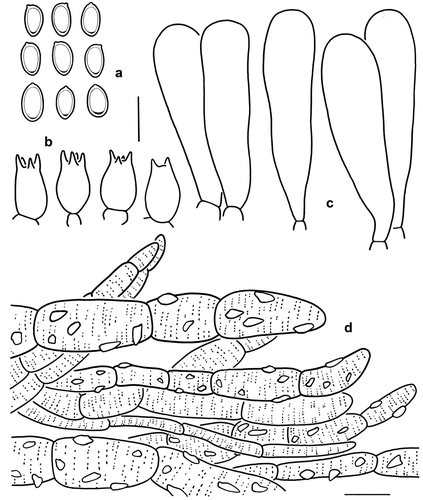
Figure 6. Microscopic features of Micropsalliota gigaspora (Holotype, KUN-HKAS 131119). (a) Basidiospores. (b) Basidia. (c) Cheilocystidia. (d) Pileus squamules. Bars: a – c = 10 μm; d = 20 μm.
Fungal Names: FN571733.
Etymology: Referring to the large basidiospores up to 9 μm long.
Types: China, Yunnan Province, Dehong Dai and Jingpo Autonomous Prefecture, Yingjiang County, Mangyun Town, Hongbeng River, on soil under evergreen broad-leaved forest, 97.601667°E, 24.449444°N, alt. 700 m, 19 July 2022, X.P. Fan 291 (KUN-HKAS 131119, holotype). GenBank: ITS = OR799891, LSU = OR799936, rpb2 = OR962231.
Diagnosis: Micropsalliota gigaspora is distinguished by small basidiomes (pilei 20–27 mm in diam., stipe 35–50 × 2–3 mm), narrow and thick annulus, clavate cheilocystidia and large basidiospores (7–9 × 4–5.5 μm).
Macroscopic description: Pileus 20–27 mm in diam., conical, convex in early stage, applanate or subumbonate with age, margin crenate, surface dry, covered with fibrillose scales, slightly recurved, dense on the disc and scattered near the margin, reddish brown (7E7), background white (1A1) or light grey (1B1), turning light brown with age. Context firm, up to 1–2 mm thick at disc. Lamellae free, crowded, with 2–4 series of lamellulae, 1–2 mm broad, light yellow (6A3) to brown (6C5). Stipe 35–50 × 2–3 mm, cylindrical, hollow; light yellow (6A3) to brown (6C5), covered with white (1A1) fibrils. Annulus persistent, superior, membranous, thick, white (1A1), edge entire, with light brown tone, 1–2 mm wide. No colour changes were observed when bruised or cut.
Microscopic description: Basidiospores [20/1/1] 7–9 × 4–5.5 μm, av. = 7.98 × 4.45 μm, Q = (1.40) 1.56–2.00, Qm = 1.80 ± 0.16, ellipsoid, with apical thickening, without germ pore, light brown, inamyloid. Basidia 11–15 (20) × 7–8 (12) μm, short-clavate, hyaline, 4-spored, sometimes 2-spored. Pleurocystidia absent. Cheilocystidia 37–50 × 7–14 μm, broadly clavate to clavate, hyaline, smooth. Pileus squamules composed of hyphae 6–19 μm in diam., hyaline, smooth, cylindrical, slightly constricted at septa, with reddish brown or light brown incrusting and membranous pigments.
Distribution: Southwestern China (Yunnan).
Habit and habitat: Solitary or scattered on soils.
Known distribution: West of Yunnan province, China.
Additional specimens examined: China, Yunnan Province, Dehong Dai and Jingpo Autonomous Prefecture, Yingjiang County, Mangyun Town, Hongbeng River, on soil under evergreen broad-leaved forest, 97.601667°E, 24.449444°N, alt. 700 m, 18 July 2022, X.P. Fan 258 (KUN-HKAS 131118).
Notes: Micropsalliota gigaspora is characterised by small basidiomes, a pileus covered with slightly recurved scales or fibrils, clavate cheilocystidia, and large basidiospores.
Micropsalliota brunneola Heinem. is morphologically similar to M. gigaspora in having similar-sized pileus and similar colour (Heinemann Citation1980). However, M. brunneola differs in forming shorter stipe (long 25 × 3.5 mm), and much smaller basidiospores (4.1–4.9 × 2.9–3.3 μm). Micropsalliota digitatocystis also have similar appearance but differ in forming larger basidiomes (pilei 16–72 mm in diam., stipe 60–90 × 5–8 mm), smaller spores (5.8–7.4 × 4–4.6 μm), and needle-like pleurocystidia (Li et al. Citation2021); M. gigaspora is also similar to several other species that have big basidiospores: M. megarubescens, however, has larger basidiomes (pilei 25–80 mm in diam.), white to cream and become light grey to greyish brown in age (Zhao et al. Citation2010). M. megaspora has smaller basidiomes (pilei 5–12 mm in diam.), and a paler fibrillose at disc (Zhao et al. Citation2010). Micropsalliota ventricocystidiata Al-Sadi & S. Hussain has a pale pileus (white to pale reddish-brown) and larger basidiomes (pilei 30–50 mm in diam., stipe 40–70 × 7–10 mm) (Al-Kharousi et al. Citation2022).
Micropsalliota gigaspora is sister to M. purpureobrunneola and M. squarrosa (this paper) in the molecular analyses (BI-PP < 0.95 ; MLBP = 85%). M. purpureobrunneola differs in forming smaller basidiomes (pilei 5–12 mm in diam.) and larger basidia (17.2–26 × 5.7–7.4 μm) (He et al. Citation2020); M. squarrosa differs in forming subcapitate cheilocystidia, which is clavate in M. gigaspora. Both M. gigaspora and M. squarrosa have large basidiospores. However, the basidiospores are longer in M. gigaspora (av. = 7.98 × 4.45 μm) but wider in M. squarrosa (av. = 7.75 × 4.90 μm); M. squarrosa staining slightly yellow (4A4) or reddish brown (7C5) when bruised but M. gigaspora with no colour changes observed when bruised or cut.
Micropsalliota longicystis T. Gao & Z.W. Ge, sp. nov.
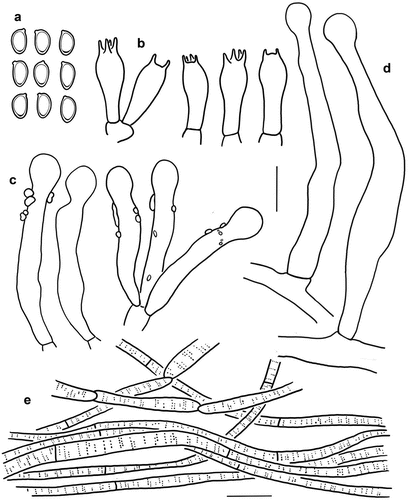
Figure 7. Microscopic features of Micropsalliota longicystis (holotype, KUN-HKAS 131121). (a) Basidiospores. (b) Basidia. (c) Cheilocystidia/pleurocystidia. (d) Caulocystidia. (e) Pileus squamules. Bars: a – d = 10 μm; e = 20 μm.
Fungal names: FN571735.
Etymology: Referring to its long caulocystidia up to 78 μm in length.
Types: China, Yunnan Province, Kunming City, Kunming Botanical Garden, on soil under moss, 102.74099494°E, 25.14476019°N, alt. 1,970 m, 12 June 2023, T. Gao 206 (KUN-HKAS 131121, holotype). GenBank: ITS = OR799897, LSU = OR799942, rpb2 = OR962257.
Diagnosis: Micropsalliota longicystis is distinguished by the white glabrous pileus, stipe with white rhizomorphs at the base, staining yellow then reddish-brown when bruised, and a dark brown or olive-green reaction with KOH, the presence of pleurocystidia and caulocystidia, and caulocystidia with a very long and narrow neck.
Macroscopic description: Pileus 5–20 mm in diam., convex, expanding to broadly convex with age, surface dry, surface silky-fibrillose, white (1A1) overall in early stage, sometimes becoming cream (3A2) to pale grey (1C3) with age. Context firm, less than 0.5 mm thick. Lamellae free, close, with 3 series of lamellulae, 2–3 mm broad, light yellow, becoming light brown (7D5–7D6) when mature, edges paler. Stipe 15–30 × 1–2 mm, cylindrical, white (1A1) to brown (2E5), surface smooth or with slightly white (1A1) fibrillose, with white rhizomorphs at the base. Annulus single, superior, persistent, edge entire, white. Pileus and stipe staining yellow then reddish brown when bruised or cut. KOH reaction dark brown or olive-green.
Microscopic description: Basidiospores [40/4/2] 5–6 × 3–3.5 μm, av. = 5.7 × 3.0 μm, Q = 1.7–2.0, Qm = 1.89 ± 0.16, ellipsoid, with apical thickening, without germ pore, light brown, inamyloid. Basidia (14) 15–17 (19) × 5–6.5 (8) μm, clavate, hyaline, 4-spored. Cheilocystidia and pleurocystidia common, similarly shaped, (25) 34–43 × 5–8 μm, clavate to clavate-capitate, sometimes subcapitate with long narrow neck, capitulum 4–5 μm in diam., hyaline, covered by light brown deposition. Caulocystidia 45–78 × 5–7 μm, tibiiform, capitate or subcapitate with long narrow neck, capitulum (3) 4–6 μm in diam., hyaline, smooth. Pileus squamules composed of hyphae 3–10 μm in diam., hyaline, smooth, cylindrical, slightly constricted at septa, with brown membranous pigments.
Distribution: Southwestern China (Yunnan).
Habit and habitat: Solitary or scattered on soils.
Known distribution: Central region of Yunnan province, China.
Additional specimens examined: China, Yunnan Province, Kunming City, Kunming Botanical Garden, on soil under moss, 102.74099494°E, 25.14476019°N, alt. 1,970 m, 17 June 2023, H. Qu Citation1075 (KUN-HKAS 131126).
Notes: Micropsalliota longicystis is characterised by a white silky-fibrillose pileus, stipe with white rhizomorphs at the base, presence of pleurocystidia and caulocystidia, and caulocystidia with a very long and narrow neck.
Micropsalliota longicystis forms a well-supported monophyletic clade (BI-PP = 1; MLBP = 100%) with Micropsalliota arginea (Berk. & Broome) Pegler & R.W. Rayner (only LSU sequences available for the Thai M. arginea zrl3090). Both species have capitate or subcapitate caulocystidia. However, M. arginea differs in forming smaller spores (4–5 × 2.5–3 μm) and smaller-sized cheilocystidia (25–35 × 3–6 μm) (Zhao et al. Citation2010).
Several species are similar in overall appearance to Micropsalliota longicystis. However, Micropsalliota allantoidea R.L. Zhao, Desjardin, Soytong & K.D. Hyde differs in forming slender basidiomes (pilei 5–10 mm in diam., stipe 10–20 × 0.5 mm) and sausage-shaped pileipellis elements (Zhao et al. Citation2010); M. pseudoarginea differs in forming a brown fibrillose-squamulose pileus, no staining reaction when bruised or cut, and a strong reddish brown reaction with KOH (Heinemann Citation1982; Zhao et al. Citation2010); Micropsalliota bifida R.L. Zhao, Desjardin, Soytong & K.D. Hyde differs in forming smaller basidiospores (3.8–5 × 2.3–3.2 μm) and bifid cheilocystidia, and strong reddish brown reaction with KOH (Zhao et al. Citation2010); Micropsalliota laeta Heinem. differs in forming bigger basidiospores (6.3–7.1 × 3.6–4 μm) and a white to light pink pileus (Heinemann Citation1980); Micropsalliota lutescens Heinem. differs in forming longer stipe (40–70 × 1.5–2 mm) and longer basidiospores (long 6.1–7.2 μm) (Heinemann Citation1980). Micropsalliota plumaria (Berk. & Broome) Höhn. differs in forming a squamulose-floccose pileus and subcapitate, broadly ventricose cheilocystidia without elongated neck (Pegler Citation1986); Micropsalliota vinaceoumbrina (A.H. Sm.) Heinem. differs in forming bigger basidiospores (6–7 × 5–5.5 μm) and bigger basidia (19–23 × 7–8 μm) (Smith Citation1944).
Micropsalliota nana T. Gao, H. Qu & Z.W. Ge, sp. nov.
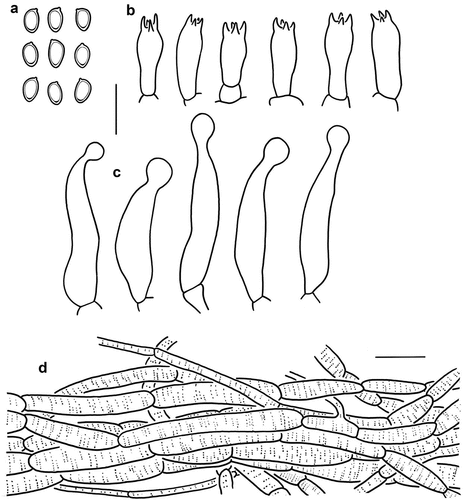
Figure 8. Microscopic features of Micropsalliota nana (holotype, KUN-HKAS 115226). (a) Basidiospores. (b) Basidia. (c) Cheilocystidia. (d) Pileus squamules. Bars: a – c = 10 μm; d = 20 μm.
Fungal Names: FN571734.
Etymology: Referring to its tiny pileus measuring 5–15 mm in diameter.
Types: China, Yunnan Province, Baoshan City, Tengchong City, Jietou Town, Goubianzhai, on soil under evergreen broad-leaved forest, 98.712175°E, 25.429414°N, alt. 1,910 m, 8 August 2019, L.R. Zhou 280 (KUN-HKAS 115226, holotype). GenBank: ITS = OR799902, LSU =OR799947, tef1 = OR962217.
Diagnosis: Micropsalliota nana is distinguished by a white, glabrous to silky pileus that turns brown when bruised or cut, small basidiospores, and capitate or subcapitate cheilocystidia.
Macroscopic description: Pileus 5–15 mm in diam., convex in early stage, expanding to plane, with obtuse umbo, surface dry, silky to fibrillose, white (1A1) when young, cream (5A2) to light yellowish grey (5B2) or light brown with age, light salmon (6A4) at disc. Context less than 0.5 mm thick. Lamellae free, moderately distant, with 2 series of lamellulae, 0.5–0.7 mm broad, white (1A1), becoming brown (7E4–7E5) when mature. Stipe 15–40 × 1–1.5 mm, cylindrical, white (1A1) when young, turning brown (6E2) with age, surface with fine white (1A1) fibrils. Annulus superior, persistent, edge entire, white (1A1) at first, brown (7E4) when mature. Staining brown (7E4) when bruised or cut.
Microscopic description: Basidiospores [20/2/1] 4.5–6 × 3–4 μm, av. = 5.2 × 3.53 μm, Q = 1.25–1.71 (2.00), Qm = 1.49 ± 0.20, ellipsoid to amygdaliform, with apical thickening, without germ pore, light brown, inamyloid. Basidia 11–20 × (4) 5–6 μm, clavate, hyaline, 4-spored. Pleurocystidia absent. Cheilocystidia 29–45 (60) × 5–13 μm, ventricose to irregularly tibiiform, capitate or subcapitate on the top, followed by a long narrow neck, capitulum 4–8 (10) μm in diam., hyaline, smooth. Pileus squamules composed of hyphae 4–15 μm in diam., hyaline, smooth, cylindrical, with membranous pigments.
Distribution: Southwestern China (Yunnan).
Habit and habitat: Gregarious on soil in broad-leaved forest.
Known distribution: Subtropical area in the west of Yunnan Province, China.
Additional specimens examined: China, Yunnan Province, Nujiang Lisu Autonomous Prefecture, Lushui City, Liuku Town, Qingshan Park, on soil by the roadside under evergreen broad-leaved forest, 98.853353°E, 25.859708°N, alt. 820 m, 3 August 2019, T.X. Xu 251 (KUN-HKAS 114619).
Notes: In the phylogenetic tree (), Micropsalliota nana forms a strongly supported clade of its own in Clade bifida. It is characterised by a white, silky pileus that turns brown when bruised or cut, small spores, and capitate or subcapitate cheilocystidia.
Micropsalliota nana can be confused with the following species by having a tiny and relatively white pileus: Micropsalliota alba Heinem. & Little Flower, M. bifida, Micropsalliota dentatomarginata R.L. Zhao, J.X. Li & M.Q. He, Micropsalliota minor J.Q. Yan, Micropsalliota pseudoarginea Heinem., and Micropsalliota subalba Heinem. & Little Flower. However, M. alba has slender basidiomes (pilei 4–8 mm in diam., stipe 20–25 × 0.6 mm), brown annulus, and bigger basidiospores (5.5–7 × 3.2–4 μm) (Heinemann and Flower Citation1983); M. bifida differs in forming cheilocystidia with two irregular toe-like lobes (Zhao et al. Citation2010); M. dentatomarginata is distinguished from M. nana by its pure white pileus and the cheilocystidia with a thickened base (Li et al. Citation2021); M. minor differs by smaller basidiomes (pilei 2.5–6 mm in diam., stipe 12–17 × 0.3–0.6 mm) and forked cheilocystidia (Yan et al. Citation2022); M. pseudoarginea differs in forming smaller spores (4–5 × 2.5–3.2 μm) and smaller cheilocystidia (15–24 × 6.5–12 μm) (Heinemann Citation1982); M. subalba differs in forming bigger basidiomes (pilei 12–18 mm in diam., stipe 28–30 × 1–1.5 mm), bigger spores (5.2–7 × 3–4 μm), and slightly white fibrillose pileus that does not change colour when bruised (Heinemann and Flower Citation1983).
In addition, Micropsalliota arginea is also similar to M. nana in demonstrating a similar overall appearance. However, M. arginea forms longer stipe (17–25 mm), smaller basidiospores (4–5 × 2.5–3 μm), and capitate or subcapitate caulocystidia (Pegler and Rayner Citation1969).
Micropsalliota squarrosa T. Gao & Z.W. Ge, sp. nov.
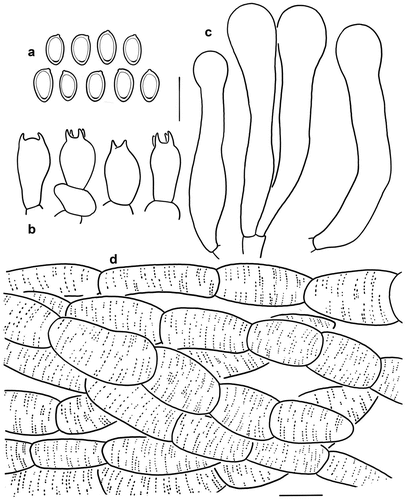
Figure 9. Microscopic features of Micropsalliota squarrosa (KUN-HKAS 128713). (a) Basidiospores. (b) Basidia. (c) Cheilocystidia. (d) Pileus squamules. Bars: a – c = 10 μm; d = 20 μm.
Fungal Names: FN571737.
Etymology: Referring to erect scales on its pileus.
Types: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Menghai County, by the side of K16 highway heading into Bulangshan Bulang Ethnic Township, on soil under evergreen broad-leaved forest, 100.356683°E, 21.561704°N, alt. 1,290 m, 23 June 2020, Y.J. Lüli 119 (KUN-HKAS 128633, holotype). GenBank: ITS = OR799915, LSU = OR799959, rpb2 = OR962250.
Diagnosis: Micropsalliota squarrosa is characterised by the brown to reddish brown pileus covered with squamules, bigger basidiospores (7–8 × 4.5–5.5 μm), clavate to clavate-capitate cheilocystidia, and tissues staining yellow or reddish brown when bruised.
Macroscopic description: Pileus 25–30 mm in diam., white (1A1), convex in early stage, plano-convex to applanate with age, covered with fibrillose brown (6E5) to reddish brown (6F4–6F6) squamules, denser at disc. Context firm, thickened at the disc, and thin near the margin. Lamellae free, crowded, with 2–4 series of lamellulae, 3–4 mm broad, white (1A1) at first, greyish white (1B1) to light brown (8E3) with age, edges paler. Stipe 55–60 × 3–4 mm, cylindrical, white (1A1) to brown (6D4), surface with white (1A1) fibrils, stipe base with white rhizomorphs. Annulus single, superior, persistent, edge entire, white (1A1). Pileus and stipe staining slightly yellow (4A4) or reddish brown (7C5) when bruised.
Microscopic description: Basidiospores [20/1/1] 7–8 (8.5) × 4.5–5.5 μm, av. = 7.75 × 4.90 μm, Q = (1.27) 1.40–1.78, Qm = 1.59 ± 0.12, ellipsoid, with apical thickening, without germ pore, light brown, inamyloid. Basidia (12) 13–15 (16) × 7–9 μm, clavate, hyaline, 4-spored. Pleurocystidia absent. Cheilocystidia (42) 45–55 × 6–9 (10) μm, clavate to clavate-capitate, some subcapitate with long narrow neck, capitulum 7–13 μm in diam., hyaline, smooth. Pileus squamules composed of hyphae 4–15 μm in diam., hyaline, smooth, cylindrical, slightly constricted at septa, with membranous pigments.
Distribution: Southwestern China (Yunnan).
Habit and habitat: Solitary or scattered on soils.
Known distribution: Tropical region of Yunnan province, China.
Additional specimens examined: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Jinghong City, Dadugang Township, on soil under evergreen broad-leaved forest, 100.91482°E, 22.28536°N, alt. 1,120 m, 27 June 2020, Y.J. Lüli 207 (KUN-HKAS 128713).
Notes: Micropsalliota squarrosa is characterised by brown to reddish brown pileus covered with squamules, bigger basidiospores (7–8 × 4.5–5.5 μm), clavate to clavate-capitate cheilocystidia, and tissues staining yellow or reddish brown when bruised or cut.
Morphologically, Micropsalliota squarrosa is similar to M. globocystis, M. digitatocystis, and M. pseudoglobocystis. However, M. globocystis differs in forming smaller basidiospores (6–7 × 3.5–4.2 μm) and staining reddish brown when bruised; M. digitatocystis differs in forming longer stipe (stipe 60–90 × 5–8 mm), smaller basidiospores (5.8–7.4 × 4–4.6 μm) and needle-like pleurocystidia (Li et al. Citation2021); M. pseudoglobocystis differs in forming larger basidiomes (pilei 25–55 mm in diam.) and forming needle-like pleurocystidia (Li et al. Citation2021).
Phylogenetically, Micropsalliota squarrosa is sister to M. purpureobrunneola and M. gigaspora. However, M. purpureobrunneola differs in forming smaller basidiomes (pilei 5–12 mm in diam.) and larger basidia (17.2–26 × 5.7–7.4 μm) (He et al. Citation2020); while M. gigaspora differs in forming clavate, rather than subcapitate cheilocystidia, and demonstrating no colour staining when bruised or cut.
Micropsalliota umbonata T. Gao & Z.W. Ge, sp. nov.
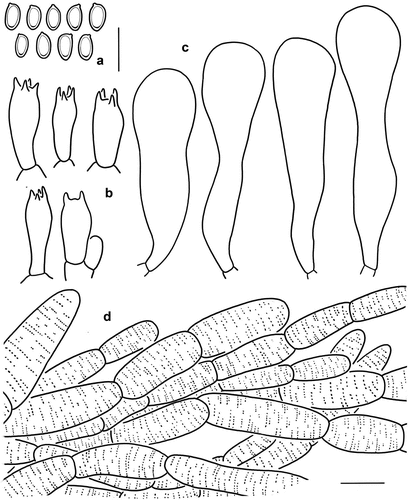
Figure 10. Microscopic features of Micropsalliota umbonata (holotype, KUN-HKAS 131131). (a) Basidiospores. (b) Basidia. (c) cheilocystidia. (d) pileus squamules. Bars: a – c = 10 μm; d = 20 μm.
Fungal Names: FN571738.
Etymology: Referring to its pileus with obtuse umbo.
Types: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Mengla County, Shangyong Township, Niupeng Village, on soil under evergreen broad-leaved forest, 101.57251°E, 21.32043°N, alt. 1,080 m, 26 June 2020, G.S. Wang 1044 (KUN-HKAS 131131, holotype). GenBank: ITS = OR799920, LSU = OR799964, rpb2 = OR962254, tef1 = OR962194.
Diagnosis: Micropsalliota umbonata is distinguished from other Micropsalliota species by yellowish brown pileus covered with fibrillose, yellowish brown to brown squamules, pileus with obtuse umbo.
Macroscopic description: Pileus with a wide range of size, 15–50 mm in diam., campanulate at first, conical or broadly conical with age, with obtuse umbo, surface dry; white (1A1) to cream (1A2) when young, with reddish brown (7D5) tone in age; covered with fibrillose, light brown (5B4), yellowish brown (5D7) or brown (7E7) squamules, dense on the disc and scattered near the margin. Context firm, up to 3 mm thick, white. Lamellae free, crowded, 2–4 mm broad, white (1A1) at first, becoming greyish white (5B2) to greyish brown (5D2), edges paler, with 4–6 series of lamellulae. Stipe 80–130 × 3–6 mm, cylindrical, hollow, smooth to tomentose, white (1A1) or with reddish brown (5F6) tone, surface heavily covered white (1A1) tomentose-flocculose fibrils. Annulus single, membranous, persistent, superior, up to 5 mm broad, white (1A1), sometimes brown (6E5) in margin. Upper part of annulus smooth or glabrous, lower part with flocculose fibrils. Pileus and stipe staining yellow (4A4) when bruised; context staining yellow (4A4) then red (7A2) or reddish brown (6E8) when cut.
Microscopic description: Basidiospores [40/2/2] (5.5) 6–7 × 3–4 μm, av. = 6.13 × 3.48 μm, Q = 1.50–2.00, Qm = 1.78 ± 0.23, ellipsoid to amygdaliform, with apical thickening, without germ pore, light brown, inamyloid. Basidia 15–19 × (5.5) 6–7 μm, clavate, hyaline, 4-spored, sometimes 2-spored. Pleurocystidia absent. Cheilocystidia 42–60 (65) × 7–10 (12) μm, apex 12–18 (22) μm in diam., broadly clavate to clavate-capitate, some subcapitate, rarely ventricose, hyaline, smooth. Pileus squamules composed of hyphae (4) 11–22 μm in diam., hyaline, smooth, cylindrical, slightly constricted at septa, with light brown membranous pigments.
Distribution: Southwestern China (Yunnan).
Habit and habitat: Cespitose, gregarious, or occasionally solitary on soil.
Known distribution: Tropical region of Yunnan Province, China.
Additional specimens examined: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Mengla County, Shangyong Township, Niupeng Village, on soil under evergreen broad-leaved forest, 101.57251°E, 21.32043°N, alt. 1,080 m, 26 June 2020, P.C. Yuan 092 (KUN-HKAS 131134); ibid., L.K. Jia 878 (KUN-HKAS 125689); ibid. G.S. Wang 1048 (KUN-HKAS 131132).
Notes: Micropsalliota umbonata is distinguished by light brown to yellowish brown pilei covered with yellowish brown to brown squamules, campanulate, conical to broadly conical pileus with obtuse umbo, and clavate to clavate-capitate cheilocystidia without a long neck.
The following species share similar morphological characters with Micropsalliota umbonata: Micropsalliota bambusicola (Heinem.) Heinem. which has shorter basidiospores (5.5–5.9 × 3.1–3.5 μm), and much narrower cheilocystidia 20–35 × 10–20 μm (Heinemann Citation1956); Micropsalliota arginophaea Heinem. have much smaller basidiomes (pilei 6–20 mm in diam.), and subcylindrical to clavate cheilocystidia with long elongated neck in most cases (Heinemann Citation1980); M. furfuracea has brown flake-like scales on the pileus (Zhao et al. Citation2010), while M. umbonata has fibrillose, yellowish brown to brown squamules.
Micropsalliota umbonata, which forms an independent lineage in Clade globocystis, is sister to M. megarubescens, M. digitatocystis, M. globocystis, M. pseudoglobocystis, and M. purpureobrunneola in the molecular analyses (). However, M. megarubescens colours white to cream and becomes light grey to greyish brown with age (Zhao et al. Citation2010); M. digitatocystis and M. pseudoglobocystis differs in having needle-like pleurocystidia (Li et al. Citation2021); M. globocystis has conical to convex or plano-convex, purple to purplish brown, greyish brown (8E3), or reddish brown (8E4) pilei (Zhao et al. Citation2010); M. purpureobrunneola differs in forming smaller basidiomes (pilei 5–12 mm in diam.) and purplish-brown pileus (He et al. Citation2020).
Key to Micropsalliota species distributed in China
1a. Pileus white to dirty white, glabrous to slightly fibrillose................................................................................2
1b. Pileus coloured, e.g. brown, red, or violet, silky to fibrillose-scaly...................................................................13
2a. Basidiomes medium-sized, pileus 20–80 mm in diam., stipe 70–120 mm long, strongly staining reddish brown when bruised or cut..........M. megarubescens
2b. Basidiomes small-sized, pileus less than 20 mm in diam., stipe less than 40 mm long.............................. 3
3a. Pileus less than 5 mm in diam..................................... 4
3b. Pileus 5–20 mm in diam................................................. 5
4a. Stipe < 15 mm long, basidiospores 4.3–5.5 × 2.7–3.3 μm.....M. pseudodelicatula
4b. Stipe > 17 mm long, basidiospores 5.5–7.5 × 3.5–4 μm.......................................M. albofelina
5a. Caulocystidia present; Stipe base with white rhizomorphs.................................................... M. longicystis
5b. Caulocystidia absent; Stipe base without white rhizomorphs.......................................................................6
6a. Cheilocystidia forked or bifid...........7
6b. Cheilocystidia simple, utriform, tibiiform, or ventricose-capitate with a long flexuous neck...............................8
7a. Basidiomes tiny, pileus 2.5–6 mm in diam.; Cheilocystidia two types, tibiiform or forked with capitate or subacute apex.................M. minor
7b. Basidiomes larger, pileus 6–21 mm in diam.; Cheilocystidia bifid with two toe-like subcapitate lobes...................................M. bifida
8a. Cheilocystidia non-capitate..........................9
8b. Cheilocystidia capitate, with a sinuous or straight neck...................................10
9a. Cheilocystidia broadly clavate or ventricose-clavate, hyaline, smooth; basidiospores 4–5 × 2.5–3.2 μmM. pseudoarginea
9b. Cheilocystidia utriform, with broadly obtuse apex, covered by hyaline deposition; basidiospores 5.3–6.2 × 3–3.5 μmM. tenuipes
10a. Cheilocystidia clavate to ventricose, capitate, without a thicked base..........11
10b. Cheilocystidia capitate, with a sinuous or straight neck and a thickened base..............................................12
11a. Pileus 5–12 mm in diam.; basidiospores 4.5–6 × 3–4 μm; flesh staining reddish brown when bruised or cut......................................................M. nana
11b. Pileus 12–18 mm in diam.; basidiospores 5.2–7.3 × 3–4 μm; flesh not staining when bruised or cut.........M. subalba
12a. Basidiospores 4–5 × 2.5–3 μm, ovoid in face view, amygdaliform in profile view..............M. ovalispora
12b. Basidiospores longer than 5 μm, wider than 3 μm, ellipsoid, sometimes amygdaliform.........13
13a. Basidiomes larger, pileus 12–15 mm in diam., stipe 25–35 mm long..........M. dentatomarginata
13b. Basidiomes smaller, pileus 3–7 mm in diam., stipe 12–19 mm long.......M. delicatula
14a. Basidiomes medium-sized, pileus 20–80 mm in diam., stipe 35–130 mm long......15
14b. Basidiomes small-sized, pileus less than 20 mm in diam., stipe less than 40 mm long.........22
15a. Pleurocystidia present, needle-like........16
15b. Pleurocystidia absent............17
16a. Basidiospores 4.5–6 × 2.5–3.2 μm; flesh staining bright yellow then reddish brown when bruised or cut........................................... M. pseudoglobocystis
16b. Basidiospores 5.8–7.4 × 4–4.6 μm; flesh staining yellowish brown when bruised or cut.......................................................... M. digitatocystis
17a. Pileus squamules flake-like, appressed, light brown to brown; flesh staining red when bruised or cut......................................... M. furfuracea
17b. Pileus squamules fibrillose-scaly, erect or recurved, reddish brown, greyish brown, purplish brown or dark brown; flesh staining yellow, reddish brown or blue.............18
18a. Pileus squamules reddish brown, purplish brown or dark brown, background grey or yellowish brown; larger squamules in margin, recurved; lamellae staining blue when bruised or cut.......M. ferruginea
18b. Pileus squamules light brown, greyish brown purplish brown or reddish brown, background white or light grey; denser at disc, scanty towards the margin; flesh staining yellow or reddish brown when bruised or cut......................................................... 19
19a. Basidiospores shorter than 7 μm, narrower than 4 μm.........................20
19b. Basidiospores longer than 7 μm, wider than 4 μm................................................................................ 21
20a. Pileus conical to broadly conical, with conspicuously obtuse umbo; squamules light brown, yellowish brown, or brown...................M. umbonata
20b. Pileus conical to broadly conical, convex or plano-convex and slightly umbonate; squamules purple to purplish brown, greyish brown or reddish-brown................................ M. globocystis
21a. Stipe 55–65 × 3–4 mm; cheilocystidia subcapitate; stipe base with white rhizomorphs, basidiospores 7–8 (8.5) × 4.5–5.5 μm, av. = 7.75 × 4.90 μm......M. squarrosa
21b. Stipe 35–50 × 2–3 mm; cheilocystidia non-capitate; stipe base without white rhizomorphs, basidiospores 7–9 × 4–5.5 μm, av. = 7.98 × 4.45 μm...................................................... M. gigaspora
22a. Partial veil cortinate, remaining as fibrillose patches along pileus margin.............. M. cortinata
22b. Partial veil forming a membranous annulus......23
23a. Pileus pink, red to violet-red......24
23b. Pileus brown to dark brown, or with reddish brown tones on the disc.......................................... 28
24a. Cheilocystidia utriform with obtuse, subcapitate, or capitate apex............................................................ 25
24b. Cheilocystidia non-utriform......26
25a. Stipe dirty white with pink tone, cheilocystidia without deposit......M. cf. roseipes
25b. Stipe white, cheilocystidia covered by light brown deposit................................ M. rufosquarrosa
26a. Cheilocystidia hyphoid, often forked, up to 60 μm long; pileipellis hyphae with membranous pigments................................................... M. wuyishanensis
26b. Cheilocystidia simple, non-hyphoid, capitate or subcapitate; pileipellis hyphae with vacuolar pigments........27
27a. Basidiomes slender (pileus 6–22 mm in diam., stipe 18–42 mm long); pileus greyish red to dull red or reddish brown; stipe white.........M. gracilis
27b. Basidiomes stout (pileus 10–18 mm in diam., stipe 15–18 mm long); pileus violet brown; stipe violet red........................M. lateritia var. vinaceipes
28a. Basidiospores 4–5.5 × 2.5–3 μm............M. arginophaea
28b. Basidiospores longer than 5.5 μm, wider than 3.5 μm..................................................................................... 29
29a. Pileus white to cream with reddish brown stains, glabrous to silky....................... M. rubrobrunnescens
29b. Pileus brown to dark brown, fibrillose to floccose or squamulose.............................................................. 30
30a. Pleurocystidia present; cheilocystidia pyriform to subglobose......................................... M. appendiculata
30b. Pleurocystidia absent; cheilocystidia capitate or subcapitate with long neck .................................... 31
31a. Pileus brown to dark brown; squamules erect or recurved; cheilocystidia ventricose with a long obtuse neck to pyriform, apex merely obtuse or seldom subcapitate............................. M. megaspora
31b. Pileus reddish brown or greyish brown; squamules appressed; cheilocystidia clavate to irregularly tibiiform, capitate or subcapitate with long narrow neck..................................... M. fimbriata
4. Discussions
The results of our phylogenetic analyses are, to some extent, consistent with the previous studies (Zhao et al. Citation2010; Wei et al. Citation2015; Li et al. Citation2021; Al-Kharousi et al. Citation2022; Patil et al. Citation2022; Yan et al. Citation2022). However, compared to the previous studies (Yan et al. Citation2022; Ivanova et al. Citation2023), the present study resolved 6 more major clades based on the ITS, LSU, rpb2, and tef1 sequence datasets, and some clades, for instance, Clade lateritia, and Clade bifida, had higher support values and were able to be combined with morphological features. Nevertheless, the backbone of the Micropsalliota phylogeny remains poorly resolved. Based on our present study, Micropsalliota consists of 11 major clades.
Species in Clade globcystis share reddish brown to greyish brown fibrillose or squamulose on the white context (except for M. megarubescens), erect fibrillose or squamulose, basidiospores generally longer than 6 μm (except for M. pseudoglobocystis), cheilocystidia clavate to capitate and almost all staining reddish brown when bruised or cut (Heinemann and Leelavathy Citation1991; Zhao et al. Citation2010; Wei et al. Citation2015; Li et al. Citation2021).
Species in Clade lateritia have small-sized to medium-sized basidiomes, that are thick-fleshy and stout, and have deep red to violet brown fibrils on the white context (except for M. rubrobrunnescens and M. rubrobrunnescens var. tibiicystis, which have a white and silky pileus with reddish-brown stains) (Zhao et al. Citation2010; Yan et al. Citation2022; Ivanova et al. Citation2023).
Clade ferruginea is currently composed of only one species, M. ferruginea. It is distinguished from all other Micropsalliota species by relatively large basidiomes with erect to recurved squamules that are brown to dark brown, and lamellae staining a slightly blue when bruised or cut.
Species in Clade furfuracea share reddish brown appressed fibrillose or squamulose on the white context, vivid red to reddish brown staining, and rather large spores with a mean of 6.9 × 3.8 μm, distinguish them from other species (Zhao et al. Citation2010).
Species in Clade pleurocystidiata are characterised by robust basidiomes, presence of large pleurocystidia and brown scales on pileus, broadly clavate, utriform to broadly utriform cheilocystidia, which is similar to pleurocystidia (Heinemann Citation1980; Heinemann and Flower Citation1983; Zhao et al. Citation2010; Patil et al. Citation2022).
Species in Clade megaspora share smalle-sized basidiomes (pilei 3–13 mm in diam., except for M. repanda), fibrillose to floccose, light yellow, yellowish brown to dark brown squamules (Heinemann Citation1980; Zhao et al. Citation2010; Ivanova et al. Citation2023).
Clade jiangxiensis is characterised by its brownish, fibrillose scales on pileus, cylindrical to subclavate cheilocystidia, ellipsoid, and sometimes amygdaliform basidiospores (Ji and He Citation2023).
Clade bifida contains 24 species with different morphological characteristics. Except for M. brunneosquamata and M. geesterani, which form robust basidiomes and thick-fleshed pileus, all species in Clade bifida are slender and very small. Most small species are white, only M. arginophaea, M. gracilis, M. roseipes, M. rufosquarrosa, and Micropsalliota suricatoides D.D. Ivanova, O.V. Morozova & T.H.G. Pham share coloured pilei (Heinemann and Flower Citation1983; Zhao et al. Citation2010; He et al. Citation2020; Li et al. Citation2021; Yan et al. Citation2022; Ivanova et al. Citation2023).
Clade albofelina is similar to Clade bifida, the only species, M. albofelina D.D. Ivanova & O.V. Morozova, is characterised by the following features: Delicate, tiny pristine white discolouring to brown basidiomes, cheilocystidia with a long neck, and well-distinguished capitulum. The most distinctive feature of this species is the presence of thin white hairs which cover the entire basidiome (Crous et al. Citation2021).
Clade cortinata contained only one species, M. cortinata, which is distinguished by cortinate partial veil that leaves remnants only on the pileus margin (Heinemann Citation1980, Citation1988; Zhao et al. Citation2010).
Clade ventricocystidiata contains only one species, M. ventricocystidiata, which is characterised by medium-sized, thick-fleshy, and stout basidiomes covered with reddish-brown squamules; amygdaliform basidiospores measuring 7.5–8.5 × 4.5–5 μm; and the cheilocystidia which are mostly ventricose, rarely more or less subcylindrical (Al-Kharousi et al. Citation2022).
In previous studies, Micropsalliota has approximately 80 species, of which 24 are known to be distributed in China (most were reported from Southwestern China). Based on multigene phylogeny and morphological studies on specimens collected from Yunnan and Hainan Provinces, seven new species and a newly recorded species of China were introduced in the present study, increasing the total number of Micropsalliota species found in China from 24 to 32. With further investigations and studies of macrofungi in China going on, more Micropsalliota species are expected to be discovered.
Acknowledgments
We are grateful to X.P. Fan, L.K. Jia, Y.J. Lüli, G.S. Wang, T.X. Xu, P.C. Yuan, and L.R. Zhou (Kunming Institute of Botany, Chinese Academy of Sciences) for providing valuable specimens and images.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Al-Kharousi M, Hussain S, Al-Muharabi MA, Al-Maqbali D, Al-Shabibi Z, Al-Balushi AH, Al-Yahya’ei MN, Al Saady N, Velazhahan R, Al-Sadi AM. 2022. Notes on the genus Micropsalliota (Agaricales, Basidiomycota) and the description of a new species from southern Oman. Phytotaxa. 543(2):113–126. doi: 10.11646/phytotaxa.543.2.2.
- Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 25(15):1972–1973. doi: 10.1093/bioinformatics/btp348.
- Chen J, Bahkali AH, Zhao RL, Hyde KD. 2016. Micropsalliota brunneosquamata, a new species from Thailand. Chiang Mai J Sci. 43(4):689–694.
- Chen YL, Pei NC, Zhang LP, Deng WQ, Wu F, Liu M. 2019. Three new records of Micropsalliota from China. Acta Agric Univ Jiangxiensis. 41(4):708–714. doi: 10.13836/j.jjau.2019082.
- Crous PW, Osieck ER, Jurjevi Ž, Boers J, Van Iperen AL, Starink-Willemse M, Dima B, Balashov S, Bulgakov TS, Johnston PR, et al. 2021. Fungal planet description sheets: 1284–1382. Persoonia. 47(1):178–374. doi: 10.3767/persoonia.2021.47.06.
- Cubeta MA, Echandi E, Abernethy T, Vilgalys R. 1991. Characterization of Anastomosis groups of binucleate Rhizoctonia species using restriction analysis of an amplified ribosomal RNA gene. Phytopathology. 81(11):1395–1400. doi: 10.1094/Phyto-81-1395.
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol. 2(2):113–118. doi: 10.1111/j.1365-294X.1993.tb00005.x.
- Ge ZW, Xu TX, Qu H, Ma YR. 2021. Three new species of Smithiomyces from tropical Asia support an amphi-Pacific disjunct distribution in the genus. Mycologia. 113(5):1009–1021. doi: 10.1080/00275514.2021.1936832.
- Ge ZW, Yang ZL, Qasim T, Nawaz R, Khalid AN, Vellinga EC. 2015. Four new species in Leucoagaricus (Agaricaceae, Basidiomycota) from Asia. Mycologia. 107(5):1033–1044. doi: 10.3852/14-351.
- Guzmán-Dávalos L. 1992. First record of the genus Micropsalliota (Basidiomycotina, Agaricaceae) in Mexico. Mycotaxon. 43:199–205.
- Guzmán-Dávalos L, Heinemann P. 1994. Micropsalliota subalpina nov. sp. (Agaricaceae) from Mexico. Bulletin du Jardin botanique national de Belgique / Bulletin van de National Plantentuin van België. 63(1/2):195–199. doi: 10.2307/3668476.
- Hall TA. 1999. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser. 41:95–98.
- He MQ, Hyde KD, Cheewangkoon R, Zhao RL. 2020. Two new species of Micropsalliota (Agaricaceae/Agaricales) from Thailand. Phytotaxa. 453(2):137–144. doi: 10.11646/phytotaxa.453.2.5.
- Heinemann P. 1956. Champignons recoltes au Congo Belge par Madame M. Goossens-Fontana II. Agaricus Fries s.s. Bull Jard Bot LÉtat Brux. 26(1):1–127. doi: 10.2307/3667096.
- Heinemann P. 1980. Les genres Agaricus et Micropsalliota en Malaisie et en Indonesie. Bulletin du Jardin botanique national de Belgique / Bulletin van de National Plantentuin van België. 50(1/2):3–68. doi: 10.2307/3667774.
- Heinemann P. 1982. Quelques Psalliotes de Nouvelle-Guinee (Papua New Guinea). Bull Jard Bot Natl Belg Bull Van Natl Plantentuin Van Belg. Bulletin du Jardin botanique national de Belgique / Bulletin van de National Plantentuin van België. 52(3/4):405–413. doi: 10.2307/3667893.
- Heinemann P. 1983. Cle de determination de Micropsalliota (Agaricaceae) et description de deux especes nouvelles. Bulletin du Jardin botanique national de Belgique / Bulletin van de National Plantentuin van België. 53(1/2):85–95. doi: 10.2307/3668030.
- Heinemann P. 1988. Novitates generis Micropsalliotae (Agaricaceae). Bulletin du Jardin botanique national de Belgique / Bulletin van de National Plantentuin van België. 58(3/4):540–543. doi: 10.2307/3668305.
- Heinemann P. 1989. Le genre Micropsalliota en Amerique tropicale et subtropicale. Bulletin du Jardin botanique national de Belgique / Bulletin van de National Plantentuin van België. 59(3/4):459–466. doi: 10.2307/3668360.
- Heinemann P, Flower S. 1983. Micropsalliota de Kerala (Inde). Bull Jard Bot Natl Belg Bull Van Natl Plantentuin Van Belg. Bulletin du Jardin botanique national de Belgique / Bulletin van de National Plantentuin van België. 53(1/2):75–84. doi: 10.2307/3668029.
- Heinemann P, Leelavathy KM. 1991. The genus Micropsalliota (Agaricaceae) in Kerala State, India. Mycol Res. 95(3):341–346. doi: 10.1016/S0953-7562(09)81245-8.
- Höhnel FXR. 1914. Fragmente zur Mykologie XVI (XVI. Mitteilung, Nr. 813 bis 875). Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften Math.-naturw. Klasse Abt I. 123:49–155.
- Ivanova D, Morozova O, Pham THG. 2023. Three new species of Micropsalliota (Agaricaceae, Basidiomycota) from central and southern Vietnam. Phytotaxa. 626(4):247–258. doi: 10.11646/phytotaxa.626.4.2.
- Ji XH, He G. 2023. A new species of Micropsalliota (Agaricales, Agaricaceae) from China. Nova Hedwig. 116(1–2):57–65. doi: 10.1127/nova_hedwigia/2023/0728.
- Kalyaanamoorthy S, Minh BQ, Wong TKF, Von Haeseler A, Jermiin LS. 2017. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat Methods. 14(6):587–589. doi: 10.1038/nmeth.4285.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 30(4):772–780. doi: 10.1093/molbev/mst010.
- Kornerup A, Wanscher JHK. 1978. The methuen handbook of colour. 3rd ed. London, UK: Eyre Methuen Ltd. Reprint.
- Largent D. 1986. How to identify mushrooms to genus I: Macroscopic features. Eureka, CA, USA: Mad River Press.
- Larsson A. 2014. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 30(22):3276–3278. doi: 10.1093/bioinformatics/btu531.
- Li JX, He MQ, Zhao RL. 2021. Three new species of Micropsalliota (Agaricaceae, Agaricales) from China. Phytotaxa. 491(2):167–176. doi: 10.11646/phytotaxa.491.2.6.
- Liu W, Chen YL, Zhang LP, Liang JF. 2022. Two new records of the genus Micropsalliota from China. Chin J Trop Crops. 43(4):703–709. doi: 10.3969/j.issn.1000-2561.2022.04.006.
- Ma YR, Liu TZ, Yu XD, Wei TZ, Ge ZW. 2022. Six new species of Leucoagaricus (Agaricaceae) from northeastern China. Diversity. 14(5):314. doi: 10.3390/d14050314.
- Matheny PB. 2005. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol Phylogenet Evol. 35(1):1–20. doi: 10.1016/j.ympev.2004.11.014.
- Parra LA, Tan Y, Xu ML, Zhou JL, Wang B, Zhao RL. 2016. A reexamination of Allopsalliota indicates synonymy with Micropsalliota (Agariceae, Agaricaceae, Agaricales, Basidiomycota). Mycoscience. 57(5):303–310. doi: 10.1016/j.myc.2016.02.005.
- Patil PB, Vaidya S, Maurya S, Patil NP. 2022. Micropsalliota pileocystidiata (Agaricaceae), a new species from Maharashtra, India. Mycoscience. 63(5):215–221. doi: 10.47371/mycosci.2022.07.001.
- Pegler DN. 1986. Agaric flora of Sri Lanka. Kew Bull. 12:1–519.
- Pegler DN, Rayner RW. 1969. A contribution to the agaric flora of Kenya. Kew Bull. 23(3):347–412. doi: 10.2307/4117177.
- Rambaut. 2018. A. FigTree Version 1.4.4. [accessed 2022 March 19]. http://tree.bio.ed.ac.uk.
- Rehner SA, Buckley E. 2005. A beauveria phylogeny inferred from nuclear ITS and EF1- sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 97(1):84–98. doi: 10.3852/mycologia.97.1.84.
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542. doi: 10.1093/sysbio/sys029.
- Silvestro D, Michalak I. 2012. raxmlGUI: A graphical front-end for RAxML. Org Divers Evol. 12(4):335–337. doi: 10.1007/s13127-011-0056-0.
- Singer R. 1975. The agaricales in modern taxonomy. Vaduz: J. Cramer.
- Smith AH. 1944. New North American Agarics. Mycologia. 36(3):242–262. doi: 10.1080/00275514.1944.12017545.
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22(21):2688–2690. doi: 10.1093/bioinformatics/btl446.
- Sun JY, Wang YS, Wang X, Wang Y, Yi ZC, Yan JQ, Huo GH, Wang LS. 2020. Two new records of Micropsalliota from Jiangxi Province. J Northeast For Univ. 48(3):120–123. doi: 10.1639/0007-2745-123.3.430.
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 172(8):4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990.
- Wang FJ, Zhou XY, Gan L. 2017. Micropsalliota lateritia, a new record of Micropsalliota from Hubei Province in China. J Central China Normal Univ. 51(5):651–654. doi: 10.19603/j.cnki.1000-1190.2017.05.016.
- Wei L, Li YH, Hyde KD, Zhao RL. 2015. Micropsalliota pseudoglobocystis, a new species from China. Mycotaxon. 130(2):555–561. doi: 10.5248/130.555.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Bruns T, Lee S Taylor J, editors. PCR - protocols and applications - a laboratory manual. Cambridge, MA, USA: Academic Press; pp. 315–322.
- Xu JC, Liu F, Li Z. 2016. Fantastic mushroom world: macrofungi of the Nabanhe National Nature Reserve. Xishuangbanna, China: Nabanhe National Nature Reserve; pp. 1–217.
- Yan JQ, Zeng ZH, Hu YP, Ke BR, Zeng H, Wang SN. 2022. Taxonomy and multi-gene phylogeny of Micropsalliota (Agaricales, Agaricaceae) with description of six new species from China. Front Microbiol. 13:1011794. doi: 10.3389/fmicb.2022.1011794.
- Zhang D, Gao FL, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT. 2020. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 20(1):348–355. doi: 10.1111/1755-0998.13096.
- Zhao RL, Desjardin DE, Soytong K, Perry BA, Hyde KD. 2010. A monograph of Micropsalliota in Northern Thailand based on morphological and molecular data. Fungal Divers. 45(1):33–79. doi: 10.1007/s13225-010-0050-4.