Abstract
A Schiff base bis(salicylidene)propylene-1,3-diamine (salpen)2 and its complex with zinc metal as bis(salicylidene)propylene-1,3-diaminatozinc(II) [Zn(salpen)2] were prepared and characterised by various spectral techniques. The zinc complex had high thermal stability (>300°C) as well as high glass transition temperature (>150°C). Optical properties of the metal complex were studied by UV–visible absorption and photoluminescence emission spectroscopy. Multilayered organic electroluminescent devices were fabricated with a structure ITO/α -NPD/Zinc(salpen)2/BCP/Alq 3/LiF/Al, using the zinc complex as an emissive material. The electroluminescence peak emission was centred at 513 nm with colour chromaticity having Commission Internationale d'Eclairage (CIE) coordinates x=0.26 and y=0.50. A bright blue-green emission with a luminance of 1990 Cd/m2 was recorded at 15 V as an optimum voltage.
1. Introduction
Luminescent metal complexes have potential applications in the development of energy-efficient, low-cost, full colour and flat panel displays. The three primary colours red, green and blue are used for full colour displays. The blue emitting materials are one of the prime colour for the development of full colour displays based on either the ‘colour changing medium’ technology or the RGB filtered white emission.[Citation1–8] The emission of blue light in terms of efficiency and durability is more problematic than other colours. This problem can be overcome by choosing selective organic compounds, dyes or polymers such as oxadiazole,[Citation9] cyclopentadiene derivatives,[Citation10] distylpyrazine,[Citation11] polyalkylfluoene,[Citation12] poly(p-phenylene),[Citation13] polypyridines, [Citation14] which emit blue light.
Metal chelate complexes of 8-hydroxyquinoline and its derivatives are widely used for their applications in organic light-emitting diodes (OLEDs).[Citation1,Citation7,Citation15] Schiff base metal complexes with transition and non-transition metals have been investigated extensively for many years because of their applications as in OLEDs, lasers, transistors and fluorescent sensors for highly specific probes.[Citation16–21] Salicylaldehyde Schiff base ligands are similar in structure with 8-hydroxyquinoline ligand in having at least one hydroxyl group, a coordination nitrogen atom and a delocalised π-conjugated system.[Citation21,Citation22] Salicylaldehyde Schiff base metal complexes exhibit good luminescent properties.[Citation16,Citation17,Citation21–23] Kim et al. [Citation17] reported the photoluminescent and electroluminescent properties of the aromatic-bridged azomethine metal complexes. However, the results indicated that the complexes were insoluble in common organic solvents and most of them were difficult to sublime so it was used less in fabrication of electroluminescent devices. Yu et al. [Citation21,Citation22] synthesised zinc complexes with 2-hydroxy,1-naphthaldehyde and flexible aromatic bridged having hetero atoms in chain and with aromatic-bridged ligand. The complexes showed blue and blue-green emissions under ultra-violet (UV) light excitation. Zinc–Schiff base complexes have shown good thermal stability and luminescence in both the solid and solution states.[Citation24] Vaschenko et al.,[Citation25] reported that zinc–Schiff base complex has a ligand with ethyl linker, they also showed that the electroluminescence (EL) of complexes was bathochromic shifted as compared with PL. In this communication, we used a higher alkyl linker for ligand formation. In order to see the effect of long alkyl chain linker, we used in this case the propyl group as a bridging group.
We report a novel bis(salicylidene)propylene-1,3-diaminatozinc complex that emits blue-green emission on UV excitation and has good thermal stability as well as high glass transition temperature suitable for OLEDs. The UV–visible absorption and fluorescence properties of the complex were investigated.
2. Experimental
2.1. Materials and instruments
Salicyldehyde and propylenediamine were purchased from Fluka. Zinc acetate dihydrate used was of analytical grade. N, N-diphenyl-N, N-bis(1-naphthyl)-1,1-biphenyl-4,4-diamine(α-NPD) and tris(8-hydroxyquinolinato) aluminium(Alq 3) were obtained from Aldrich and the solvents used during the synthesis were of high purity.
An Fourier transform infrared spectroscopy (FTIR) model, Alpha Bruker, Germany, was used to run the infrared spectra. C, H, N analysis was performed using an Elemental Analyzer Perkin-Elmer 2400 CHN. Thermal gravimetric analysis (TGA) and differential thermal analysis (DTA) were done by a Mettler Toledo TGA/SDTA851e instrument. The excitation and emission spectra of the complex were recorded in methanol using the spectrophotometer Horiba Jobin YVON Fluolog Model FL 3-11. The EL and PL spectra were recorded on a high-resolution USB fibre optic spectrometer Model HR2000. For V−I, a Keithley 2400 electrometer was used.
2.2. Synthesis and characterisation of the ligand and zinc complex (ZnL)
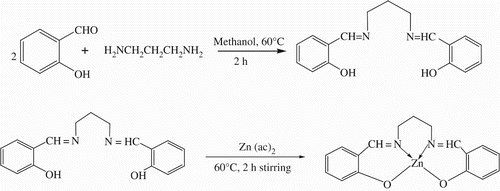
The ligand (H 2L) was prepared by adding drop wise solution of propylene-1,3-diamine (0.3 g, 2 mM) in 20 ml of absolute methanol to 50 ml methanolic solution of salicylaldehyde (1 g, 4 mM) in a conical flask with magnetic stirring at 60 °C for 2 h. The off-white precipitates formed were filtered and washed with deionised water. The compound was recrystallised with methanol and dried in an oven.
(salpen)2: yield 87%; melting point 54–56 °C; IR(KBr): cm −1 3051, 2944, 1679, 1602, 1546, 1209, 600 to 800 cm−1; 1HNMR: (400 MHz, CHCl 3) δ 13.15 (s 2H), 8.34 (s 2H), 7.31–6.80 (m 8H), 3.63 (m 4H), 1.81 (m 2H) ppm; Anal. Calc. C, 72.34; H, 6.38; N, 9.93; Found C, 72.28; H, 6.40; N, 9.96.
The ligand bissalicylidenepropylene-1,3-diammine (0.564 g, 2 mM) was taken in a conical flask in 50 ml methanol and warmed at 60 °C, then an aqueous solution of zinc acetate (0.438 g, 2 mM) in 5 ml of distilled water was added drop wise with constant stirring and the mixture was refluxed for 2 h. The metal complex was precipitated out from the solution. The precipitate was filtered and washed with deionised water and then recrystallised with methanol and then dried in an oven to give a pure off-white complex. The metal complex was then purified by the vacuum sublimation technique, maintaining a pressure of 10−6 torr by providing a primary current of 0.60 A having a temperature in the range of 80–105 °C. The yield of the ligand was ∼ (87%) and the yield of the complex was ∼ (85–90%). The complete synthetic route of the metal complex is shown in .
[Zn(salpen)2]: yield ∼ 85–90%; melting point 295–297 °C; IR(KBr): cm −1 2904, 1622, 1545, 1190, 600 to 800 cm−1; 1HNMR: (400 MHz, DMSO) δ 1.87 (m 2H), 3.68 (m 2H), 3.83 (m 2H), 6.80–7.25 (m 8H), 8.34 (s 2H) ppm; Anal. Calc. C, 59.06; H, 4.63; N, 8.106; Found C, 59.01; H, 4.65; N, 8.12.
2.3. Characteristics of the ligand and complex
The chemical structures were confirmed by a combination of elemental analysis, FTIR and 1HNMR.
The elemental analysis data indicated the formula of the compounds to be (salpen)2 and [Zn(salpen)2].
Schiff base ligand showed absorption bands due to stretching vibrations at 3051 cm−1, which we believe is related to the hydrogen-bonded
stretch. This stretch was absent in the complex, in agreement with the proposed structure. The absence of these bands confirmed the formation of the complex by deprotonation of the ligand.
stretching vibrations shifted from 1679 to 1622 cm−1 and
stretching vibration shifted from 1209 to 1190 cm−1 in the zinc complex compared with the ligand after complexation. This shift was due to donation of electron density from the ligand to the metal. The characteristic peaks from 600 to 800 cm−1 showed the presence of benzene rings.
The 1HNMR spectra of Schiff base showed signal due to hydroxyl protons at 13.15 ppm. The signal due to protons disappeared in the zinc complex, which indicated that there was binding through the oxygen atom of the ligand with deprotonation. There was downfield shift in proton peaks due to donation of electron density from the ligand to metal centre, indicating the formation of the reported complex.
2.4. Fabrication of electroluminescent devices
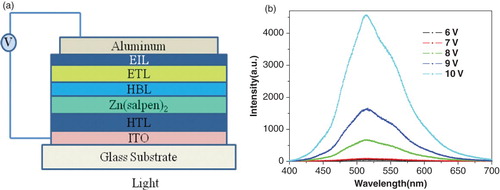
Thin-film electroluminescent devices containing the Zn complex were prepared using Zn(salpen)2 as an EL material, as shown in of the device. Indium–tin oxide- (ITO) coated glass substrate with a sheet resistance of 20 Ω was patterned and cleaned using deionised water, acetone, trichloroethylene and isopropyl alcohol sequentially for 20 min each using an ultrasonic bath and dried in a vacuum oven. The following layers were deposited on this substrate sequentially under high vacuum ( torr) at a deposition rate of 0.1 A°/s, where the thickness of the deposited films was monitored in situ using a quartz crystal monitor. Thirty nanometre of N, N-diphenyl-N, N-bis(1-naphthyl)-1,1-biphenyl-4,4-diamine (α-NPD) as the hole transporting layer; 35 nm of Zn(salpen)2 as the emitting layer; 6 nm of 2,9-dimethyl,4,7-diphenyl-1,10-phenanthroline (BCP) as the hole and exciton blocking layer, 28 nm of tris(8-hydroxyquinolinato)aluminium (Alq 3) as the electron transport layer and a cathode composed of 1 nm lithium fluoride and 100 nm aluminium were used to complete the device structure.
The device fabricated thus had the following configurations:
ITO/α-NPD(30 nm)/Zinc(salpen)2(35 nm)/BCP (6 nm)/Alq 3(28 nm)/LiF(1 nm)/Al (100 nm).
3. Results and discussion
3.1. Thermal characteristics (TGA & DTA)
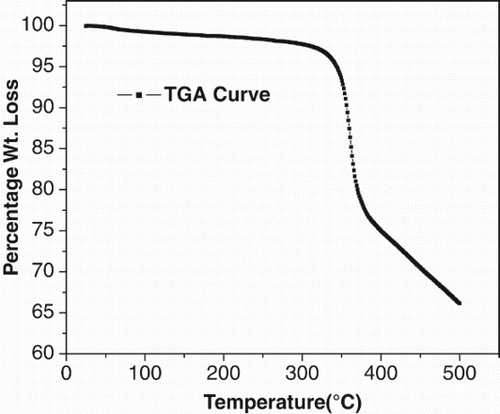
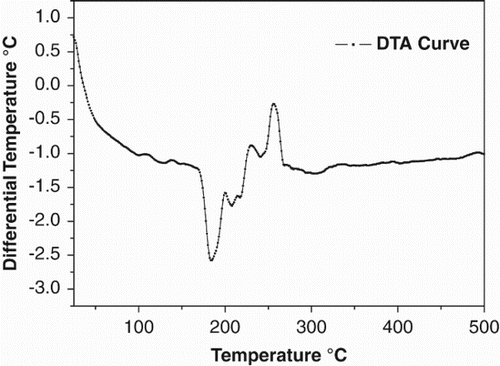
The TGA and the DTA of the zinc complex were carried out in nitrogen atmosphere at a temperature range of 0–500 °C as shown in . The onset temperature of weight loss was 315 °C, and temperature for nearly 20% weight loss was at 360 °C. Above 380 °C the complex lost all of its weight continuously and decomposed completely. Hence, the material exhibited excellent thermal stability, showing thereby that the complex is stable up to 315 °C. In the DTA curve shown in , between 150 °C and 200 °C temperature one endothermic peak was observed with no corresponding mass loss observed in the TGA. We therefore believe that this transition is due to a phase transition and represents a glass transition temperature in the compound.
3.2. Optical characterisation
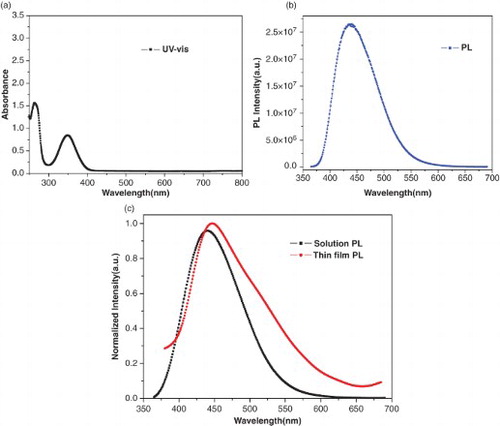
The zinc complex was chemically stable, thermally stable and also optically stable as shown by the photoluminescent spectra taken after a week. The complex showed good PL upon excitation with UV–visible light. The UV–visible absorption and PL emission spectra obtained in ethanol and PL in thin film are shown in (a)–(c). The UV–visible absorption peaks of the complex were observed at 265 and 349 nm. These absorption bands are due to ligand centred and n−π* electronic transitions. Upon excitation at these wavelengths, these materials fluoresced at 440 nm in the visible spectra. The complex showed good luminescence in both the solid and solution states. The complex showed a better PL quantum yield as compared with Alq 3, the relative PL quantum yield in ethanol was 1:1.7, using Alq 3 as the standard. There are no
transitions in zinc complexes and the emission of light is assigned as relaxation from a higher energy level to the lower energy level due to intra-ligand transitions. The molar absorptivity of the complex at absorption wavelength was ∼ 4940 M−1 cm−1 at 349 nm. The thin-film PL of the zinc complex was recorded by deposition of a thin film of thickness 200 °A on the glass substrate by the thermal deposition technique. The peak position was found at 445 nm on excitation at 350 nm. The Commission Internationale d'Eclairage (CIE) coordinates for the emitted colour was x=0.20 and y=0.23.
3.3. Electroluminescence characteristics
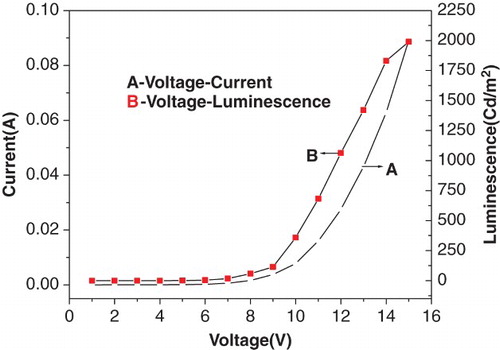
The EL characteristics were recorded using a regulated voltage supply with current limitation connected to the ITO (positive) and aluminium (negative) electrodes and the voltage was raised until electroluminescence appeared as shown in . The colour emitted by the fabricated devices was bluish green with an EL peak emission at 513 nm. The EL intensity of the device increased with an increase in voltage from 6 to 10 V. The turn-on voltage of the device was 7 V, where the voltage was taken as the voltage required in achieving the luminance of 10 Cd/m2.
Here, the variability in PL and EL peaks may originate from molecular conformational change.[Citation26] This shift can be due to interaction with hole transport materials. Therefore, the materials can be a good emission source for colour tunable applications in full colour displays.
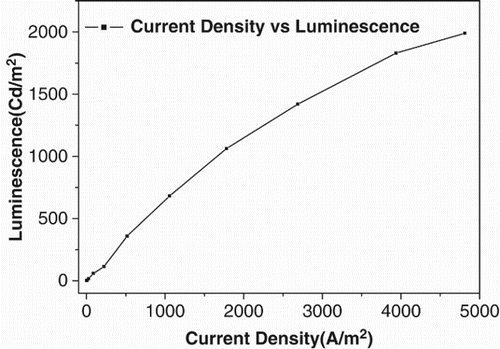
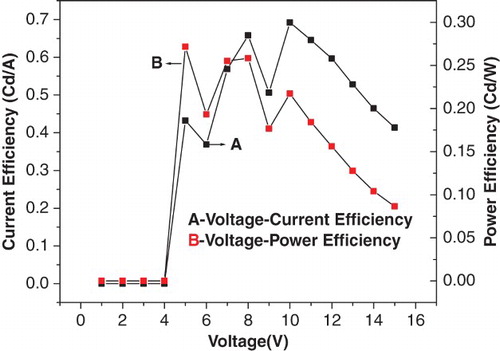
The current–voltage characteristics of the device containing the synthesised complex as emitting material are shown in . The current–voltage characteristic showed that the current started rising above 7 V indicating the onset of light emission (threshold voltage). Current intensity increased with the increase in voltage. The luminescence–voltage characteristics of the device containing synthesised zinc complex are shown in and . The bright bluish green light emitted from this EL device was above the threshold voltage. The current density–voltage curve is shown in . The EL device had a maximum brightness of 1990 Cd/m2 at 15 V with a current density of 4812.5 A/m2. The luminescence increased with an increase in the injection current as well as bias voltage. This showed that the device had very good brightness. The device showed a maximum current efficiency of 0.69 Cd/A and a maximum power efficiency of 0.26 lm/W at 10 V. It was also inferred from the figure that the chromaticities of the emitted colour were independent of the operating voltage: x=0.26, y=0.50 ().
4. Conclusion
A zinc–Schiff base complex was synthesised and characterised. Based on this material, organic light-emitting device was fabricated as an emissive layer. The excitons formed in the emissive layer showed EL centred at 513 nm, during the operation of the EL device. The chromaticities of the device were found to be independent of the operating voltage. In the present case, the maximum brightness obtained was 1990 Cd/m2 at 15 V with a bright bluish green emission. The results show that synthesised zinc complexes can be used as a promising emission source for organic light-emitting devices application.
Acknowledgements
The authors gratefully recognise the financial support from the University Grant Commission (UGC) MRP-40-51/2011(SR) and JRF to VN by Council of Scientific and Industrial Research (CSIR), New Delhi, India.
References
- Tang CW, Van Slyke SA. Organic electroluminescent diodes. Appl Phys Lett. 1987;51:913–915. doi: 10.1063/1.98799
- Service R. Organic LEDs look forward to a bright, white future. Science. 2005;310:1762–1763. doi: 10.1126/science.310.5755.1762
- Adachi C, Tokito S, Tsutsui T. Organic electroluminescent device with a three-layer structure. Jpn J Appl Phys. 1988;27:L713–L715.
- Sheets JR, Antoniadis H, Hueschen M, Leonard W, Miller J, Moon R, Roitman D, Stocking A. Organic electroluminescent devices. Science. 1996;273:884–888. doi: 10.1126/science.273.5277.884
- Wu CC, Sturm JC, Register RA, Tian J, Dana EP, Thompson ME. Efficient organic electroluminescent devices using single-layer doped polymer thin films with bipolar carrier transport abilities. IEEE Trans Electron Devic. 1997;44:1269–1281. doi: 10.1109/16.605468
- Shih HT, Lin CH, Shih HH, Cheng CH. High-performance blue electroluminescent devices based on a biaryl. Adv Mater. 2002;14:1409–1412. doi: 10.1002/1521-4095(20021002)14:19<1409::AID-ADMA1409>3.0.CO;2-O
- Yeh SJ, Wu MF, Chen CT, Song YH, Chi Y, Ho MH, Hiu SF, Chen CH. New dopant and host materials for blue-light-emitting phosphorescent organic electroluminescent devices. Adv Mater. 2005;17:285–289. doi: 10.1002/adma.200401373
- Tao XT, Suzuki H, Wada T, Sasabe H, Miyata S. Lithium tetra-(8-hydroxy-quinolinato) boron for blue electroluminescent applications. Appl Phys Lett. 1999;75:1655–1657. doi: 10.1063/1.124832
- Nohara M, Hasegawa M, Hosakawa C, Tokailin H, Kusumoto T. A new series of electroluminescent organic compounds. Chem Lett. 1990;19:189–190. doi: 10.1246/cl.1990.189
- Kido J, Hongawa K, Okuyama K, Nagai K. Bright blue electroluminescence from poly(N-vinylcarbazole). Appl Phys Lett. 1993;63:2627–2629. doi: 10.1063/1.110402
- Hosokawa C, Tokalin H, Higashi H, Kusumoto T. Efficient electroluminescence of distyrylarylene with hole transporting ability. J Appl Phys. 1995;78:5831–5833. doi: 10.1063/1.359650
- Uchida M, Ohmori Y, Morishima C, Yoshino K. Visible and blue electroluminescent diodes utilizing poly(3-alkylthiophene)s and poly(alkylfluorenes). Synth Met. 1993;55:4168–4173. doi: 10.1016/0379-6779(93)90576-I
- Grem G, Leditzky G, Ullrich B, Leising G. Realization of a blue-light-emitting device using poly(p-phenylene). Adv Mater. 1992;4:36–37. doi: 10.1002/adma.19920040107
- Gebler DD, Wang YZ, Blatchford JW, Jessen SW, Lin LB, Gustafson TL, Wang HL, Swager TM, MacDiarmid AG, Epstein AJ. Blue electroluminescent devices based on soluble poly(pyridine). J Appl Phys. 1995;78:4264–4266. doi: 10.1063/1.359890
- Qiu Y, Shao Y, Zhang DQ, Hong XY. Preparation and characterization of high efficient blue-light emitting materials with a secondary ligand for organic electroluminescence. Jpn J Appl Phys. 2000;39:1151–1153. doi: 10.1143/JJAP.39.1151
- Hamada Y, Sano T, Fujita M, Fujii T, Nishio Y, Shibata K. Blue electroluminescence in thin films of azomethin-zinc complexes. Jpn J Appl Phys. 1993;32: L511–L513.
- Kim SM, Kim JS, Shin DM, Kim YK, Ha Y. Synthesis and application of the novel azomethine metal complexes for the organic electroluminescent devices. Bull Korean Chem Soc. 2001;22:743–747.
- Costamagna J, Vargas J, Lattorre R, Alvarado A, Mena G. Coordination compounds of copper, nickel and iron with Schiff bases derived from hydroxynaphthaldehydes and salicylaldehydes. Coordin Chem Rev. 1992;119:67–88. doi: 10.1016/0010-8545(92)80030-U
- Garnovskii AD, Nivorozhkin AL, Minkin VI. Ligand environment and the structure of Schiff base adducts and tetracoordinated metal-chelates. Coordin Chem Rev. 1993;126: 1–69. doi: 10.1016/0010-8545(93)85032-Y
- Luo H, Franwick PE, Green MA. Synthesis and structure of a novel Cu(II) complex with a monoprotic tetradentate Schiff base ligand. Inorg Chem. 1998;37:1127–1130. doi: 10.1021/ic9708632
- Yu T, Su W, Li W, Hong Z, Hua R, Li M, Chu B, Li B, Zhang Z, Hu ZZ. Synthesis, crystal structure and electroluminescent properties of a Schiff base zinc complex. Inorg Chim Acta. 2006;359:2246–2251. doi: 10.1016/j.ica.2006.01.019
- Yu T, Zhang K, Zhao Y, Yang C, Zhang H, Qian L, Fan D, Dong W, Chen L, Qiu Y. Synthesis, crystal structure and photoluminescent properties of an aromatic bridged Schiff base ligand and its zinc complex. Inorganica Chimica Acta. 2008;361:233–240. doi: 10.1016/j.ica.2007.07.012
- Kotova OV, Eliseeva SV, Averjushkin AS, Lepnev LS, Vaschenko AA, Rogachev YA, Vitukhnovskii AG, Kuzmina NP. Zinc(II) complexes with Schiff bases derived from ethylenediamine and salicylaldehyde: the synthesis and photoluminescent properties. Russian Chem Bull. 2008;57:1880–1889. doi: 10.1007/s11172-008-0254-x
- Eltaye NE, Teoh SG, Adnan R, Teh JB, Fun H-F. Synthesis, crystal structure and luminescent properties of some Zn(II) Schiff base metal complexes: experimental and computational study. J Fluoresc. 2011;21:1393–1400. doi: 10.1007/s10895-010-0822-y
- Vashchenko AA, Lepnev LS, Vitukhnovskii AG, Kotova OV, Eliseeva SV, Kuz'mina NP. Photo- and electroluminescent properties of zinc(II) complexes with tetradentate Schiff bases, derivatives of salicylic aldehyde. Opt Spectrosc. 2010;108:463–465. doi: 10.1134/S0030400X10030227
- Xua B, Hao Y, Fang X, Wang H, Liu X. Optical and electrical properties of [N,N-bis(salicylidene)ethylenediamine]zinc as an electroluminescent material. Appl Phys Lett. 2007; 90:1–3.