Abstract
A series of polyfluorene-based polymers with a range of weight percentages (w/w) of a platinum(II)-containing porphyrin, 5,15-dimesityl-10,20-diphenylporphyrinato platinum(II) (MPP(Pt)), were synthesised and incorporated into organic light-emitting diodes. All polymers showed emission predominantly in the red/NIR region with only those polymers with porphyrin w/w of less than 2% showing residual tails at wavelengths lower than 600 nm, indicating increased emission from the porphyrin as w/w increases. The 2% loading of MPP(Pt) gave the highest efficiency LED (0.48%) and light output (2630 mW/m2).
1. Introduction
Phosphorescent emitters are of great interest in organic light-emitting diodes (OLEDs) due to their potential to achieve near to 100% internal quantum efficiency.[Citation1] Upon incorporation of heavy metal atoms with strong spin–orbit coupling such as platinum(II) or iridium(III), the system is distorted such that radiative transitions from triplet states, that is, where ΔS ≠ 0, become feasible. Intersystem crossing from any excited singlet state is also more favourable, resulting in radiative decay from T1 regardless of the original spin of the exciton. Since the first investigation of the electroluminescence (EL) from Pt(II)–porphyrin complexes [Citation2] and Ir(ppy)3,[Citation3] many groups have reported the use of such complexes in blend with a polymer host. However, phosphorescent dopant aggregation and phase separation are disadvantages of blend architecture, due to the increased phosphorescent quenching rate.[Citation4,Citation5] While synthetically more challenging, copolymerisation overcomes these disadvantages.[Citation6,Citation7] Having the dopant covalently bonded to the polymer will both increase the rate of energy transfer and reduce loss processes by maintaining the absorbing and emitting moieties at fixed distance, thus preventing intermolecular interaction between dopant molecules. Solubility of the whole system is also increased facilitating low-cost solution-processed device fabrication. In particular, the incorporation of Pt(II)-complexed porphyrins into host polymers has been demonstrated to be a viable route, that is, as a side chain in 2-methoxy-5-(20-ethylhexyloxy)-1,4-phenylene vinylene (MEH-PPV).[Citation8–10] These materials are promising candidates for deep-red [Citation11] OLEDs with spectral components extending even into the near-infrared (NIR),[Citation12–15] the focus of increasing attention for application in the biomedical, security and communication sectors. Still few investigations have been reported for the incorporation of Pt(II)–porphyrin directly in the polymer backbone.[Citation16–18] Of the few previously reported polymers, porphyrins are generally incorporated by the β-pyyrollic position.[Citation16,Citation18] Meso incorporation into a polyfluorene polymer has previously been achieved by Xiang but was studied only in the context of oxygen sensing and was synthesised via a less controlled (compared to Suzuki) Yamamoto reaction.[Citation17]
Here, we investigate the inclusion of Pt(II)–porphyrin, a dimesityl diphenyl porphyrin platinum (MPP-Pt) in a polyfluorene host polymer, poly(9,9-di-n-octylfluorenyl-2,7-diyl) (PFO) where the phosphorescent dopant is covalently linked to the fluorescent host via phenyl groups (PF-MPP(Pt)). We also characterised the steady-state and time-resolved photoluminescence (PL) of the materials, which were then incorporated in light-emitting diodes.
2. Synthesis
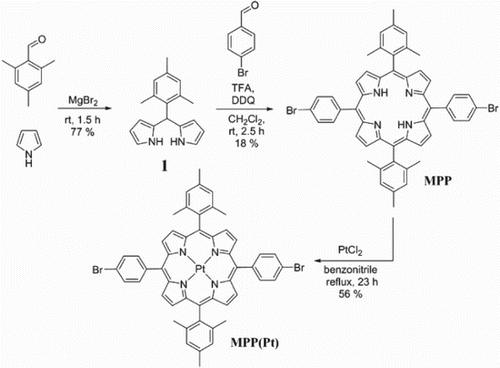
The polymer that forms the basis of this work, PF-MPP(Pt), was prepared via Suzuki–Miyaura cross-coupling reaction of monomers 2, 3 and MPP(Pt) (Scheme 2). Flourene monomers 2 and 3 were synthesised from a literature procedure from fluorene.[Citation19] MPP(Pt) was synthesised as in Scheme 1 in three steps from pyrrole and mesitaldehyde using a modified procedure of Lindsey that allows the synthesis of trans-A2B2 porphyrins from a relevant dipyrromethane and aldehyde.[Citation20] Mesityldipyrromethane 1 was formed by stirring mesitaldehyde with MgBr2 using pyrrole as solvent. Dipyrromethane 1 was subsequently reacted with bromobenzaldehyde in the presence of TFA and oxidised with DDQ to give MPP as purple crystals. Complexation of the free base porphyrin with PtCl2 in refluxing benzonitrile resulted in MPP(Pt) as a red powder.
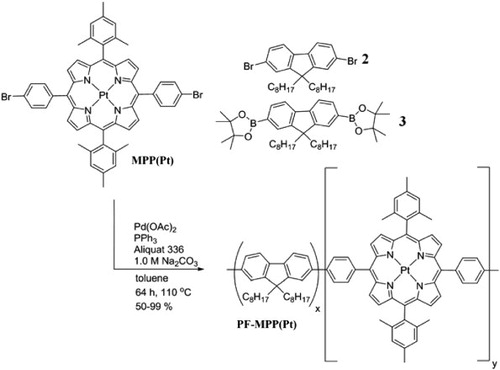
Suzuki polymerisation of the monomers was realised using Pd(OAc)2 with PPh3 as catalyst in the presence of Aliquat 336 and base (Scheme 2). PF-MPP(Pt) was prepared with MPP(Pt) in several weight percentages (w/w), controlled by varying the ratio of the monomers. The amount of diboronic ester fluorene 3 was always constant while the ratio of fluorene dibromide 2 and MPP(Pt) was varied to control the w/w of porphyrin in the final polymer. MPP(Pt) was incorporated with w/w of 0.5%, 1.0%, 2.0% and 5.0% into PF MPP(Pt), giving a range of products that were analysed together (P1, P2, P3 and P4, respectively). The novel polymers were isolated as dark red solids and were purified by Soxhlet extraction using acetone, hexane and finally chloroform. Gel permeation chromatography of the polymers showed a molecular weight (Mw) of 15.0–23.0 kDa with PDIs of 1.6–1.9.
Attempts to determine the percentage incorporation of MPP(Pt) into each of the polymers were made by 1H NMR spectroscopy. shows how the ratio of porphyrin to PFO signals changes with increasing porphyrin incorporation and it can clearly be seen that the percentage incorporation increases with increased porphyrin feed ratio, and does so in a manner concurrent with the feed ratios. The estimated incorporation values are shown in and are based on the ratio between the alkyl protons closest to fluorene and the most downfield pyrrolic proton peaks, these being the most isolated. As the feed ratio is increased, so does the incorporation ratio by an equivalent amount. This shows good evidence that the polymers are as expected relative to each other and represent a good range of porphyrin incorporation. The values are lower than the corresponding feed ratio, however, low incorporation to feed ratios have been shown before on similar systems.[Citation21] It is thought that this discrepancy is primarily a result of errors associated with the NMR spectra integration, rather than lower than the expected porphyrin incorporation, due to the good solubility of MPP(Pt) in toluene. By comparing the MPP(Pt) peaks in the polymers to monomeric MPP(Pt) and MPP, the peaks may be tentatively identified. The four protons a–d come at 8.1, 8.4, 8.9 and 9.1 ppm and can be seen as the intensity of the aromatic region is increased. All four shifts are further downfield than MPP(Pt) as a monomer. Proton e is expected to be under the much larger PFO peaks. The furthest downfield peaks (8.9 and 9.1 ppm) have been assigned as c and d and the remaining peaks at 8.1 and 8.4 ppm have been assigned as a and b. These assignments are based on the ordering of peaks of previous compounds (heteroaromatic protons were further downfield than aromatic). As the level of porphyrin incorporation into the polymer is increased from 0.5% to 5.0%, the relative intensities of the proton peaks increase, qualitatively showing that the four porphyrin-containing polymers have a good range of w/w.
Figure 1. H1 NMR spectra in CDCl3 of PF-MPP(Pt) containing 0.5% (P1, purple, top), 1.0% (P2, cyan, second from top), 2.0% (P3, green, second from bottom) and 5.0% (P4, maroon, bottom) w/w of MPP(Pt). PF observed peaks (top right) were set to the same level. Peaks corresponding to porpyhin protons are shown in dotted boxes.
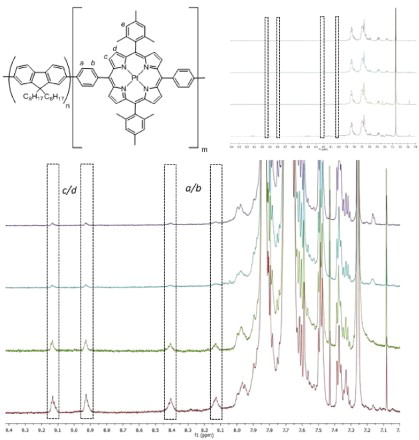
Table 1. Synthetic results of polymerisation.
Methods for spectroscopy and LEDs are reported in the Electronic Supplementary Information (ESI).
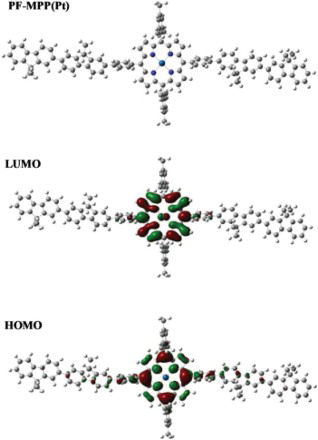
The energy levels and molecular orbitals of PF-MPP(Pt) were calculated by DFT B3LYP in silico using basis set 6–31G* for C, H and N and LanL2DZ for Pt. shows the HOMO and LUMO levels of an approximation of a porphyrin-containing portion of the polymer. To reduce calculation times, the octyl chains of PF have been replaced with methyl groups.
What is most apparent from the MO calculations is the degree of localisation of the molecular orbitals to either the porphyrin or fluorene portion of the molecule. The twist from porphyrin to phenyl to fluorene is such that conjugation across the entire molecule is broken. While this will have an overall negative impact on mobilities of the system, it does mean that the basic electronic characteristics of both moieties are likely to be only mildly affected. However, the HOMO, and to a smaller degree the LUMO, do both show a mild degree of delocalisation from the porphyrin to the main polymer chain, despite the large twist. It is expected therefore that energy transfer will be more facilitated than the analogous blend of porphyrin and PFO. Significant LUMO contribution from the Pt d-orbitals can be observed indicating that its role not only facilitates inter-system crossing but that the electronic transitions have metal-to-ligand charge transfer characteristic.
3. Results and discussion
We report the absorption and emission spectra for the copolymers P1–P4 thin films in (b) and 3(c). The copolymers show an intense featureless band at 380 nm due to the absorption of the PFO host polymer. In addition, the samples show two features at about 512 and 540 nm that we attribute to the Q-bands of the metalloporphyrin.[Citation22] These bands increase in intensity as the concentration of MPP-Pt increases and are clearly distinguishable for loadings as low as 1%.
Figure 3. (a) Chemical structure of the PFO-MPP(Pt) copolymers with different MPP(Pt) loading: 0.5% for P1, 1% for P2, 2% for P3 and 5% for P4. (b) Normalised absorption spectra of the copolymers film on fused silica glass. The inset shows MPP(Pt) absorption features, the curves are stacked with Y-offset following the increase in the MPP(Pt) content. (c) Normalised PL spectra of the copolymer films. The ‘*’ indicates the monochromator second-order transmission of the excitation wavelength. (d) PL time decay taken at the most intense peak emission wavelength of the MPP(Pt) (665 nm) following excitation at 371 nm.
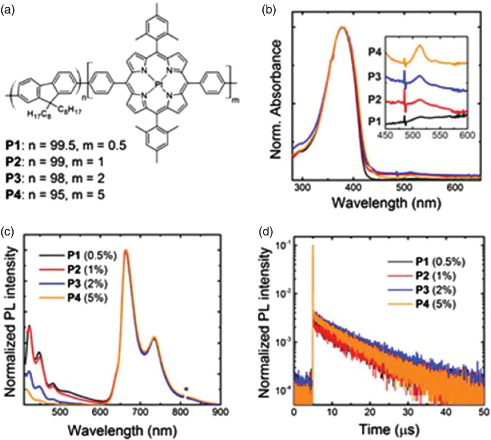
Interestingly, given the small dopant loading, the PL spectra show a dominant emission in the red and NIR region with three components at about 665, 735 and 805 nm and an emission shoulder at 645 nm that we assign to the MPP(Pt) segment. The copolymers also present a lower intensity emission at shorter wavelengths with vibronic peaks at ∼442, 467 and 497 nm that we assign to PFO. Interestingly, when increasing the porphyrin content, the intensity of these peaks reduces relative to the porphyrin emission, therefore suggesting an efficient energy transfer from PFO to the porphyrin. Indeed, the good spectral overlap between the MPP(Pt) absorption and the PFO emission (Fig. S1) corroborates this hypothesis: both the Soret band (peaking at 402 nm) and the Q-band of the Pt(II)–porphyrin significantly overlap with the PFO emission. We also note a weak fluorescence emission band between 500 and 600 nm that can be related to the presence of fluorenone defects or aggregates/excimers whose presence is further validated by the TCSPC data.[Citation23,Citation24] In fact, the radiative lifetime of PFO taken at 550 nm (Fig. S2) can be fit with a bi-exponential function yielding time constants of ∼400 ps and 6.6 ns that are consistent with PFO exciton and fluorenone excimer respectively.[Citation25] The radiative lifetime taken near the peak emission wavelength of the PFO (440 nm) also confirms the presence of efficient energy transfer from the PFO to the porphyrins. In fact, the lifetime of the pure PFO of about ∼400 ps is reduced upon loading with the MPP(Pt) and drops below the instrument detection limit (∼150 ps). The PL lifetime taken at the emission wavelength of the MPP(Pt) emission (665 nm) are reported in (d). The copolymers show lifetime decays in the microsecond scale indicating the triplet nature of the emissive state. Specifically, we found time constants of 9.3 µs (P1), 9.2 µs (P2), 9.2 µs (P3) and 8.6 µs (P4).
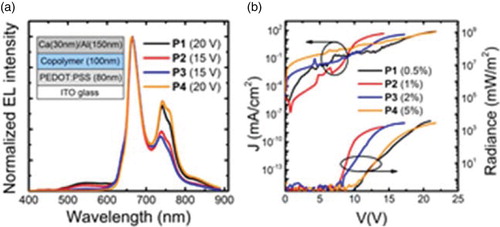
Figure 4. (a) Normalised EL spectra for the copolymers. The LED structure is reported. (b) LED characteristics: current density and radiance versus voltage. The active layer thickness is ∼100 nm and the device area is 3.5 mm2.
The PL efficiency is around 4.9% for the MPP(Pt) loading of 0.5% (P1) and slightly decreases (4.5%) upon increasing the amount of porphyrin up to 2% (P2-P3). A drop to a PL efficiency of 2.5% is seen for higher porphyrin content (P4). The trend is consistent with the lifetime values suggesting an increase in the non-radiative deactivation of the exciton for porphyrin concentrations higher than 2%. Note that although these values may appear relatively low in absolute terms, they are comparable to values reported in the literature for similar polymers.[Citation18]
We also incorporated the copolymers in LEDs with ITO/PEDOT:PSS as anode and Ca/Al as cathode. We report the EL spectra in (a) in which we note that the copolymers display an emission predominantly in the red and NIR region. In particular, the EL of P1 peaks at 665, 736 and 818 nm with a shoulder at 642 nm. We do not observe any shifts of the EL for higher MPP(Pt) loadings nor compared to the PL spectra. For copolymer P1 and P2, however, we note a weak EL emission at wavelength below 600 nm. When increasing the content of porphyrin, the intensity of the emission tail decreases with little or no intensity left for loading above 1%. Such emission may be related to oxidative defects on the PFO [Citation23] and to the emission from MPP(Pt) singlet state, as reported for similar Pt(II)–porphyrin complexes at 580 nm.[Citation26,Citation27] For MPP(Pt) loadings above 1%, copolymers show a pure electrophosphorescence (P3 and P4).
The current–voltage and radiance–voltage characteristics are shown in (b), and a summary of the LEDs performance is reported in . The 2% loading of MPP(Pt) (P3) gives the highest efficiency LED (0.48%) and light output (2630 mW/m2). The result is particularly encouraging compared to what has been reported in the literature up to now, especially when taking into account that the LEDs are not optimised for charge collection and extraction.[Citation16,Citation18] We note an overall improvement of the performance (i.e. lower turn-on voltage, higher efficiency and light output) when increasing the porphyrin loading up to 2%. However, the LEDs performance lowers when the loading exceeds 5%. Given the partial overlap of the MPP(Pt) absorption with the PFO emission, we speculate that the EL emission at low voltages is mainly due to charge trapping in the MPP(Pt) segment rather than energy transfer. However, upon a further increase in the applied voltage, charges can be injected directly into the PFO levels and can subsequently migrate to the MPP(Pt) moiety and contribute to the phosphorescence emission.
Table 2. Summary of LEDs performances.
4. Conclusions
We have prepared a series of conjugated copolymers containing MPP(Pt), a platinum(II)-complexed porphyrin, directly into the PFO backbone via the meso position. Emission of the polymers at 665 nm was shown to have a lifetime decay on the microsecond scale, indicating phosphorescence for all porphyrin-containing polymers, with decreased fluorescence from the PFO backbone as the w/w of MPP(Pt) was increased. The polymers were incorporated into OLEDs, and 2% w/w of MPP(Pt) was shown to have an efficiency of 0.48% and a light output of 2630 mW/m2. Pure MPP(Pt) electrophosphorescence is achieved for loading as low as 2%.
Supplemental data
Supplemental data for this article can be accessed 10.1080/21606099.2015.1047473.
SI_-_Freeman_et_al._Deep-Red_Manuscript-corrected_HB1__2_.docx
Download MS Word (337.5 KB)Acknowledgements
We thank the EC Seventh Framework Programmes (No. 264694 (GENIUS), N. 607585 (OSNIRO), N. 643238 (SYNCHRONICS)), the Royal Society and the EPSRC. F.C. gratefully acknowledges the Wolfson Foundation and the Royal Society for the award of a Wolfson-Royal Society Merit Award.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
References
- Minaev B, Baryshnikov G, Agren H. Principles of phosphorescent organic light emitting devices. Phys Chem Chem Phys. 2014;16:1719–1758. doi: 10.1039/C3CP53806K
- Baldo MA, O'Brien DF, You Y, Shoustikov A, Sibley S, Thompson ME, Forrest SR. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature. 1998;395:151–154. doi: 10.1038/25954
- Baldo MA, Thompson ME, Forrest SR. High-efficiency fluorescent organic light-emitting devices using a phosphorescent sensitizer. Nature. 2000;403:750–753. doi: 10.1038/35001541
- Lane PA, Palilis LC, O'Brien DF, Giebeler C, Cadby AJ, Lidzey DG, Campbell AJ, Blau W, Bradley DDC. Origin of electrophosphorescence from a doped polymer light emitting diode. Phys Rev B. 2001;63:235206. doi: 10.1103/PhysRevB.63.235206
- Sudhakar M, Djurovich PI, Hogen-Esch TE, Thompson ME. Phosphorescence quenching by conjugated polymers. J Am Chem Soc. 2003;125:7796–7797. doi: 10.1021/ja0343297
- Chen X, Liao JL, Liang Y, Ahmed MO, Tseng HE, Chen SA. High-efficiency red-light emission from polyfluorenes grafted with cyclometalated iridium complexes and charge transport moiety. J Am Chem Soc. 2003;125:636–637. doi: 10.1021/ja0211151
- Sandee AJ, Williams CK, Evans NR, Davies JE, Boothby CE, Kohler A, Friend RH, Holmes AB. Solution-processible conjugated electrophosphorescent polymers. J Am Chem Soc. 2004;126:7041–7048. doi: 10.1021/ja039445o
- Morgado J, Cacialli F, Friend RH, Iqbal R, Yahioglu G, Milgrom LR, Moratti SC, Holmes AB. Tuning the red emission of a soluble poly(p-phenylene vinylene) upon grafting of porphyrin side groups. Chem Phys Lett. 2000;325:552–558. doi: 10.1016/S0009-2614(00)00725-9
- Iqbal R, Moratti SC, Holmes AB, Yahioglu G, Milgrom LR, Cacialli F, Morgado J, Friend RH. Synthesis of porphyrin-PPV copolymers for application in LEDs. J Mater Sci – Mater Electron. 2000;11:97–103. doi: 10.1023/A:1008913027972
- Morgado J, Cacialli F, Iqbal R, Moratti SC, Holmes AB, Yahioglu G, Milgrom LR, Friend RH. Förster energy transfer and control of the luminescence in blends of an orange-emitting poly(p-phenylenevinylene) and a red-emitting tetraphenylporphyrin. J Mater Chem. 2001;11:278–283. doi: 10.1039/b005478j
- Fenwick O, Sprafke JK, Binas J, Kondratuk DV, Di Stasio F, Anderson HL, Cacialli F. Linear and cyclic porphyrin hexamers as near-infrared emitters in organic light-emitting diodes. Nano Lett. 2011;11:2451–2456. doi: 10.1021/nl2008778
- Li P, Fenwick O, Yilmaz S, Breusov D, Caruana DJ, Allard S, Scherf U, Cacialli F. Dual functions of a novel low-gap polymer for near infra-red photovoltaics and light-emitting diodes. Chem Commun. 2011;47:8820–8822. doi: 10.1039/c1cc12752g
- Steckler TT, Fenwick O, Lockwood T, Andersson MR, Cacialli F. Near-infrared polymer light-emitting diodes based on low-energy gap oligomers copolymerized into a high-gap polymer host. Macromol Rapid Comm. 2013;34:990–996. doi: 10.1002/marc.201300240
- Steckler TT, Lee MJ, Chen Z, Fenwick O, Andersson MR, Cacialli F, Sirringhaus H. Multifunctional materials for OFETs, LEFETs and NIR PLEDs. J Mater Chem C. 2014;2:5133–5141. doi: 10.1039/c4tc00342j
- Tregnago G, Flechon C, Choudhary S, Gozalvez C, Mateo-Alonso A, Cacialli F. Virtually pure near-infrared electroluminescence from exciplexes at polyfluorene/hexaazatrinaphthylene interfaces. Appl Phys Lett. 2014;105:143304. doi: 10.1063/1.4898135
- Hou Q, Zhang Y, Li FY, Peng JB, Cao Y. Red electrophosphorescence of conjugated organoplatinum(II) polymers prepared via direct metalation of poly(fluorene-co-tetraphenylporphyrin) copolymers. Organometallics. 2005;24:4509–4518. doi: 10.1021/om050059k
- Xiang H, Zhou L, Feng Y, Cheng J, Wu D, Zhou X. Tunable fluorescent/phosphorescent platinum(II) porphyrin–fluorene copolymers for ratiometric dual emissive oxygen sensing. Inorg Chem. 2012;51:5208–5212. doi: 10.1021/ic300040n
- Zhuang WL, Zhang Y, Hou Q, Wang L, Cao Y. High-efficiency, electrophosphorescent polymers with porphyrin–platinum complexes in the conjugated backbone: synthesis and device performance. J Polym Sci Pol Chem. 2006;44:4174–4186. doi: 10.1002/pola.21522
- Yang R, Tian R, Yan J, Zhang Y, Yang J, Hou Q, Yang W, Zhang C, Cao Y. Deep-red electroluminescent polymers: synthesis and characterization of new low-band-gap conjugated copolymers for light-emitting diodes and photovoltaic devices. Macromolecules. 2005;38:244–253. doi: 10.1021/ma047969i
- Lindsey JS. Synthetic routes to meso-patterned porphyrins. Acc Chem Res. 2009;43:300–311. doi: 10.1021/ar900212t
- Jiang B, Jones WE. Synthesis and characterization of a conjugated copolymer of poly(phenylenevinylene) containing a metalloporphyrin incorporated into the polymer backbone. Macromolecules. 1997;30:5575–5581. doi: 10.1021/ma970227n
- Mink LM, Neitzel ML, Bellomy LM, Falvo RE, Boggess RK, Trainum BT, Yeaman P. Platinum(II) and platinum(IV) porphyrin complexes: synthesis, characterization, and electrochemistry. Polyhedron. 1997;16:2809–2817. doi: 10.1016/S0277-5387(97)00026-0
- Becker K, Lupton JM, Feldmann J, Nehls BS, Galbrecht F, Gao DQ, Scherf U. On-chain fluorenone defect emission from single polyfluorene molecules in the absence of intermolecular interactions. Adv Funct Mater. 2006;16:364–370. doi: 10.1002/adfm.200500550
- Pei QB, Yang Y. Efficient photoluminescence and electroluminescence from a soluble polyfluorene. J Am Chem Soc. 1996;118:7416–7417. doi: 10.1021/ja9615233
- Sims M, Bradley DDC, Ariu M, Koeberg M, Asimakis A, Grell M, Lidzey DG. Understanding the origin of the 535 nm emission band in oxidized poly(9,9-dioctylfluorene): the essential role of inter-chain/inter-segment interactions. Adv Funct Mater. 2004;14:765–781. doi: 10.1002/adfm.200305149
- Kwong RC, Sibley S, Dubovoy T, Baldo M, Forrest SR, Thompson ME. Efficient, saturated red organic light emitting devices based on phosphorescent platinum(II) porphyrins. Chem Mater. 1999;11:3709–3713. doi: 10.1021/cm9906248
- Ponterini G, Serpone N, Bergkamp MA, Netzel TL. Comparison of radiationless decay processes in osmium and platinum porphyrins. J Am Chem Soc. 1983;105:4639–4645. doi: 10.1021/ja00352a020