Abstract
Aim
To examine adherence, performance, and safety of self-administered aerobic endurance exercise when exercise intensity was prescribed and self-monitored with the Borg RPE scale in patients with chronic kidney disease (CKD), and the relationship between performed exercise and change in walking distance.
Materials and Methods
97 men and 50 women (age 66 ± 14 years, measured GFR 22 ± 8 mL/min/1.73m2) were prescribed 60 min aerobic endurance exercise/week at RPE 13–15. The 6-minute walk test was measured at 0, 4, 8 and 12 months of exercise.
Results
100 patients completed the study, 80% reported exercise intensity at 12 months, 74% performed exercise within the prescribed RPE. Median RPE was 13 (13–15). Median duration was 56 (33–109) minutes/week. Patients with a short walking distance at baseline performed significantly less minutes of exercise/week (p = 0.039). There was no correlation between weekly duration and change in walking distance. No exercise-related incidents were recorded. Walking distance improved significantly by 30 ± 56 metres (p < 0.001).
Conclusions
The Borg RPE scale is useful, acceptable, simple and safe for prescribing and monitoring intensity of self-administered aerobic endurance exercise in patients with CKD. A RPE of 13–15 improved walking distance in well-functioning and deconditioned patients, within a wide range of weekly duration of exercise.
Introduction
Chronic kidney disease (CKD) is a global health problem with an estimated prevalence of around 11% and is more common among the elderly [Citation1]. Patients with CKD lose strength and aerobic capacity and become increasingly physically inactive as the disease progresses [Citation2]. The consequences are deleterious as a low level of physical functioning and inactivity for patients with CKD is associated with poor clinical outcomes and increased mortality [Citation3]. Although most studies focus on patients on renal replacement therapy, several studies have shown that the decline in physical function starts relatively early in the course of CKD [Citation4–8] and has an impact on activities in daily life, such as walking capacity [Citation8–9]. In the natural progression of kidney disease, inactive patients can have lost up to 50% of their aerobic capacity by the time they are in need of dialysis treatment [Citation10–11]. According to a Cochrane review there is evidence that exercise is beneficial for patients with CKD [Citation12] and several studies have shown improvement in walking distance after exercise interventions [Citation13–17]. Although focus has been on centre-based, supervised exercise interventions, usually with relatively short intervention periods, some recent studies have found improvement in walking distance after self-administered exercise with interventions between 6 and 12 months [Citation15–17]. However, it remains to be elucidated how exercise should be prescribed regarding intensity and duration to be both effective and safe when exercise is to be self-administered for a sustainable longer period.
A method for prescribing exercise intensity is the Borg rating of perceived exertion (RPE) scale, designed for estimating exertion, fatigue and breathlessness during physical activity [Citation18]. The Borg RPE scale has been found useful for prescribing and self-monitoring exercise training intensity for healthy subjects and for patients with heart failure [Citation19–20]. Although the Borg RPE scale is often recommended to evaluate exertion in aerobic exercise training in patients with CKD and many clinical trials use the Borg RPE to describe the intensity of the performed exercise [Citation12], there are to our knowledge no studies evaluating the Borg RPE scale as a tool to prescribe exercise intensity and as an instrument for self-monitoring exercise training in patients with CKD.
The RENEXC trial, on which the material for this study is based, included patients with CKD stages 3 to 5, not on renal replacement therapy, irrespective of age and comorbidities in order to represent clinical reality in CKD care. The Borg scale was used to prescribe aerobic endurance exercise at a ‘somewhat strenuous’ to ‘strenuous’ intensity, and the patients used the Borg scale to self-monitor their exercise intensity. This level of intensity was deemed to be well tolerated. As the exercise was performed self-administered at home or in a nearby gym according to each patient’s preference, the level of intensity was chosen based on the assumption that it mirrored requirements of ordinary daily life for this group of patients and would minimise risk of injury. The intervention period was 12 months and we aimed to create a training program that could be realistically implemented and integrated in the patient’s daily life.
The aims of this study were firstly to examine adherence, performance, and safety of self-administered aerobic endurance exercise when exercise intensity was prescribed and self-monitored with the Borg RPE scale, and secondly the relationship between the performed exercise and change in walking distance in patients with CKD.
Materials and methods
Study design
This is a sub-study of the RENEXC-trial (RENal EXerCise), a single-centre, prospective, randomised controlled trial. In the RENEXC-trial two treatment arms were compared, one group performing aerobic endurance exercise in combination with strength training and the other performing aerobic endurance exercise in combination with balance training. Complete study design and primary data analysis of RENEXC have been presented previously [Citation15]. In this sub-study data from all patients who performed aerobic endurance exercise in the RENEXC-trial were analysed. Data collection began in November 2011 and was completed in January 2017. Two physiotherapists (p1 and p2) were involved in prescription and follow up and collection of data, p1 between November 2011 and February 2013 (54 baseline, 38 four-month, 27 eight-month and 11 twelve-month measurements) and p2 between February 2013 and January 2017 (all remaining measurements).
Patients
Patients were consecutively recruited from the outpatient clinic of the Nephrology Department at Skåne University Hospital, Lund, Sweden. Inclusion criteria were adults ≥18 years of age, with an estimated glomerular filtration rate <30 mL/min/1.72 m2, not on renal replacement therapy. After inclusion glomerular filtration rate was measured (mGFR) using Iohexol clearance. Comorbidity was accepted as our aim was to represent clinical reality in the CKD population. Exclusion criteria were unstable cardiovascular disease, severe neurological or orthopaedic disorders, uncontrolled hypertension, severe electrolyte disturbances, inability to communicate in Swedish or expected start in renal replacement therapy within one year of study start.
Exercise intervention
The patients were prescribed aerobic endurance exercise with either additional strength or balance training. The goal was for the patients to perform self-administered aerobic endurance exercise at an intensity corresponding to a RPE on the Borg scale between 13 and 15 ‘somewhat strenuous’ to ‘strenuous’, for 60 min or more/week. The additional strength or balance training was to be performed at a RPE of 13–17, ‘somewhat strenuous’ to ‘very strenuous’, 90 min/week. Each training session was preceded by warm-up exercises. The patient could choose to perform exercise at home or at a nearby gym. Aerobic endurance exercise consisted of activities such as walking, jogging, swimming, cycling etc. The research physiotherapist prescribed the exercise program individually regarding type, duration/week and intensity in accordance with each patient’s baseline status and randomisation. If the patient chose to perform exercise at a gym, the physiotherapist would instruct the patient on location, if the exercise was to be performed at home the instruction took place at the outpatient clinic at the hospital. The physiotherapist followed up the exercise program through phone contact every week during the first three months and every second week for the remaining months of the study. During each follow-up session she discussed and adjusted the exercise program in regard to intensity and duration/week according to each patient’s performance and tolerance in order to intensify the training program or adapt to disease deterioration. For patients who improved during the intervention period, an activity such as walking at a brisk pace might be rated as ‘strenuous’ in the beginning of the intervention period but after improvement, the RPE for the same activity would instead be rated as ‘somewhat strenuous’. To maintain the RPE within the prescribed goal of 13–15, the aerobic endurance activity was intensified in accordance with each individual patient’s progression. In contrast, during periods of deterioration, for example if the patient had been inactive due to sickness, adaptation of the training program would mean reducing the intensity, to make it possible for the patient to perform the aerobic endurance activity within RPE 13–15. Duration was adapted in a similar way, if the patient at the beginning of the intervention period was not able to reach 60 min of aerobic endurance exercise/week, duration would be increased in accordance with the patient’s improvement, and decreased in the case of deterioration. Adjustments to the exercise program were also made in conjunction with the tests of physical performance after 4 and 8 months of exercise.
Borg Rating of Perceived Exertion scale (RPE)
The Borg RPE scale is a self-assessed scale from 6 to 20. It is used for estimating exertion, fatigue and breathlessness during physical activity [Citation18]. The Borg RPE scale was used to prescribe the exercise intensity during the study. The Borg RPE scale was also used as an instrument for the patients to self-monitor the RPE of the performed exercise, aerobic endurance, strength and balance, respectively, and was reported in the self-kept training diaries. When prescribing the intensity for aerobic endurance exercise corresponding to a RPE of 13–15 we aimed to reflect the requirements of activities in daily life, ensuring that the training program would be safe to perform when self-administered.
Training diary
The patients reported the performed exercise in regard to intensity (RPE) and duration (minutes) of each exercise session in a self-kept training diary. This was sent to the research physiotherapist at regular intervals. The limitations of a self-kept exercise diary were taken into consideration [Citation21] and the physiotherapist performed a weekly or biweekly follow up to complete the exercise log or to collect the exercise adherence data [Citation22].
6-Minute walk test (6MWT)
This submaximal, functional test measures the distance a patient can walk, indoors on a flat surface, for a period of six minutes. Walking aids are allowed. The 6MWT is performed in a 30 m marked hospital corridor. The patients are instructed to walk as many metres as possible at a self-chosen pace, and standardised encouragement is given according to protocol [Citation23]. The 6MWT was performed at baseline and after 4, 8 and 12 months of exercise.
Statistical analysis
Data were analysed using R software (R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics are presented with mean ± SD or median values with interquartile range. Change in walking distance was assessed using the Student’s paired t-test. The relationships between variables were analysed with univariate and multivariate linear regressions. Relationship between non-parametric data were analysed with Spearmans correlation coefficient. The patients’ walking distance at baseline, relative to their predicted values, and difference in walking distance in metres after 12 months of exercise is shown in a Bland Altman plot. Change in walking distance after 12 months of exercise for patients performing different durations of exercise per week is shown in a descriptive figure. All analyses are performed per protocol. p-values p < 0.05 were considered significant. Predicted values for healthy individuals for the 6MWT, taking age, sex, weight and height into account, were calculated as follows; 6-minute walking distance for men (7.57 × height, cm) – (5.02 × age) – (1.76 × weight, kg) − 309 m and for women (2.11 × height, cm) – (2.29 × weight, kg) – (5.78 × age) + 667 m [Citation24].
Ethical considerations
The RENEXC-trial was approved by the Regional Ethical Review Board in Lund: 2011/369 and adhered to the Helsinki declaration. All patients received written and oral information and gave a written informed consent.
The RENEXC trial was registered at www.ClinicalTrials.gov; NCT02041156.
Results
Patients
describes the flow of patients through the study. 217 patients were assessed for eligibility and 151 patients were included. Three patients discontinued before baseline assessment and one patient did not perform the 6MWT at baseline and was therefore not analysed in this sub-study.
Figure 1. Flow of patients across various phases of the trial. Recontinued shows patients performing the 6MWT after not performing the 6MWT at the previous control. One patient did not perform the 6MWT at baseline and was therefore not analysed in this sub-study.
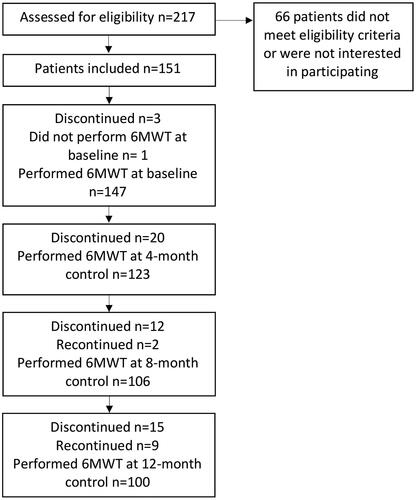
Thus, a total of 147 patients (97 men and 50 women; mean age: 66 ± 14 years; range: 19 − 87 years) performed the 6MWT at baseline. The mean mGFR for these patients was 22 ± 8 mL/min/1.73 m2. MGFR was used for staging CKD: 25 patients were classified with CKD stage 3 (mGFR 30–59 mL/min), 95 patients with mCKD stage 4 (mGFR 15–29 mL/min) and 27 patients with CKD stage 5 (mGFR <15 mL/min). Baseline clinical characteristics and comorbidity burden are given in . Some patients were not able to perform a full six minutes of walking during the 6MWT and needed to terminate the test before they had walked for 6 min (n = 9 at baseline, n = 7 at the 4-month test, n = 7 at the 8-month test and n = 3 at the 12-month test). These patients had a significantly higher prevalence of diabetes mellitus (p = 0.008) in comparison with the patients who could perform a full 6 min of walking. Out of the nine patients who terminated the 6MWT prematurely at baseline, seven patients did not perform a 6MWT at the 12-month control. Patients who discontinued the study (n = 47) had a significantly shorter walking distance (p ≤ 0.001) at baseline and a significantly higher prevalence of diabetes mellitus (p = 0.018) than the completers.
Table 1. Clinical characteristics of 147 patients at baseline.
Adherence to the self-administered aerobic endurance program and exercise performance
Adherence and exercise performance are shown in . The aerobic endurance exercise program was well tolerated with 89% of the patients reporting RPE during the first four months of the study and 80% at 12 months. A similar decrease can be seen in reported duration/week, with 95% of the patients reporting duration during the first four months of the study and 86% of the patients at 12 months.
Table 2. Adherence and exercise performance.
The median intensity of the self-administered aerobic endurance exercise throughout the study was RPE 13 ‘somewhat strenuous’. Most patients performed exercise within the prescribed RPE 13–15 during the intervention, 78% during the first four months and 74% for the remaining study period. Two thirds of the patients who did not perform exercise within the prescribed RPE, performed exercise at a lower intensity (RPE <13), one third of the patients performed exercise at a higher intensity (RPE >15).
Although aerobic endurance exercise had a set goal for exercise duration of 60 min/week, each patient received an individually prescribed exercise duration depending on their baseline status. The median duration for aerobic endurance exercise was 73 min/week during the initial four months of the study and a median of 60 min/week at 12 months (). There was a wide range in aerobic exercise duration, 44% of the patients performed less than 60 min/week during the first 4 months of the study, 42% at 8 months and 48% at 12 months (). There was a decline in total training duration (aerobic endurance exercise in combination with either strength or balance training) during the intervention period ().
Exercise related incidents
No exercise-related injuries or incidents during the self-administered exercise period were reported in the self-kept training diary or during the continuous follow up performed by the research physiotherapist.
Walking distance
Mean walking distance during the 6MWT at baseline, 4, 8 and 12 months is shown in . Mean walking distance recorded during the 6MWT at baseline was 402 metres, which corresponds to 79% of the predicted norm for healthy individuals. The walking distance ranged from 60 to 690 metres. After 12 months of exercise there was a significant increase in walking distance, resulting in a walking distance of 460 metres, corresponding to 89% of the predicted norm for healthy individuals, and ranging from 150 to 735 metres. Walking distance improved significantly by an average of 30 ± 56 metres (p < 0.001).
Table 3. Results for the 6MWT and change in walking distance after 4, 8 and 12 months of exercise.
Relationship between exercise performance and walking distance
We found no statistically significant association between the walking distance at baseline as percentage of the predicted norm and the intensity (RPE) of the performed exercise, that is patients could perform exercise within the prescribed RPE irrespective of their walking distance at baseline. There was no significant association between the intensity (RPE) of the performed exercise and change in walking distance after 12 months of exercise, that is a higher RPE during the intervention period did not correspond to a greater improvement in walking distance.
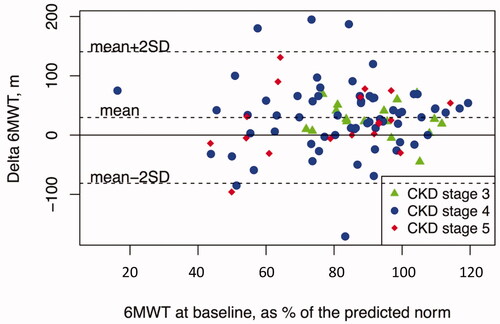
We found a positive correlation between the walking distance at baseline as percentage of the predicted norm and the duration (minutes/week) of performed aerobic endurance exercise during the study (p = 0.039), meaning that patients with a shorter walking distance at baseline performed less minutes of aerobic endurance exercise/week. However, there was no significant relationship between patient’s walking distance at baseline as percentage of the predicted norm and the change in walking distance after 12 months of exercise, see . also highlights the patients in the different stages of CKD, showing that there was a wide heterogeneity within the different stages of CKD in regard to baseline walking distance and change in walking distance after exercise. Patients in CKD stages 4–5 had a wider distribution of baseline walking distance, as well as heterogeneity in regard to change in walking distance after 12 months of exercise in comparison to patients in CKD stage 3. The magnitude of improvement was not correlated to the patient’s baseline status.
Figure 2. The patients’ performance in the 6MWT at baseline relative to the predicted norm in relation to the difference in walking distance in metres after 12 months of exercise.
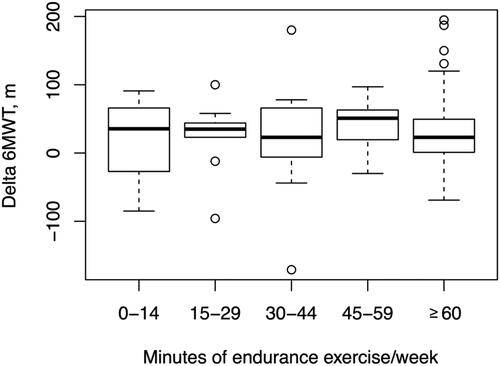
Effect of weekly duration of aerobic endurance exercise on walking distance
Although there was a wide range in weekly exercise duration, the patients’ median RPE was within the prescribed intensity (RPE 13–15) irrespective of exercise duration. Results for the 6MWT for patients who performed different durations of exercise, 0–14, 15–29, 30–44, 45–59 and ≥60 min/week, respectively, are shown in .
Table 4. Increase in 6MWT after 12 months of training grouped according to the different levels of weekly duration of aerobic endurance exercise.
Walking distance after 12 months of exercise increased in all groups. The lowest increase (4%) in walking distance was found in the group who performed less than 15 min of aerobic endurance exercise/week. The greatest increase in walking distance (8%) was found in the group who performed 45–59 min of aerobic endurance exercise/week. For patients performing 60 min or more/week change in walking distance increased with 6%. We found no significant relationship between weekly duration of aerobic endurance exercise and change in walking distance after 12 months of exercise, nor did we find a significant relationship between cumulative minutes of aerobic endurance exercise in combination with balance or strength training and difference in walking distance after 12 months, that is patients who performed a longer duration of exercise did not show a greater improvement in walking distance. Change in walking distance after 12 months of exercise for patients with different durations of aerobic endurance exercise per week is shown in .
Discussion
The aim of this study was to examine adherence, performance, and safety of self-administered aerobic endurance exercise when exercise intensity was prescribed and self-monitored with the Borg RPE scale, as well as examining the relationship between the performed exercise and change in walking distance in patients with CKD. We found that our training program enabled an excellent adherence to exercise intensity and duration and was safe to perform. A major finding of this study was that patients performing self-administered aerobic endurance exercise during 12 months, with a self-monitored exercise intensity of RPE 13–15 on the Borg scale, significantly improved walking distance, despite a wide range of weekly duration of exercise.
Adherence to the self-administered aerobic endurance program and exercise performance
Our training program had an excellent level of adherence during the 12-month intervention period, both regarding number of patients reporting performed exercise intensity (80%) and the performance of the aerobic endurance exercise within the prescribed exercise intensity (74%). There was a minor decline in number of patients reporting exercise and we found a decrease in weekly duration of the performed exercise during the study. However, the majority of the patients were able to perform exercise within the prescribed RPE 13–15 on the Borg scale throughout the intervention period. The high level of adherence can be explained by the training program being specific, measurable, achievable, realistic, and timed. These characteristics are well recognised when writing SMART goals to attain behavioural change [Citation25]. While weekly duration of exercise is simple to specify and easily measured, determining and measuring exercise intensity without the use of heart rate monitors is not as straightforward and although the Borg RPE scale is a recognised method to prescribe exercise intensity it is not regularly used to self-monitor exercise intensity in patients with CKD. We have shown that the Borg RPE scale is a simple method for the physiotherapist to prescribe exercise training and for the patient to evaluate and self-monitor exercise intensity, moreover studies show that interventions encouraging patients to self-monitor their behaviour have higher success rates regarding behavioural change [Citation26]. In order to promote a training program that was acceptable and realistic for the patients to perform and that could be sustained for a longer period, we prescribed exercise based on each individual’s baseline status. Furthermore, the type of aerobic endurance exercise was adapted to each patient’s preference, regarding type of activity (walking/cycling/cross-trainer etc.) and whether it was to be performed at home or in a nearby gym. The high adherence we observed can also be explained by the continuous follow-up by phone contact by the research physiotherapist. Using the Borg RPE scale to describe the subjective experience of exertion made it possible to adjust the exercise intensity via phone contact, enabling modification of the training program in response to each individual’s progression. The phone contact with the research physiotherapist also provided encouragement and motivation for continued exercise, as well as providing patients with an opportunity to ask any questions that might arise during training. This can have had a central role in maintaining motivation over a longer training period, as was concluded in a recent review [Citation27]. Several studies have reported similar levels of adherence to exercise interventions in non-dialysis dependent patients with CKD. Rossi et al. [Citation14] reported that 73% of patients attended all exercise sessions, and 27% of the patients completed 50% of the sessions during a 12 week intervention period when exercise was to be performed 2 times/week. The overall adherence, excluding drop-out, was 79%. Greenwood et al. [Citation13] reported that 59% of patients attended a minimum 50% of exercise sessions during their 12 week intervention period where two sessions/week were performed supervised and one session/week was home-based. Alp Ikizler et al. [Citation28] reported that 85% of patients completed more than 50% of the exercise sessions, and 49% completed more than 75% of the exercise sessions that were performed 3 times/week during a 4 month intervention period. Excluding drop-outs they had a 95% overall adherence to the training program. However, it has also been shown that home-based training can prove more difficult to maintain, with adherence declining during the intervention period [Citation16]. The Landmark study [Citation16], one of the largest exercise studies in patients with CKD not on dialysis treatment, had an initial period of 8 weeks supervised training with high adherence (70%). This was followed by home-based training for 10 months where patients were contacted regularly to monitor their adherence. During the home-based phase of the trial physical activity levels initially increased during the first 6 months, then decreasing to baseline levels at 12 months. The Landmark study then concluded that ongoing supervision may be more effective [Citation16].
Although we found a decrease in patients reporting exercise and in the performed duration of exercise, we have shown that is possible to maintain a high level of adherence to an individually prescribed, self-administered exercise program when patients are encouraged to self-monitor their exercise supported by regular phone-contact during a 12 month period. With the number of patients with CKD increasing, it is essential to find a feasible way to keep patients motivated to perform self-administered exercise. The EXCITE trial, comprising patients on dialysis treatment, was home-based and showed a high adherence of 89% during the 6-month intervention period, supported by regular encouragement by dialysis staff [Citation17]. Follow up and regular encouragement through phone contact, as used in the RENEXC study, is time and cost efficient and may be easier to incorporate into clinical practice than ongoing supervised exercise.
A large proportion of the included patients completed the 12 month intervention period (68%) and the number of patients who discontinued the study (n = 47) was according to the expected drop-out rate in the power calculation for the RENEXC-trial. Patients who discontinued the study had a significantly higher prevalence of diabetes, and a significantly shorter walking distance at baseline than study completers. Interestingly, the patients with diabetes also had a lower degree of heart rate variability at baseline, indicating a higher degree of cardiac autonomic dysfunction [Citation29]. Though we did not systematically collect data regarding reasons for discontinuing the study, some patients reported discontinuing due to illness, some due to lack of motivation and some patients discontinued the study when starting renal replacement therapy. Three patients died, for reasons not related to the study. While exercise duration was adapted to the patients’ baseline status but with the goal of reaching 60 min/week, exercise intensity was set to correspond to RPE 13–15 on the Borg scale for all patients. In the EXCITE study [Citation17], the largest study to date of exercise in patients on dialysis treatment, patients showed significant improvement in walking distance after a low intensity walking program of 60 min/week where exercise intensity was adapted according to the patients’ baseline results in the 6MWT. As many of the patients included in our study had relatively high levels of deconditioning, among whom there was a high prevalence of diabetes and a short baseline walking distance, it is possible that a lower intensity exercise would have been beneficial to motivate the more debilitated patients to remain in the study. Thus, it is important for practitioners to be extra observant and support these patients in performing exercise by adapting the exercise prescription.
Safety
When prescribing exercise intensity corresponding to a RPE of 13–15 we aimed to guide the patients in performing exercise that would be no more strenuous than activities in daily life, such as stair climbing or walking at a brisk pace, ensuring that the training program would be safe to perform when self-administered. As there were no exercise related incidents reported throughout the study, we conclude that it is safe to perform self-administered aerobic endurance exercise at RPE 13–15 for patients with CKD.
Walking distance at baseline and change in walking distance after exercise
The patients’ mean walking distance at baseline corresponded to 79% of the predicted walking distance for healthy individuals. This is in agreement with previous studies, showing that decline in aerobic capacity begins early in CKD [Citation4–8], emphasising that exercise should be an early part of treatment in these patients. The wide range of the baseline walking distance shows the heterogeneity of our patients, highlighting the need for individually prescribed exercise programs. As heterogeneity is representative for patients in CKD stages 3–5, our results can be extrapolated to apply to the CKD 3–5 population. After 12 months of exercise walking distance had improved significantly for patients who completed the trial. In our previous publication from the RENEXC-trial we found that walking distance improved significantly in an intention to treat analysis of all randomised patients [Citation15]. Improvement in walking distance after an exercise intervention is in accordance with several recent studies [Citation13,Citation14,Citation16,Citation17]. While these studies have shown a more rapid improvement in walking distance, the majority of these interventions were supervised and centre-based [Citation13,Citation14,Citation16]. It can be argued that supervised training is seemingly more efficient and can be performed at a higher intensity, but in this study, we were able to show that patients were able to maintain training during 12 months and integrate exercise in their daily routine promoting for a sustainable exercise routine.
Relationship between exercise performance and walking distance
We found no significant association between walking distance at baseline and RPE of the performed exercise, therefore we conclude that patients irrespective of their performance at baseline were able to perform exercise within the prescribed interval of RPE 13–15. Furthermore, we found no significant association between RPE of the performed exercise and change in walking distance, that is a higher exercise intensity does not necessarily result in a greater improvement in walking distance.
Duration of exercise and change in walking distance
There was a wide range of duration regarding performed minutes of exercise/week. Just a little more than half of the included patients were able to perform 60 min or more of aerobic endurance exercise/week. However, we found that the walking distance at baseline had a positive correlation to the weekly duration of exercise performed, i.e. the duration was adapted to each individual. This suggests that the 6MWT is useful not only to evaluate walking capacity, but also to prescribe individualised duration of aerobic endurance exercise for patients with CKD. As there was no correlation between weekly duration of exercise and change in walking distance, this indicates that deconditioned patients with a short walking distance at baseline can increase walking capacity when performing a shorter weekly duration of somewhat strenuous to strenuous exercise (RPE 13–15), whereas patients with a longer baseline walking distance must perform a longer duration of exercise to increase walking capacity. Warburton and Bredin conclude in their article that ‘The shape of the dose-response relationship is such that the greatest relative health benefits are observed in physically inactive individuals who become more physically active’ and that health benefits can be achieved at low volumes of exercise [Citation30]. This is in accordance with our findings in patients with CKD. For patients with little experience of exercise, an aerobic exercise prescription at a lower weekly duration could mean a reduced threshold for attempting exercise and a higher adherence to the training program. This is important information for healthcare professionals working with patients with CKD as many patients are elderly, inactive and struggle to find motivation to start an exercise regime while contending with symptoms of CKD. Our results show that it is possible for a deconditioned patient to maintain and improve walking distance even if they perform as little as 15 min of aerobic endurance exercise/week at an RPE of 13–15 on the Borg scale, when the natural progression for an inactive patient would be a decrease in walking distance as kidney function declines. This can be of clinical importance. Loellgen et al. [Citation31] conclude that change from a sedentary lifestyle to engaging in regular activity with moderate intensity levels shows positive health benefits and risk reduction for all-cause cardiac mortality. In the previously mentioned EXCITE-study, patients not only showed significantly improved walking distance after a ‘mini dose’ of exercise, but also that increase in walking distance resulted in fewer hospitalisations and reduced mortality [Citation32].
Relevance for physiotherapy
Our results contribute to increased knowledge of self-administered exercise prescription in patients with CKD, when exercise is prescribed by a physiotherapist but performed independently in the patient’s home or at a gym. The results can contribute to the development of guidelines for aerobic endurance exercise in patients with CKD 3–5. With increasing numbers of patients with CKD it is essential to find a sustainable way to integrate individually prescribed exercise into routine treatment of these patients. Using the Borg RPE scale to prescribe and self-monitor safe and efficient exercise intensity promotes patient autonomy, and saves time and resources for the patient and for the health care system, as follow up and adjustment of the exercise program can be performed through phone contact.
Limitations and strengths
The study has limitations. Firstly, we had no sedentary control group.
As exercise prescribed by a physiotherapist is standard care in our clinic, it would not have been ethical or practically possible to randomise patients to a sedentary control group. Another limitation is the self-reported exercise diaries that could have been improved if combined with other more objective measures, such as accelerometers. However, when the study was initiated accelerometers were not commonly available in clinical use. Our study has several strengths. We had a 12-month intervention period, a wide inclusion of patients no matter age or number of comorbidities, in order to be representative of the general CKD population, and a training program that was self-supervised. These factors contribute to showing that it is feasible and possible to integrate aerobic endurance exercise into the daily life of patients with CKD.
Conclusion
Exercise prescription for self-administered exercise with continuous follow up through phone-contact and self-monitored exercise intensity with the Borg RPE scale resulted in excellent adherence during a 12-month period to the prescribed intensity of RPE 13–15 on the Borg scale and a good adherence to the goal duration of 60 min/week. The Borg RPE scale is a useful, acceptable, simple and safe method for prescribing and monitoring intensity of self-administered aerobic endurance exercise in patients with CKD. The patients showed a significant increase in walking distance after 12 months of aerobic endurance exercise. There was a substantial proportion of patients with short exercise duration/week. However, even relatively low doses of exercise at the prescribed intensity improved walking capacity in deconditioned patients with CKD. This is valuable information when prescribing self-administered aerobic endurance exercise to inactive and debilitated patients with CKD.
Acknowledgements
The authors would like to thank all participants in the RENEXC-trial, as well as the renal failure nurses Carina Holmesson and Marianne Liljenborg and medical secretary Ann-Charlotte Malmberg for their time and support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765.
- Zelle D, Klaassen G, van Adrichem E, et al. Physical inactivity: a risk factor and target for intervention in renal care. Nat Rev Nephrol. 2017;13(3):152–168.
- Painter P, Roshanravan B. The association of physical activity and physical function with clinical outcomes in adults with chronic kidney disease. Curr Opin Nephrol Hypertens. 2013;22(6):615–623.
- Padilla J, Krasnoff J, Da Silva M, et al. Physical functioning in patients with chronic kidney disease. J Nephrol. 2008;21(4):550–559.
- Hellberg M, Höglund P, Svensson P, et al. A decline in measured GFR is associated with a decrease in endurance, strength, balance and fine motor skills. Nephrology. 2017;22(7):513–519.
- Clyne N, Jogestrand T, Lins L-E, et al. Progressive decline in renal function induces a gradual decrease in total hemoglobin and exercise capacity. Nephron. 1994;67(3):322–326.
- Hiraki K, Yasuda T, Hotta C, et al. Decreased physical function in pre-dialysis patients with chronic kidney disease. Clin Exp Nephrol. 2013;17(2):225–231.
- Johansen K, Painter P. Exercise in individuals with CKD. Am J Kidney Dis. 2012;59(1):126–134.
- Plantinga LC, Johansen K, Crews DC, et al. Association of CKD with disability in the United States. Am J Kidney Dis. 2011;57(2):212–227.
- Beasley CR, Smith DA, Neale TJ. Exercise capacity in chronic renal failure patients managed by continuous ambulatory peritoneal dialysis. Aust N Z J Med. 1986;16(1):5–10.
- Painter P, Messer-Rehak D, Hanson P, et al. Exercise capacity in hemodialysis, CAPD, and renal transplant patients. Nephron. 1986;42(1):47–51.
- Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev. 2011;10:CD003323236.
- Greenwood SA, Lindup H, Taylor K, et al. Evaluation of a pragmatic exercise rehabilitation programme in chronic kidney disease. Nephrol Dial Transplant. 2012;27(Suppl 3):iii126–34.
- Rossi AP, Burris DD, Lucas FL, et al. Effects of a renal rehabilitation exercise program in patients with CKD: a randomized, controlled trial. Clin J Am Soc Nephrol. 2014;9(12):2052–2058.
- Hellberg M, Höglund P, Svensson P, et al. Randomized controlled trial of exercise in CKD – the RENEXC study. Kidney Int Rep. 2019;4(7):963–976.
- Howden EJ, Coombes JS, Strand H, et al. Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis. 2015;65(4):583–591.
- Manfredini F, Mallamaci F, D’Arrigo G, et al. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol. 2017;28(4):1259–1268. Erratum in: J Am Soc Nephrol. 2018;29(7):2028.
- Borg G. Borg's perceived exertion and pain scales. Champaign, IL (US): Human Kinetics; 1998.
- Scherr J, Wolfarth B, Christle JW, et al. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol. 2013;113(1):147–155.
- Carvalho VO, Bocchi EA, Guimarães GV. The Borg scale as an important tool of self-monitoring and self-regulation of exercise prescription in heart failure patients during hydrotherapy. Circ J. 2009;73(10):1871–1876.
- Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(sup2):1–14.
- King AC, Taylor CB, Haskell WL, et al. Strategies for increasing early adherence to and long-term maintenance of home-based exercise training in healthy Middle-aged men and women. Am J Cardiol. 1988;61(8):628–632.
- ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117.
- Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;185:11384–11387.
- Bovend'Eerdt TJ, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: a practical guide. Clin Rehabil. 2009;23(4):352–361. Erratum in: Clin Rehabil. 2010;24(4):382.
- Michie S, Ashford S, Sniehotta FF, et al. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health. 2011;26(11):1479–1498.
- Clyne N, Anding-Rost K. Exercise training in chronic kidney disease-effects, expectations and adherence. Clin Kidney J. 2021;14(Suppl 2):ii3–ii14.
- Alp Ikizler T, Robinson-Cohen C, Ellis C, et al. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol. 2018;29(1):250–259.
- Clyne N, Hellberg M, Kouidi E, et al. Relationship between declining glomerular filtration rate and measures of cardiac and vascular autonomic neuropathy. Nephrology. 2016;21(12):1047–1055.
- Warburton DE, Bredin SS. Reflections on physical activity and health: What should we recommend? Can J Cardiol. 2016;32(4):495–450.
- Loellgen H, Zupet P, Bachl N, et al. Physical activity, exercise prescription for health and home-based rehabilitation. Sustainability. 2020;12(24):10230.
- Torino C, Manfredini F, Bolignano D, et al. Physical performance and clinical outcomes in dialysis patients: a secondary analysis of the EXCITE trial. Kidney Blood Press Res. 2014;39(2–3):205–211.