?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present research was carried out to look into therapeutic insight of biosynthesized silver nanoparticles (AgNPs) by leaf extract of Byttneria herbacea Roxb (BH). The analysis of biosynthesized BH-AgNPs by UV-visible spectroscopy shows an intense surface plasmon resonance (SPR) peak at 422 nm initially and 437 nm after 30 min which certainly reveals the formation of BH-AgNPs. Fourier Infra-red Spectroscopy (FT-IR) reveals that BH-AgNPs are biosynthesized by using different bioactive compounds like O-H stretch of free hydroxyl alcohol and phenols, N-H bond of primary amines present in the leaf extract. Transmission Electron Microscope (TEM) analysis revealed that BH-AgNPs are almost spherical in nature with an average size range from of 2 nm to 12 nm. The particle size analysis by Dynamic Light Scattering (DLS) reveals that the BH-AgNPs are poly-dispersed in nature with an average size of 8 nm ± 2 nm, with a negative zeta potential value of −21 mV which reveals the biosynthesized BH-AgNPs are very stable. The BH-AgNPs (Byttneria herbacea -AgNPs) revealed excellent free radical scavenging activity and exceptional antimicrobial activity. The anti-proliferative and cytotoxic studies in KB oral cancer cells revealed biosynthesized BH-AgNPs can employ as future novel therapeutic agents in cancer treatment and other biomedical applications.
Introduction
The present era of research is mainly focussed on Nano-materials and Nanostructures derived from physical, chemical and biological sources that play an important role in nanotechnology for their promising industrial, pharmaceutical and biomedical applications. Over the past few decades, the metal nanoparticles fabricated from different noble metals exhibited distinct biological, physical and chemical properties. Among these metal nanoparticles silver nanoparticles (AgNPs) became the most commonly investigated, interesting and challenging nano-materials suitable for various potential therapeutic applications [Citation1–2]. At present, the nano-sized particles less than 100 nm in size are gaining attention abundantly due to their new applications in various industries like medical, health care, food and pharmaceutics. Presently, in the past two decades, it clearly revealed that silver nanoparticles attained great interest due to their shape, size and size distribution which plays an important role in optical, electromagnetic, electrical, thermal, catalytic and biological properties [Citation3–6]. Currently, widespread research on silver nanoparticles reveals excellent anticancer and antimicrobial properties [Citation7–10].
Worldwide the cancer-related deaths are a major concern. Non-communicable diseases (NCD) like cancer is emerging as major public health problems in India The most common cancers in men are lung cancer, mouth or oral cancer, stomach and oesophagus and among women wide spread cancers are breast cancer, cervix and uteri cancer. India is highly populated country with 1.417 million populations, according to a study in the year 2022 more than 1.39 million new cancer cases were registered and 7,70,230 deaths due to cancer in the year 2020. In India, the incidence of cancer cases is likely to increase from 1.46 million in 2022 to 1.57 million in 2025. Most prevalent cancer among the population is breast cancer, oral cancer, cervical cancer, lung cancer, stomach cancer and colorectal cancer. Oral cancer is the most common cancer among men and women in the world, Especially in India more than 16.1% of men and 10.4% of women are prone to oral cancer when compared with other cancers [Citation11]. Keeping in-view above oral cancer prevalence, we have initiated our present work. The green synthesized metal nanoparticles exhibit numerous biomedical applications. Green synthesis of metallic nanoparticles has gained a lot of interest due to its bio-safe and eco-friendly with low-cost simple method [Citation12–13]. This efficiently reduces the use of toxic chemicals to the maximum and protects the environment. In recent years, the green synthesis of silver nanoparticles was reported using different plant extracts which exhibit numerous functions such as antimicrobial, wound healing, anti-inflammatory and anticancer studies [Citation14–19]. Recent studies revealed that silver nanoparticles (AgNPs) fabricated by aqueous leaf extracts of Aristolochia bracteolate revealed significant larvicidal and pupicidal efficiency on larvae and pupa of Culex quinquefasciatus mosquito vector. Chloroxylon swietenia leaf extract-mediated silver nanoparticles act as both food dye degrading and antibacterial agents against Staphylococcus nepalensis and Pseudomonas stuteria food-borne pathogens. The leaf extract mediated AgNPs by Ctenolepis garcini L., showed good antibacterial activity against both gram-positive and gram-negative bacteria and also revealed excellent antioxidant activity followed by anticancer potential against HepG2 cell line. The silver nanoparticles with Azadirachta indica aqueous kernel extract have good anti-inflammatory activity as well as anti-diabetic activity. The biosynthesized of silver nanoparticles (AgNPs) from Kigelia africana leaves (Lam.) Benth. extract revealed good cytotoxicity against RINm5F insulinoma cell line and the expression of the Peroxisome proliferator-activated receptor gamma (PPARγ) gene was upregulated in the RINm5F insulinoma cell line confirming the AgNPs have good anti-diabetic efficacy. [Citation20–24]. The green synthesised silver nanoparticles are said have excellent anticancer properties which clearly disclose with the above studies in various cancers cell lines in both in vitro and in vivo. Though they are several studies on various cancers, very few studies are there in oral cancer anticancer studies. So an important medicinal plant Byttneria Herbacea leaf extract was selected for our study to green synthesis silver nanoparticles and to characterize them by different advanced spectroscopy methods and look into their biomedical applications like antibacterial activity, antioxidant activity and most importantly to explore the in vitro anticancer properties in KB oral cancer cell line.
The present selected plant species Byttneria herbecea Roxb () is a procumbent aromatic plant that grows up to 215 cm with 4–10 cm long woody root-stocks which is perennial in nature which has been collected from the Seshachal hills of Tirumala, Tirupati, Andhra Pradesh, India. It is also recognized as an endemic plant to the Indian Peninsular region by the Wildlife Institute of India, Dehradun s Envis Bulletin [Citation25]. The identified plant was confirmed and authenticated [Herbarium No.NN2122/PURSESVU/2020] by Dr. N.Nagaraju, Associate Professor and Head Retd, Department of Botany, Sri Venkateswara Arts and Science College, Tiruapti, Andhra Pradesh and a voucher specimen has been deposited in the department. The previous reports on the phytochemical analysis of different plant parts of B. herbacea revealed that the plant parts are rich in flavonoids, steroids, phenol, alkaloids, cardiac glycosides, tannins, fatty acid & lipid, volatile oil, carotenoids, saponin, gum & mucilage, anthraquinones, anthocynin & iridoids [Citation26]. Byttneria herbacea Roxb., leaves, roots and stem are widely used as ethnic medicine to cure various disorders like dysentery and impaction by using leaves [Citation27], treat leprosy[Citation28], relief against asthma [Citation29], retention of the placenta [Citation30], treatment of fractures in limbs [Citation31] for treatment of leucorrhoea and inflammation [Citation32] wound healing, swellings and relief of body pains by root paste [Citation33–35] and finally, they also possess good antioxidant potential [Citation36]. National Medicinal Plant Board [Citation37] enlisted B. herbacea as medicinal plant in India. Based upon the above biomedicinal properties the Byttneria herbecea Roxb is selected for further studies on green synthesis of silver nanoparticles.
2. Material and methods
2.1. Collection of plant material
Fresh leaves of Byttneria herbacea Roxb. were collected from Talakona forest region of Seshachala hills of Tirumala, Tirupati and they are washed thoroughly tap water quite a few times and finally followed by distilled water [Citation9–10]. The washed leaves were air-dried on coarse filter paper for weekdays, after that the dried leaves were macerated into a fine powder with the help of an electric blender.
2.2. Biosynthesis of BH-AgNPs
15 grams of fresh macerated leaf powder was added to 150 ml of Milli Q water and the sample was heated at 70° C for one hour in a hot water bath and left overnight. Next day the aqueous leaf extract of Byttneria herbacea Roxb was the filter through muslin cloth followed by whatmann no 1 filter paper. This filtered extract was stored at 4 °C in the refrigerator for further studies.
The biosynthesis of silver nanoparticles (AgNPs) was carried out by the protocol [Citation9–10]. The biosynthesized BH-AgNPs colloidal solution was centrifuged at 12000 rpm per 15 min, the obtained BH-AgNPs pellet was washed thrice with Milli Q water by repeating the centrifugation step to remove the unbound plant bio-molecules on the surface of the BH-AgNPs. The final pellet of biosynthesized BH-AgNPs was dissolved in sterile Milli Q water and used for further spectral characterization and biomedical applications.
2.3. Spectral Characterization of BH-AgNPs
The biosynthesized BH-AgNPs were further spectral characterized by various spectroscopic methods. The biosynthesized silver nanoparticles were analyzed in UV-visible Spectrometer, and recorded its surface Plasmon resonance (SPR) peak was by sampling 1 µL − 3 µL of the sample at regular time intervals at room temperature at the resolution of 1 nm between 220 nm −750 nm on the Nanodrop 8000 (Thermo Scientifics, USA). Fourier transform infra-red spectroscopy (FTIR) analysis of Byttneria herbacea Roxb leaf extract and biosynthesized BH-AgNPs was carried out using Bruker Tensor 27 (Thermo Scientifics, USA) to discover the bioactive compounds present in the leaf extract and which bioactive compounds are actively involved bio-reduction and biosynthesis of BH-AgNPs. Particle size and Zeta potential analysis were carried out by dynamic light scattering (DLS) method by using Nanopartica Horiba SZ-100, (Japan), to find out the size distribution and stability of biosynthesized BH-AgNPs in the purified aqueous sample. HR-TEM analysis was carried out to find out the shape and size of biosynthesized BH-AgNPs by coating a drop of the biosynthesized BH-AgNPs on carbon-coated copper grid, the sample was dried under a mercury lamp for 5 min prior to TEM analysis. Energy Dispersive X-ray (EDAX) analysis was carried-out to know the elemental percentage of silver ions and other elements present in biosynthesized BH-AgNPs. Both TEM and EDAX analysis was carried out using Transmission Electron Microscope (FEI-Tecnai G2 20 Twin, Vellore Institute of Technology (VIT), Vellore.
2.4. Antimicrobial Analysis of BH-AgNPs
The antibacterial competence of bio-synthesized BH-AgNPs was determined against six bacterial strains of both gram-negative bacteria such as Escherichia coli (Donor) rifampin resistant, Escherichia coli (Mutant) streptomycin resistant, Escherichia coli (recipient) streptomycin resistant, (Medox, Biotech India Pvt. Ltd), Escherichia coli and gram-positive such as Staphylococcus aureus and Bacillus megatorium, by using Agar disc-diffusion method standardized by previous studies [Citation38–39]
2.5. Antioxidant activity of BH-AgNPs by DPPH method
Free radical scavenging activity of the BH-AgNPs was determined by using 2, 2′-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay according to the method described by Mittal et al. [Citation40]. The DPPH stock solution was prepared by dissolving 4 mg of DPPH in100 ml of methanol and stored at 20 °C. DPPH solution (2 ml) was added to 1 ml of a methanol solution containing test samples of leaf extract of Byttneria herbacea Roxb and biosynthesized BH-AgNPs at different concentrations of 25 µg/mL, 50 µg/mL, 75 µg/mL, and 100 µg/mL. The DPPH radical scavenging activity (RSA) was measured by determining the absorbance at 517 nm, using ascorbic acid as standard and the antioxidant activity.
2.6. In vitro MTT assay for cytotoxic activity of BH-AgNPs
Cell viability was quantified by performing MTT assay as described by well-known Scientists [Citation9,Citation10,Citation41,Citation42]. Seeded 200 μL KB cancer cell suspension in a 96-well plate at the required cell density (20,000 cells per well) and allowed the cells to grow for about 24 h. After 24 h different concentrations of BH-AgNPs 25 µL, 50 µL, 100 µL and 200 µL along with standard drug Camptothecin 7.5 µM concentration were added and incubated on the plate for 24 h at 37 °C in a 5% CO2 atmosphere. After the incubation period, add MTT reagent to a final concentration of 0.5 mg/mL of total volume. The plate was wrapped with aluminium foil to avoid exposure to light, keep the plate in an incubator and incubate for 3 h and then 100 μL of DMSO the solubilization solution was added. The plate was read in ELISA reader at 570 nm and 630 nm was used as a reference wavelength. The IC50 value was determined by using a linear regression equation, i.e. Y = Mx + C. Here, Y = 50, M and C values were derived from the viability graph.
2.7. Analysis of cell cycle study by BH-AgNPs
KB oral cancer cells are cultured in a 6-well plate at a density of 2 x 105 cells/2 ml and were added and incubated in a CO2 incubator overnight at 37 °C for 24 h. Aspirate the spent medium and treat the cells with IC50 value of BH-AgNPs at the concentration of 89.25 µL/mL and along with standard drug Camptothecin 7.5 µM concentration was added in 2 ml of culture medium, except in cell control and incubate the cells for 24 h. The cells were centrifuged and fixed with 1 ml of cold 70% ethanol. The cell pellet was washed twice with PBS to ensure that only DNA is stained with Propidium iodide (PI stains all nucleic acids), so the cell pellet is treated with 50 μL of Ribonuclease A to get rid of RNA and finally add 400 μL PI solution per million cells directly to cells and mix well. Finally incubate the cells for 5 to 10 min at room temperature in dark and then analyze the samples by flow cytometry.
2.8. Dual fluorescence study of apoptosis by BH-AgNPs on KB oral cancer cell
The KB oral cancer cells are cultured in a 6-well plate explained as above. The stained cells were observed under a fluorescence microscope with a filter cube with Excitation 560/40 nm and Emission 645/75 nm for EtBr (Ethidium bromide) and Excitation 470/40 and Emission 525/50 for Acridine orange and Hoechst 33258 staining fluorescence microscopy Excitation 352/461 nm and Emission 510/40 nm. Images were overlaid using Image-J Software v1.48.
2.9. Apoptosis analysis of BH-AgNPs on KB cell lines
The KB oral cancer cells are cultured in a 6-well plate explained as above. At the end of the treatment, remove the medium from all the wells and give a PBS wash and add 5 μL of FITC Annexin V and gently vortex the cells and incubate for 15 min at RT (25 °C) in the dark, after incubation finally add 5 μL of PI and 400 μL of 1X Binding Buffer to each tube and vortex gently and analyze the samples by flow cytometry immediately.
2.10. Caspase 3 expression study by BH-AgNPs on KB cell lines
The KB oral cancer cells are cultured in a 6-well plate explained as above. At the end of the treatment, remove the medium from all the wells and give a PBS wash. The cells were fixed in 1 ml of prechilled cold 70% ethanol. The cells are pelleted at a higher speed and then aspirate the supernatant careful not to lose the pellet, then wash the pellet twice with PBS and then finally add 5 μL of FITC Caspase 3 antibody. Mix thoroughly and incubate for 30 min in the dark at Room Temperature (20° to 25 °C). The cells were washed with 1X PBS with 0.1% sodium azide once and then finally add 0.5 ml of PBS and mix thoroughly and analyze the samples by Flow Cytometry.
3. Results and discussion
3.1. Uv- Visible spectral analysis of BH-AgNPs
The UV visible data proved that the aqueous leaf extract of Byttneriea herbacea has capability to change 0.02 M silver nitrate solution into the ionic form Ag+, the diluted colourless leaf extract reacts with 0.02 M silver nitrate solution and reduce to ionic form Ag+ to Ag0 which is confirmed by the colour change of the solution to dark brown colour (). The surface plasmon resonance spectrum (SPR) peak of biosynthesized BH-AgNPs was obtained at 422 nm, the same sample was read after 30 min revealed the SPR at 437 nm (). The results were similar to the previous reports on the green synthesis of silver nanoparticles from various plants extracts, the SPR peak was reported to increase its intensity with an increase in time intervals and concentration of AgNO3 from 0.001 M to 0.005 M. Likewise, the biosynthesized AgNPs by different plant extract like leaf extract Boerhavia erecta, Artemisia annua, Decaschistia crotonifolia, and flower extracts of Aerva lanata and Ferulago macrocarpa [Citation15, Citation43–46] revealed similar results.
3.2. Ftir analysis of biosynthesized BH-AgNPs
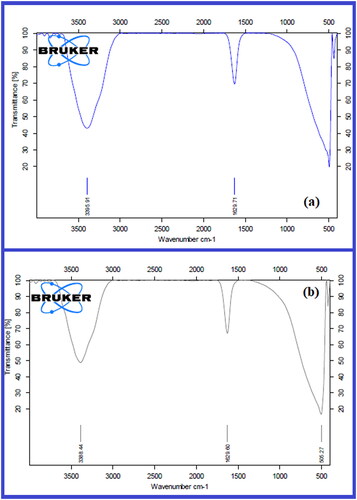
FTIR analysis of Byttneriea herbacea leaf extract and biosynthesized BH-AgNPs were shown in (. The IR peaks for Byttneriea herbacea leaf extract found at 3395.44, 1629.71, 510.17 cm−1. The peak at 3395.44 corresponds to O–H stretch of free hydroxyl alcohol and phenols, the peak at 1629.71 cm−1 is due to N-H bond of primary amines. The bio-synthesised BH-AgNPs showed some prominent peaks in FTIR analysis such as 3388.44, 1629.60 & 506.27 cm−1. The peaks at 3388.44 cm−1 were due to O-H bonds of alcohol and phenols the intensity of the peak is reduced when compared with the extract, peak at 1629.60 cm−1 corresponds to the stretch of N-H bending of primary amines and 506.27 cm−1 due to aliphatic iodo compounds of C-I stretch. FTIR results revealed that the different bioactive molecules like flavonoids, terpenoids, quinuones, polyphenols and steroids of the aqueous leaf extract of Byttneriea herbacea may perhaps involve in the reduction of silver nitrate to BH-AgNPs by capping and stabilization. The FTIR results were alike to previous reports on green synthesis of silver nanoparticles by various plant extracts of Artemisia Annua, Decaschistia crotonifolia, Aerva lanta, Ferulago macrocarpa, etc. [Citation9–10, Citation38–39, Citation47–48].
3.3. Particle size and zeta potential analysis of biosynthesized BH-AgNPs
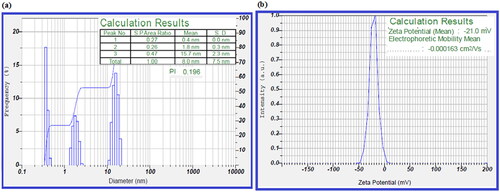
The particle size analysis of BH-AgNPs was carried out using Dynamic light scattering (DLS). The results were calculated by the scattered light intensity with time, the size of biosynthesized BH-AgNPs was in the range of 1 nm − 17 nm. 10% of particles were less than 1 nm, 50% of them were 2.2 nm and 90% of them were 15.7 nm in range with an average size found to be 8 nm ± 2 nm (. The biosynthesized BH-AgNPs revealed poly disperse index (PDI) around 0.196, which concludes that the particles are polydispersed in nature. Zeta potential analysis is a physical property that gives the net surface charge of the nanoparticles. The net surface charge of biosynthesized BH-AgNPs was negatively charged which was detected to be around −21 mV shown in (). The results of the surface charge of BH-AgNPs revealed that they have a high zeta potential value of −21 mV which gives more stability to biosynthesized BH-AgNPs in a colloidal solution. It is concluded that BH-AgNPs are well dispersed without any agglomeration due to high zeta potential value [Citation9–10, 17].
3.4. Tem and EDAX analysis of biosynthesized BH-AgNPs
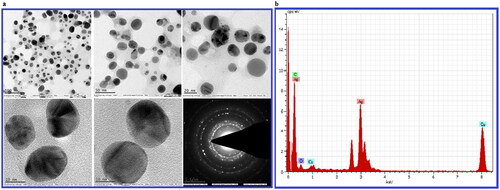
The size and morphology of biosynthesized BH-AgNPs were carried out by transmission electron microscopy, the results reveal the sizes of BH-AgNPs were range of 1 nm to 8 nm ± 2 nm with spherical shape with an average size 8 ± 2 nm, () apparently the results were similar to DLS data. Similar type of result was observed from an earlier report [Citation17]. The crystalline nature of biosynthesized BH-AgNPs was further detected by selected area diffraction pattern (SAED) by TEM analysis showing well-defined sharp spots in a symmetric manner around the bright spots. The Energy dispersive X-ray analysis of BH-AgNPs reveals the elemental composition of the biosynthesized BH-AgNPs, it reveals the highest proportion peak of Ag obtained at 3.0 KeV, followed by some minor peak of C, O, Cu. EDAX data shows the weight percentage of elemental silver is 69.53%, Cu 13.25%, carbon 16.25% and oxygen 0.98% ().The results were similar to our previous reports [Citation9–10].
3.5. Bactericidal activity of biosynthesized BH-AgNPs
Presently many researchers are working on antimicrobial activity using different metal nanoparticles because the bacterial strains are becoming very resistant to antibiotics. So it is important to find out an alternative source for the future against these bacterial strains which can overcome the present problem. So in the present study, an attempt has been made to study the antibacterial activity of biosynthesised BH-AgNPs. The results revealed that the biosynthesized BH-AgNPs showed excellent antibacterial activity against six bacterial species viz., Gram –ve antibiotic-resistant Escherichia coli (Donor strain) rifampin resistant, and Escherichia coli (Recipient strain) streptomycin-resistant and Escherichia coli (mutant strain) streptomycin resistant and Escherichia coli and Gram + ve Staphylococcus aureus, Bacillus megatrium. The zone of inhibition (ZOI) of BH-AgNPs at different concentrations of 10 mcg, 20 mcg, 30 mcg and 40 mcg along with standard antibiotic Amoxyclav 30 mcg (Himedia-SD063) in different bacterial cultures was shown in and ZOI values in mm were measured and tabulated in by comparing with standard antibiotic Amoxyclav 30 mcg. From the above results, it is concluded that the BH-AgNPs have an excellent and efficient antibacterial activity when compared with standard antibiotics shown in . It also reveals that lower concentrations revealed an excellent zone of inhibition which equivalent to the concentration of standard antibiotic. The higher concentrations of BH-AgNPs were superlative than the standard antibiotic Amoxyclav 30 mcg. According to previous studies, it’s well known that metal nanoparticles exhibit very good antibacterial activity, especially silver nanoparticles revealed superior antibacterial activity due to their physicochemical properties. The shape and size of silver nanoparticles play an important role in these processes, they cause lethal effect by three mechanisms, when they come in contact with the surface of bacteria, they attach to the cell surface and disturb the cell wall and enter the bacterial cell and cause disturbance in permeability and respiration. Thus induce DNA damage and ROS stress and also stops the production of ATP, which finally lead to uncontrollable ion transport through the cell membrane and finally leads to bacterial cell death. So it is concluded that BH-AgNPs exhibited exceptional antibacterial activity due to their spherical shape and very minute size of less than 10 nm. So BH-AgNPs can useful as excellent antibacterial agents in the future [Citation9–10, Citation13–14, Citation17, Citation46–50]. As per recent studies, Chloroxylon swietenia leaf extract-mediated silver nanoparticles act as very good antibacterial agents against Staphylococcus nepalensis and Pseudomonas stuteria food-borne pathogens in which the nanoparticles are varied shape and size. The AgNPs biosynthesized by Ctenolepis garcini L. also showed good antibacterial activity against both gram-positive and gram-negative bacteria with different sizes and shapes [Citation21–22].
Figure 5. a. Antibacterial activity of biosynthesized BH- AgNPs on Gram negative antibiotic resistant E.coli strains E.coli Mutant strain, E.coli Donor strain, E.coli recipient strain, and gram positive Staphylococcus aureus and Bacillus megaterium at different concentrations of 10mcg, 20 mcg, 30mcg and 40mcg and centre Ab- Antibiotic (Amoxyclav 30mcg, Himedia-SD063) Fig 5.b. Graphical representation of Antibacterial activity of green synthesized BH- AgNPs on Gram negative antibiotic resistant E.coli strains E.coli Mutant strain, E.coli Donor strain, E.coli recipient strain, and gram positive Staphylococcus aureus and Bacillus megaterium and antibiotic Amoxycillin 30 mcg Himedia-SD063 Fig 5.c. Antioxidant activity of biosynthesized BH- AgNPs by DPPH method µg/mL.

Table 1. Antimicrobial Activity of BH-AgNPs.
3.6. Dpph antioxidant activity of biosynthesized BH-AgNPs
It is well known that certain types of unstable molecules consist oxygen, which readily reacts with other molecules in a cell, these unstable molecules are known as reactive oxygen species (ROS) which readily and easily reacts with DNA, RNA, proteins and lipids of the cell and cause cell death. These different reactive oxygen species cause oxidative stress in various human cells and cause disorders like inflammation, cancer and ageing and other neurodegenerative disorders. So it is very important and useful to find out suitable antioxidants which can reduce ROS. In the present study, in vitro antioxidant activity of the BH-AgNPs was determined by DPPH free radical scavenging assay. The results revealed that BH-AgNPs exhibited concentration-dependent scavenging activity against both DPPH [ & ]. Byttneriea herbacea leaf extract (BH-LE) and BH-AgNPs showed the maximum inhibition of 54.12% and 74.78%, respectively against DPPH radicals at the highest concentration of 250 µg/mL used in this assay. The DPPH activity of BH-AgNPs is greater than the standard ascorbic acid 70.26% at the highest concentration. From this assay, it can be concluded that the BH-AgNPs have efficient scavenging activity. There are several other studies reported previously, where the silver nanoparticles exhibited excellent antioxidant activity. [Citation9–10, Citation14–15,Citation48, Citation51]. So, it reveals that the biosynthesized BH-AgNPs can be useful as future antioxidant agents.
Table: 2. DPPH antioxidant activity of biosynthesized BH-AgNPs.
3.7. In vitro MTT assay for cytotoxic activity of BH-AgNPs
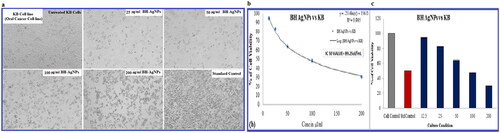
The results of MTT assay revealed the cytotoxic effect and cell viability of KB oral cancer cell lines treated with BH-AgNPs and Camptothecin (standard drug). The cells were treated with BH-AgNPs for 24 h with a range of concentrations (). The IC50 value was calculated after 24 h. The IC50 value was calculated by using a linear regression equation, i.e. Y = Mx + C. Here, Y = 50, M and C values were derived from the viability graph (). The IC50 value of BH-AgNPs was calculated by statistical data of cell cytotoxicity studies reveal that IC50 value of 89.25 µL/mL. The cytotoxicity of biosynthesized BH-AgNPs is dosage-dependent manner, as the concentration increase the cell viability decreases shown in . The results showed that BH-AgNPs have anticancer nature and revealed good cytotoxic potency against KB human Oral cancer cells. Similarly, Nepeta deflersiana-mediated silver nanoparticles show toxicity against Human Cervical Cancer Cells (HeLA), Seripheidium quettense-mediated green synthesis of biogenic silver nanoparticles also showed very good theranostic applications against cancer [Citation52–53]. The AgNPs by leaf extract of Ctenolepis garcini L revealed excellent anticancer potential against HepG2 cell line and the biosynthesized silver nanoparticles (AgNPs) from Kigelia africana leaves (Lam.) Benth. extract revealed good cytotoxicity against RINm5F insulinoma cell line. [Citation22, Citation24]. So in keeping view of the above studies, it’s very essential to determine the molecular mechanism behind anticancer properties of the BH-AgNPs and conducted further studies.
Figure 6. a. MTT assay of cytotoxic studies of green synthesized BH-AgNPs on KB –Human oral carcinoma cell line and its Morphological changes of KB cells observed under an inverted light microscope. a. Untreated KB cell line b. KB cell line cells treated with 25 µg of BH-AgNPs c. KB- cell line treated with 50 µg of BH-AgNPs d. KB cell lines treated with 100 µg of BH-AgNPs e. KB cell lines treated with 200µg of BH-AgNPs and f. Standard control (Camptothecin 7.5 µM). Fig.6.b and c. Comparative percentage of Cell viability studies of green synthesized BH-AgNPs on KB –Human oral carcinoma cell line by MTT assay and the IC50 VALUE is calculated to be = 89.25µL/mL.
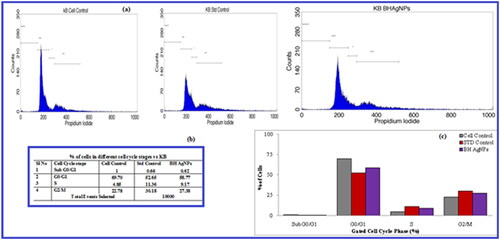
3.8. Cell Cycle assay by BH-AgNPs on oral cancer cell lines
The BH-AgNPs detected have substantial cell inhibition, the study was conducted by selecting the IC50 concentrations of 89.25 µL/mL, against the KB cell lines and evaluated Cell Cycle study results by Flow Cytometry. The cells treated with Std Control and BH-AgNPs with a concentration of 89.25 µL/mL. The results revealed follows: the % of Cells get arrested in the different stages of their life cycle. In Sub G0/G1 phase (Apoptotic phase), 1%, 0.66% and 0.62% of cells get arrested in Untreated, Standard and BH AgNPs respectively. In G0/G1 phase (Growth Phase), 69.70%, 52.65% and 58.77% of cells get arrested in Untreated, Standard and BH AgNPs respectively. In S phase (synthetic phase), 4.85%, 11.36% and 9.17% of cells get arrested in Untreated, Standard and BH-AgNPs respectively. On the other hand, in G2/M phase, 22.78%, 30.18% and 27.38% of cells get arrested in Untreated, Standard and BH-AgNPs respectively. BH-AgNPs exhibit prominent Cell Cycle phase arrest similar to the std Control, Camptothecin on KB cells. . In the present study also the BH-AgNPs showed cell cycle arrest at G2/M stage which leads to induced cell death, thus proves to BH-AgNPs as effective anticancer agents against oral cancer cells. A report on Nepeta deflersiana extract-mediated AgNPs also showed that the cell cycle and apoptosis were induced by SubG1 arrest and lead to apoptotic/necrotic cell death in HeLA cells [Citation52]. Similar type of results were observed by AgNPs biosynthesized by Hydrastis canadensis and Thuja occidentalis induce differential cytotoxicity through G2/M arrest in A375 cells [Citation54]. Our earlier leaf extract of Argyeria nervosa also showed cell cycle arrest at the G2/M stage, further leads to induced cell death [Citation10]. The present study concludes that BH-AgNPs was confirmed to be a competent anticancer agent against KB oral cancer cells.
Figure 7. a.b.c. Cycle cell assay of green synthesized BH-AgNPs on KB –Human oral carcinoma cell line (a): Flow cytometric histograms showing the phases of cell cycle distribution in the KB cell line treated with BH AgNPs with IC50 values and standard drug camptothecin at IC50 value compared to the control. (b) Table showing the % of Cells gets arrested in the different stages of their life cycle. (c) Overlay showing the % of cells gets arrested in the different stages of their life cycle.
3.9. Dual fluorescence assay by BH-AgNPs
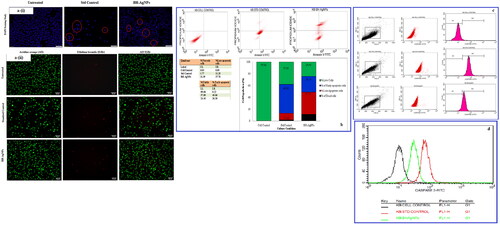
The results revealed the apoptotic nature of BH-AgNPs by fluorescent staining when compared with treated KB cells to untreated cells, they show more number of early apoptotic cells and late apoptotic cells and necrotic cells with chromatin condensation and blebbing of the cell membrane in the human oral carcinoma cells was very clearly observed (. The final results were similar to that of standard drug Camptothecin treated cells indicating that BH-AgNPs have very good anticancer activity. Similarly, the biosynthesized AgNPs using leaf extract of Flemingia wightiana, Saccharina japonica and Asian Spider Flower showed apoptotic cells and late apoptotic cells with blebbing of the cell membrane, chromatin condensation, followed by necrotic cells [Citation55–57].
Figure 8. a(i). Effect of green synthesized BH-AgNPs on nuclear damages in KB oral cancer cells were observed with Hoechst 33258 staining under fluorescence microscopy. (A) Control (untreated), (B) Standard Control (7.5 µg/mL) and (C) BH-AgNPs (89.25 µg/mL) for 24 h. Fig.8. a(ii). Analysis of Dual Fluorescent staining of Acridine orange and Ethidium bromide on KB oral cancer cells of (A) Untreated, (B) Standard Control and (C) BH-AgNPs used to identify apoptosis-associated changes of cell membranes during the process of apoptosis and also to accurately distinguish cells in different stages of apoptosis Fig. 8.b. Apoptotic assay of green synthesized BH-AgNPs on KB –Human oral carcinoma cell line by Flow cytometry assay Figure. 8.c. d. Expression of Apoptotic protein Caspase 3 anlaysis on KB –Human oral carcinoma cell line treated with green synthesized BH-AgNPs.
3.10. Apoptotic assay by annexin V-FTIC using BH-AgNPs
The apoptosis assay of BH-AgNPs by flow cytometry was shown in . The results revealed that KB cancer cell lines treated with BH-AgNPs at conc. 89.25 µL/mL and Camptothecin (7.5 µM) along with controls reveal that early apoptosis is 26.38% for BH-AgNPs and 49.06% for Camptothecin is considerably lower. But the rate of late apoptosis induced by both BH-AgNPs and Standard Camptothecin was 37.78% and 11.28% which was appreciably increased, but the percentage of necrotic cells was also appreciably increased as follows 11.39% for BH-AgNPs and 1.77% for Standard control. It is well known that KB oral cancer cells which show early apoptosis are FITC Annexin V positive and PI negative; but in case of late apoptosis both FITC Annexin V and PI positive. We hereby concluded that the KB cells treated with IC50 concentration of BH-AgNPs and Camptothecin (7.5 µM) reveals that the total apoptosis cells in Q1(75.55% of necrotic cells or dead cells) Q2 (62.11% of late apoptosis cells) and Q4(% of early apoptosis) and Q3 (% of live cells) 24.45% and 37.89% indicating that the BH-AgNPs can be an outstanding anticancer agent against KB oral cancer cells. The biosynthesized silver nanoparticles (BH-AgNPs) showed significant apoptotic nature then the standard drug shown in (. The final observations and results recommended and concluded that the test compound, BH- AgNPs induces significant necrosis and in human oral cancer cells. The previous reports on in vitro studies in MCF 7 cancer cells and other cancer cell lines by silver nanoparticles also revealed similar results [Citation58–59]. So, we recommend that the BH-AgNPs may have good therapeutic potential against human oral cancer; further preclinical studies have to be done to confirm the mechanism of action on KB oral cancer cells.
3.11. Expression of caspase 3 in KB oral cancer cell line
Finally, an important experiment was performed to find out the expression of apoptosis protein caspase 3. The caspase 3 expression was evaluated with the dose of IC50 BH-AgNPs at conc. 89.25 µg/mL and standard drug (Camptothecin) at the concentration of 7.5 µM. The results after 24 h were represented in . From the histogram of the results it’s clear that the cells are gated in M1 and M2 phase, of which M1 refers to negative expression and M2 phase refers to positive expression of Caspase 3. So in the current study, both the treated and untreated cells were gated in both phases and analyzed using Cell Quest Software, Version 6.0. The analysis of the study revealed that Caspase 3 expression is very low in untreated cells, i.e. 9.90 Mean Fluorescence Intensity (MFI), but its very high in the case standard drug, i.e. 71.16 MFI, whereas the cells treated with biosynthesized BH-AgNPs revealed 50.04 MFI of Caspase 3 expression after 24 h. From the results, BH-AgNPs treated cells showed content and significant apoptotic potential with high expression of Caspase 3. The expression of apoptotic protein caspase 3 was high with the standard drug Camptothecin when compared with BH-AgNPs. Similar type of results was observed by earlier researchers who worked on the expression of Caspase 3 by silver nanoparticles synthesized by Argyreia nervosa leaf extract [Citation10]. An another important study on Caspases expression was done by using silver nanoparticles green synthesized by Mentha arvensis on different cancer cells like MCF-7 and Hep 2 cells also revealed the induction of apoptosis by caspase-9, which confirms the activation of caspase 3 and induce oxidative stress in cancer cells and cause cell death due apoptosis [Citation59]. So, in the present study, we can conclude that the biosynthesized BH-AgNPs comprise promising therapeutic prospects against human oral cancer-derived diseases. Even though there is a range of studies on in vitro anti-cancer effect of AgNPs on diverse cancers, other than very few studies were done on KB oral cancer cell lines.
4. Summary and conclusions
In the current study, it is concluded that biosynthesized AgNPs by Byttneriea herbacea have revealed SPR band initially at 422 nm and 437 nm after 30 min, which confirms the green synthesis of BH-AgNPs. The FT-IR analysis confirmed that various bioactive compounds of leaf extract functional groups have actively participated in the reduction of BH-AgNPs. TEM data revealed the particles are in size range from 1 nm ± 2 nm to 10 nm ± 2 nm, and the results were similar to that of Dynamic Light Scattering results. The BH-AgNPs was stable with a negative zeta potential value of −21 mV. The biosynthesized BH-AgNPs also revealed significant free radical scavenging activity and outstanding antimicrobial activity against gram + ve and gram –ve bacteria. The cytotoxic and anti-proliferative studies against KB oral cancer cells reveal an astounding cytotoxic effect with an IC50 value 89.25 µg/ml. Additional studies were carried out on KB oral cancer cells by BH-AgNPs to look for insights into it anticancer effects by means of Cell cycle assay, Apoptosis assay and Caspase activity by flow cytometry. The final results revealed that the biosynthesized BH-AgNPs showed magnificent beneficial applications by arresting the cell cycle and inducing apoptosis in KB oral cancer cell lines. So we conclude that the BH-AgNPs have possible therapeutic potential against human oral cancer-derived diseases and can employ as novel anticancer therapeutic agents. Finally, we report that there are very few studies are noticed KB oral cancer cell lines using AgNPs.
Authors Contributions
The authors GKS, VSK and VNC work planning and design; Conceptualization VSK and VNC; Execution- GKS VSK, SAG and GH; Data analysis VSK, VNC, JP, SAG; Draft Preparation- GKS VSK, SAG and VNC; editing and revision- VSK, VNC, JP
Acknowledgements
The authors GKS and VSK are grateful to DST PURSE centre, Sri Venkateswara University, Tirupati, for providing the facility to carry out a part of the research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All the data related to our research article is present in the manuscript submitted
Additional information
Funding
References
- Zhang XF, Liu ZG, Shen W, et al. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. IJMS. 2016; 17(9):1534.
- Zhang Z, Shen W, Xue J, et al. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res Lett. 2018;13(1):54.
- Lee SH, Jun B-H. Silver nanoparticles: synthesis and application for nanomedicine. IJMS. 2019;20(4):865.
- Khodashenas B, Ghorbani HR. Synthesis of silver nanoparticles with different shapes. Arabian J Chem. 2019; 12(8) :1823–1838.
- Tarannum N, Gautam, YK, Divya . Facile green synthesis and applications of silver nanoparticles: a state-of-the-art review. RSC Adv. 2019;9(60):34926–34948.
- Srikar SK, Giri DD, Pal DB, et al. Green synthesis of AgNPs: a review. GSC. 2016;06(01):34–56.
- Rafique M, Sadaf I, Rafique MS, et al. A review on green synthesis of AgNPs and their applications. Artif Cells Nanomed Biotechnol. 2017;45(7):1272–1291.
- Almatroudi A. Silver nanoparticles: synthesis, characterisation and biomedical applications. Open Life Sci. 2020;15(1):819–839. pp.
- Gaddam SA, Kotakadi VS, Subramanyam GK, et al. Multifaceted phytogenic silver nanoparticles by an insectivorous plant Drosera spatulata Labill var. Bakoensis and its potential therapeutic applications. Sci Rep. 2021;11(1):21969.
- Subramanyam GK, Gaddam SA, Kotakadi VS, et al. Argyreia nervosa (samudra pala) leaf extract mediated silver nanoparticles and evaluation of their antioxidant, antibacterial activity, in vitro anticancer and apoptotic studies in KB oral cancer cell lines. Artif Cells Nanomed Biotechnol. 2021; 49(1) :635–650.
- Ferlay J, Soerjomataram I, Ervik M. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries; Sept 2018.
- Kotakadi VS, Gaddam SA, Kotha P, et al. Bio-inspired multifunctional zinc oxide nanoparticles by leaf extract of Andrographis serpilifolia and their enhanced antioxidant, antimicrobial, and antidiabetic activity—a 3-in-1 system. Part Sci Technol. 2022;40(4):485–499.. Taylor and Francis
- Gaddam SA, Kotakadi VS, Subba Rao Y, et al. Efficient and robust biofabrication of silver nanoparticles by cassia alata leaf extract and their antimicrobial activity. J Nanostruct Chem. 2014;4(1):82–88.
- Kotakadi VS, Gaddam SA, Venkata SK, et al. Biofabrication and spectral characterization of silver nanoparticles and their cytotoxic studies on human CD34 +ve stem cells. Biotech. 2016;6:216–221.
- Palle SR, Penchalaneni J, Lavudi K, et al. Green synthesis of silver nanoparticles by leaf extracts of Boerhavia erecta and spectral characterization and their antimicrobial, antioxidant ad cytotoxic studies on ovarian cancer cell lines. Lett Appl Nano BioScience. 2020;9(3):1165–1176.
- Pal S, Nisi R, Stoppa M, et al. Silver-functionalized bacterial cellulose as antibacterial membrane for wound-healing applications. ACS Omega. 2017;2(7):3632–3639.
- Venkata SK, Gaddam SA, Kotakadi VS, et al. Multifunctional silver nanoparticles by fruit extract of terminalia belarica and their therapeutic applications: a 3-in-1 system. Nano BioMed ENG. 2018;10(3):279–294.
- Slavin YN, Asnis J, Häfeli UO, et al. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol. 2017;15(1):1–20.
- Jia M, Zhang W, He T, et al. Evaluation of the genotoxic and oxidative damage potential of silver nanoparticles in human NCM460 and HCT116 cells. Int. J. Mol. Sci. 2020;21(5):1618.
- Mathiyazhagan Narayanan SP, Natarajan D, Alahmadi TA, et al. Phyto-fabrication of silver nanoparticle using leaf extracts of Aristolochia bracteolata Lam and their mosquito larvicidal potential. Process Biochem. 2022; 121 :163–169.
- Nguyen Thi Anh Nga VB, Raghavendra RS, Alshiekheid M, et al. Green fabrication of silver nanoparticles using Chloroxylon swietenia leaves and their application towards dye degradation and food borne pathogens. Food Chem Toxicol. 2022; Volume 165:113192.
- Narayanan M, Divya S, Natarajan D, et al. Green synthesis of silver nanoparticles from aqueous extract of Ctenolepis garcini L. and assess their possible biological applications. Process Biochem. 2021; 107: 91–99.
- Lan Chi NT, Narayanan M, Chinnathambi A, et al. Fabrication, characterization, anti-inflammatory, and anti-diabetic activity of silver nanoparticles synthesized from Azadirachta indica kernel aqueous extract. Environ Res. 2022; 208:112684.
- Manimegalai Sengani BV, Banerjee M, Choudhury AA, et al. Evaluation of the anti-diabetic effect of biogenic silver nanoparticles and intervention in PPARγ gene regulation. Environ Res. 2022; 215(Pt 3):114408.
- Bulletin E. Special habitats and threatened plants of India: wildlife and protected areas, Vol.11. Chandrabani, Dehradun: wildlife institute of India.. 2008.
- Somkuwar SR, Kamble RB, Chaturvedi A. Extending geographic distribution of Byttneria herbacea Roxb in Maharashtra state, India. J. Chem. Bio. Phy. Sci. Sec. B. 2014;4(3):3257–3261.
- Sreeramulu N, Suthari S, Ragan A, et al. Ethno-botanicomedicine for common human ailments in Nalgonda and Warangal districts of Telangana, Andhra Pradesh. India Annals of Plant Sci. 2013;2:223.
- Jain SP, Gupta N, Saini S, et al. Ethnomedicobotanical survey of Chhindwara Distrlct, Madhya Pradesh International Seminar on “Multidisciplinary Approaches in Angiosperm Systematics” Ethnobotany and Medicinal plants. 621.
- Chandra Babu N, Naidu MT, Venkaiah M. Ethnobotanical plants of kotia hills of Vizianagaram district. A P India J Phytol. 2010;2:76–82.
- Patel JV, Rohit M, Patel DS. Indigenous traditional knowledge (ITK) of pastoral community in banni region. Kachchh, Gujarat. 2003.
- Prusti AB. Ethnobotanical exploration of Malkangiri district of Orissa, India. Ethnobotanical Leaflets. 2007;11:122–140.
- Ashutosh KM, Mishra PK, Jyoti K, et al. Traditional botanical wisdom of Birhore tribes of Jharkhand. Ind J. Tradit Knowl. 2010;9:467–470.
- Dey A, Gupta B, Jitendra, ND Traditional phytotherapy against skin diseases and in wound healing of the tribes of Purulia district, West Bengal, India. J. Med. Plants Res. 2012;6:4825–4831.
- Mallik BK, Panda T, Padhy RN. Traditional herbal practices of the ethnic people of Kalahandi district of Odisha. Asian Pacific J. Trop. Biomedic. 2012;2:988–994.
- Suthari S, Sreeramulu N, Omkar K, et al. The climbing plants of Northern Telangana in India and their ethnomedicinal and economic uses. Ind. J. Plant Sci. 2014;3:95.
- Somkuwar SR, Dongre UJ, Chaudhary RR, et al. In-vitro screening of an antioxidant potential of Byttneria herbacea Roxb. Int.J.Curr.Microbiol.App.Sci. 2014;3(8):622–629.
- National Medicinal Plant Board Medicinal plants of India. Pharmacol. Rev. 2012;48:3–9.
- Subbaiah Kotakadi V, Aparna Gaddam S, K. Venkata S, et al. Ficus fruit-mediated biosynthesis of silver nanoparticles and their antibacterial activity against antibiotic-resistant E.coli strains. CNANO. 2015;11(4):527–538.
- Kotakadi VS, Gaddam SA, Sucharitha Venkata K, et al. New generation of bactericidal silver nanoparticles against different antibiotic-resistant Escherichia coli strains. Appl Nanosci. 2015;5(7) :847–855.
- Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31(2):346–356.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
- Alley MC, F M Ludiere DA, Monks A, et al. Validation of an automated microculturetetrazolium assay (MTA) to assess growth and drug sensitivity of human tumor cell lines. Proc. Am. Assoc. Cancer Res. 1986;27:389.
- Adoni M, Yadam M, Gaddam SA, et al. Antimicrobial, antioxidant, and dye degradation properties of biosynthesized silver nanoparticles from Artemisia Annua L. Lett Appl NanoBioScience. 2021;10(1):1981–1992.
- Palithya S, Gaddam SA, Kotakadi VS, et al. Biosynthesis of silver nanoparticles using leaf extract of Decaschistia crotonifolia and its antibacterial, antioxidant, and catalytic applications. Green Chem Lett and Rev. 2021;14(1):137–152.
- Palithya S, Gaddam SA, Kotakadi VS, et al. Green synthesis of silver nanoparticles using flower extracts of Aerva lanata and their biomedical applications. Part Sci Technol. 2022;40(1):84–96.
- Azarbani F, Shiravand S. Green synthesis of silver nanoparticles by Ferulago macrocarpa flowers extract and their antibacterial, antifungal and toxic effects. Green Chem Lett Rev. 2020;13(1):41–49.
- Cao X, Zhu L, Bai Y, et al. Green one-step synthesis of silver nanoparticles and their biosafety and antibacterial properties. Green Chem Lett Rev. 2022;15(1):28–34.
- Ojemaye MO, Okoh SO, Okoh AI. Silver nanoparticles (AgNPs) facilitated by plant parts of crataegus ambigua becker AK extracts and their antibacterial, antioxidant and antimalarial activities. Green Chem Lett Rev. 2021;14(1):51–61.
- Khatami M, Sharifi I, Nobre MAL, et al. Waste-grass-mediated green synthesis of silver nanoparticles and evaluation of their anticancer, antifungal and antibacterial activity. Green Chem Lett Rev. 2018;11(2):125–134.
- Yu X, Li J, Mu D, et al. Green synthesis and characterizations of silver nanoparticles with enhanced antibacterial properties by secondary metabolites of Bacillus subtilis (SDUM301120. Green Chem Lett Rev. 2021;14(2):190–203.
- Hajebi S, Tabrizi MH, Moghaddam MN, et al. Rapeseed flower pollen bio-green synthesized silver nanoparticles: a promising antioxidant, anticancer and antiangiogenic compound. J Biol Inorg Chem. 2019;24(3):395–404.
- Al-Sheddi ES, Farshori NN, Al-Oqail MM, et al. Anticancer potential of green synthesized silver nanoparticles using extract of nepeta deflersiana against human cervical cancer cells (HeLA). Bioinorg Chem Appl. 2018;2018(1):9390784.
- Muhammad Qasim N, Zohra T, Khalil AT, et al. Seripheidium quettense mediated green synthesis of biogenic silver nanoparticles and their theranostic applications. Green Chem Lett Rev. 2019;12(3):310–322.
- Das S, Das J, Samadder A, et al. Biosynthesized silver nanoparticles by ethanolic extracts of Phytolacca decandra, Gelsemium sempervirens, Hydrastis canadensis and Thuja occidentalis induce differential cytotoxicity through G2/M arrest in A375 cells. Colloids Surf B Biointerfaces. 2013;101:325–336.
- Netala VR, Bethu MS, Sana S, et al. Eco-friendly synthesis of silver nanoparticles using leaf extract of Flemingia wightiana: spectral characterization, antioxidant and anticancer activity studies. SN Appl Sci. 2020;2:884.
- V.m S, Pandurangan M, Kim D, et al. Green synthesis: in-vitro anticancer activity of silver nanoparticles on human cervical cancer cells. J Clust Sci. 2016;27(2):671–681.
- Pannerselvam B, Durai P, Thiyagarajan D, et al. Facile synthesis of silver nanoparticles using Asian spider flower and its in vitro cytotoxic activity against human breast carcinoma cells. Processes. 2020;8(4):430.
- Tabrizi MH, Karimi E, Namvar F, et al. Silver–palm pollen nanocomposite exhibits antiproliferative, antioxidant, and proapoptotic properties on MCF-7 breast cancer cells. Res Chem Intermed. 2018;44(11):6537–6548.
- Banerjee PP, Bandyopadhyay A, Harsha SN, et al. Mentha arvensis (Linn.)-mediated green silver nanoparticles trigger caspase 9-dependent cell death in MCF7 and MDA-MB-231 cells. Breast Cancer. 2017;9:265–278.