 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In photodynamic therapy (PDT), internalization and uptake of the photosensitizer (PS) by the cells is a passive process that relies on the enhanced permeability and retention (EPR) effect of tumour tissues due to their vasculature, increased LDL receptors, and decreased lymphatic drainage in vivo. But as worries about PDT resistance grow, using passive techniques to administer PSs is becoming less and less viable. According to reported resistance mechanisms, it is necessary to improve PS delivery by changing PS absorption and bioavailability in order to enhance the therapeutic outcome. Therefore, in this study, a multifunctional photosensitizing agent with specific monoclonal antibodies (mAbs) to E6 oncoproteins was developed for PDT of human papillomavirus (HPV)-transformed cancer cells. Using PEGylated Gold Nanoparticles (PEGy-AuNP) at the core, anti-E6 mAbs and phthalocyanines were bound together. This compound demonstrated enhanced internalization of PS, resulting in enhanced PDT effects. In spite of being demonstrated in vitro, the substance in this work is intended for in vivo application, and conclusions are drawn to suggest possible outcomes for in vivo models based on observed data. By making PSs more bioavailable, facilitating their entry into cells, and preventing efflux through intracellular binding, this strategy may reduce cellular resistance to PDT.
Introduction
Cancer therapy has been revolutionized in recent years due to the emergence of nanomedicine and drug delivery systems (DDS). From chemotherapy to other biological therapies, nanomaterials have been keenly investigated for their roles in enhancing therapy. Furthermore, the presence of immunogenic proteins in cancer and the consequent incorporation of the corresponding ligand-targeting molecules like immunoglobulins or their mimics leads to a more targeted and specific drug delivery. In photodynamic therapy (PDT), the use of nanomaterials and immunoglobulins has also transformed the way PDT is administered, though this has only been supported by a few in vitro investigations thus far [Citation1,Citation2]. Similar methodologies have also been reported as photoimmunotherapy (PIT) in other studies [Citation3,Citation4]. The need for incorporation of these carrier molecules is mainly due to the limitations of most photosensitizers (PSs), whether natural or synthetic, in achieving efficacious effects in their nude form. When taken into the body, notable limitations of PSs include ineffective cellular uptake, circulatory clearance before reaching the target site, and many more. Hence, the need to alter the delivery of PSs to tumour cells to modulate PDT performance is worthwhile.
In this present study, a novel multicomponent photosensitizing compound was designed and constructed for targeting human papillomavirus (HPV)-transformed cancer cells by recognition and binding to intracellular E6 oncoproteins. Various methods for coupling metallic nanoparticles (NPs), e.g., gold (Au), to monoclonal antibodies have been substantially investigated in many studies [Citation5–8]. Moreover, AuNPs can be functionalized in varying ways to achieve a desired function and outcome. Surface functionalization with polyethylene glycol (PEG) makes AuNP more stable, increases their biological mimicry and circulation time, and does increase the surface area for coupling other molecules. Using PEG-functionalized AuNPs (PEGy-AuNPs) with the PEG moiety containing carboxylic terminals enables coupling of monoclonal antibodies (mAbs) to the carboxylic acid end by strong amide bonds. Hence, as described in this study, PSs and mAbs can both be loaded on PEGy-AuNPs to form a multicomponent and multifunctional scaffold that achieves numerous functions and increases PDT efficacy. This type of design creates a molecular scaffold that increases cellular uptake and PS load in the target cells, and when taken in vivo, this design prolongs circulation time and avoids elimination and immune clearance [Citation9].
Previous DDS studies in PDT had focused on PS delivery using specific mAbs to extracellular antigens and outer membrane surface markers [Citation1,Citation2]. While this approach has shown an increase in cytotoxicity upon PDT, at least in vitro, it locates PSs on cell surfaces and might impair cellular uptake, especially if the coupling is so strong and there is no dissociation of PS from the scaffolds after the mAbs bind strongly to the surface antigens. This may limit the effect of PDT in some cases, as the most significant effect of PDT is caused by organelle damage when PSs localize in essential organelles, e.g., mitochondria, endoplasmic reticulum (ER), and ribosomes. In some cases, a dissociation agent may be required to enable PS uptake by the cells. In this present study, therefore, the design, development, and usability of a delivery vehicle for intracellular targets are described for the first time in PDT to avoid the limitations of extracellular accumulation of PS. Moreover, a modified technique of coupling three components, i.e., PS, mAbs, and PEGy-AuNPs, without reducing the concentration or function of the PS is described and the outcome is a stable, three-component photosensitizing agent that is readily internalized by HPV-transformed cells, in vitro. These findings have set foot on a new direction in PDT, where PS can be delivered to intracellular compartments to make PDT more efficacious.
Material and methods
Sample preparation
Gold NPs of 10 nm diameter, carboxylic acid factionalized, PEG 3000 coated (Product No. 765430), OD 50 of an unspecified molar concentration were purchased from Sigma-Aldrich®. Aliquots of 200 µL in 800 µL PBS from the stock solution were prepared and each used for a single experiment. The PS, aluminium (III) phthalocyanine chloride tetrasulfonic acid, AlPcS4Cl, with a molecular weight of 895.2 g/mol (Frontier Scientific), was prepared to a stock concentration of 100 mM in PBS and stored in the dark at 4 °C. The Anti-HPV16 + HPV18 E6 [C1P5] ab70 (Abcam) with a size of 16.5 kDa were aliquoted in volumes of 100 µL and stored at +4 °C short term and −20 °C long-term. Each vial contained 100 µg of antibody.
Buffer preparation
The isoelectric points (pIs) of the individual compounds were used to prepare the appropriate buffer for conjugation. To induce pre-concentration (i.e., the electrostatic interaction of the molecules onto the surface of the AuNP), a buffer was prepared by dissolving MES (2-(N-morpholino)ethanesulphonic acid) (Merck) in PBS with 0.05% Tween 20 [Polyoxyethylene (20) sorbitan monolaurate] and correcting to pH 5.5. At this pH, the amino groups of the mAbs were maintained below the pI with a net positive charge, and the carboxyl groups on the AuNPs were maintained above their pI, resulting in a, net negative charge. The PS was weakly negative at this pH. Preconcertation was therefore induced by having the buffer at pH 5.5.
Molecular scaffolding of the compound
Because the loading capacity of the PS onto the AuNPs is not predetermined, concentrated PS was used to achieve the maximum loading onto the AuNPs. A starting concentration of the anti-E6 mAb was 50 μg/mL in the reaction mix, and the PEGylated AuNPs were used as a 1:5 dilution of the original reagent. All reactions were done at room temperature in MES buffer at pH 5.5 under sterile conditions. EDC (N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride) [Sigma Aldrich, 03449-5 G] and NHS (N-hydroxysuccinimide) [Sigma Aldrich, 56485-1 G] were used for coupling via the EDC/NHS coupling chemistry. In working volumes of 1 mL, 0.4 mg of EDC was added directly to 1 mL of PEGy-AuNPs. This was followed by the addition of 0.6 mg of NHS and then mixing by low speed votexing for 15 min at room temperature. After activation of the COOH terminal of the PEGy-AuNP, semi-stable NHS esters are formed, which are readily available for reaction with primary amines (-NH2) on the mAbs to form amide crosslinks. Excess EDC products and unreacted NHS were removed by centrifugation at 6000 × g for 90 min. 50 μg/mL of anti E6 mAbs was then added to the activated PEGy-AuNPs, and the sample was mixed for 2.5 h at room temperature. Immediately after, PS at a concentration of 3 mM was added to the reaction tube. The mixture was agitated by continuous vigorous shaking using a Votex (Vortex-Genie 2, Scientific Industries) at 400 RPM for 15 h. Centrifugation at 6000 × g for 90 min was done to remove the supernatant containing unbound PS. The contents were then re-suspended in 1 mL of 1× PBS and stored at 4 °C. Another scaffold comprising PS bound to AuNPs without anti-E6 mAb was also prepared using the same method except for the activation of COOH and the addition of the mAbs.
Chemical characterization of the molecular scaffold
Assessment of PS loading concentration using UV-Vis spectrophotometry
UV-Vis spectrophotometry was used to measure the concentration of PS loaded onto the AuNPs. At the outset, a standard curve of the PS in PBS was plotted to show that the PS in PBS obeys Beer-Lamberts law, i.e., that the absorbance (A) of the PS is directly proportional to the concentration and the length of the light path through the solution. Standard concentration dilutions from 55 µM down to 35 µM at 5 µM decrements were prepared and read using the Jenway UV-Vis Spectrophotometer (Lasec) at 672 nm to measure A, and data were recorded and plotted on the standard curve. Having confirmed Beer-Lambert’s law, the concentration of the PS was confirmed by measuring the A of the conjugated material at 672 nm and using EquationEquation (1)(1)
(1) to determine the concentration of the PS on the AuNP.
(1)
(1)
Determination of optical properties using UV-Vis spectrophotometry
Optical properties were measured by scanning the single compounds’ and molecular scaffolds’ electromagnetic spectrum from 400 to 800 nm at 2 nm wavelength intervals in order to determine the peak absorption wavelengths. Samples were prepared by diluting the PS, AuNPs, and the molecular scaffolds from their stock solutions to a low concentration that achieved an absorbance value between 0.2 and 0.9. A of the diluted samples was measured using the UV-Vis spectrophotometer as shown in ‘Assessment of PS loading concentration using UV-Vis spectrophotometry’ section. The spectrum results were captured and plotted onto a line graph for interpretation.
Determination of particle size and HRTEM with energy dispersive X-ray spectroscopy (EDX) analysis
The JEM-2100 High Resolution Transmission Electron Microscope (HR-TEM) (JEOL Ltd.) was used to obtain images of the single particle and scaffold compound. Samples were loaded onto carbon-coated 200-mesh Cu TEM grids (Lot# 1261229, SPI Supplies) by dropwise pipetting until the grids were fully covered with solution. The grids were left to air dry in the dark for 2 h. Once dried, the grids were loaded onto the microscope, and images were captured. To further analyse the compounds, the JEM-2100 HR-TEM is equipped with an Energy Dispersive X-ray Spectroscopy (EDX) feature for analysing single elements available on the loaded Cu grids. The EDX spectroscopic analysis achieves both the elemental and qualitative analysis of the compounds present in the sample. An electron beam irradiates the area to be analysed using generated X-rays, and the elements are detected and semi-quantified, indicating the type and intensity of elements in the projected field of view. Hence, this feature was used to ascertain the presence of single elements forming the compounds.
Determination of particle charge and stability using zeta potential
Though scaffolding is done at pH 5.5, the net charge of the molecular scaffold was determined at physiologic pH (pH 7.4) to ascertain the charge of the compound in physiological terms. Charge was measured using the Zetasizer Nano Series (Malvern Instruments). Zeta potential (ZP) was measured using laser doppler electrophoresis on the instrument, and an internal formula calculates the ZP. To measure stability for physiological conditions, and in storage, ZP was measured at both 37 °C, pH 7.4 and at refrigeration temperatures. Results were captured as ZP values and used directly without further analysis.
Determination of chemical bonds using FT-IR
Fourier-transformed Infrared (FT-IR) spectroscopy was performed using the Spectrum 100 FT-IR Spectrometer (PerkinElmerTM). IR spectra ware captured by preparing samples in a homogenous mixer with dry Potassium Bromide (KBr) powder followed by high pressure compression into a semi-thick round disc. The discs were then placed directly on the optical system to be read. The PS was used in its original powder form; however, the conjugates in PBS were spun down at high speed (10,500 × g) on the NovaFuge B115-18R (Senova Biotech Co. Ltd.) for several hours to remove most PBS and a thick sediment with less solvent was used. The data was read at 4000–400 cm−1 and the obtained plots were smoothed and cleaned using the Spectrum software, and images were used as exported.
Assessment of uptake and internalization
To assess the uptake and internalization of the molecular scaffold, the red autofluorescence of the PS on the scaffold was detected by fluorescence microscopy. Cells were cultured as described on 22 × 22 glass coverslips, then treated with 10 µM of the molecular scaffold. After a 12 h incubation, the cells were washed three times with warm HBSS, followed by fixation with cold 4% paraformaldehyde. Immediately after fixing, 100 nM mitotracker (Invitrogen M7514) was loaded onto the cover slips for staining mitochondria, and 2 µg/mL 4’6-diamidine-2-phenylindole DAPI solution was used to counterstain. The coverslips were washed four times before loading inverted on a glass slide with a drop of oil. Images were captured using 40× oil immersion objective on a Carl Zeiss Axio Z1 microscope (Carl Zeiss MicroImaging GmbH), with 490Ex/516Em filters for green and 649Ex/670Em for red fluorescence.
Photo-toxicity of the molecular scaffold on HPV-transformed SiHa cells
To determine the photo-toxic effects of the molecular scaffold, E6+ HPV-transformed SiHa cells (ATCC® CCL-2.1™) derived from a squamous cell carcinoma of the cervix were cultured at a concentration of 2 x 105 cells in 3 mL of Dulbecco’s Minimum Essential Medium (DMEM) (Sigma-Aldrich®), which was supplemented with 10% Foetal Bovine Serum (FBS), in a 37 °C-humidified incubator, with 5% of atmospheric CO2 and 85% humidity. The cells were left to attach for 8 h and then treated with the respective elements and concentrations as shown in . A 12-h incubation was allowed pre-irradiation. Irradiation was done in 1 mL of medium at 10 J/cm2 using a semiconductor diode laser emitting a wavelength of 673.2 nm (Oriel Corporation), supplied by the National Laser Centre (NLC) of South-Africa. Cellular responses were conducted to assess the extent of damage by detection of morphology using an inverted light microscope (Wirsam, Olympus CKX41) and measurement of proliferation using the CellTiter-Glo luminescence assay (AnaTech: Promega, PRG7571) measured on the VICTOR Nivo Multimode Microplate Reader, part # HH35000500, Perkin Elmer).
Table 1. Experimental groups and concentrations used for PDT to assess the effects of the molecular scaffold on SiHa cells.
Statistical analysis
Statistical considerations were instrument dependent and based on the requirements of each assay and the availability of statistical methods per given set of results. Repeatable experiments were repeated three times (n = 3) and are reported as such below. All quantitative assays were performed in duplicate, and an average of the results was used. Statistical analysis was performed using SigmaPlot software version 14.0, on which all calculations, including the mean, standard deviation, standard error, and significant changes, were done. Astudent t-test was performed to determine the statistical difference between the control and experimental groups. Statistical significance is shown in graphs as (*) for p < .05, (**) for p < .01 and (***) for p < .001. The standard error on all plotted graphs is represented by error bars.
Results
Optical properties and concentration measurement
Spectrophotometric analysis demonstrated the maximum absorption of the single molecules and molecular scaffolds, as shown in and . shows AlPcS4Cl with maximum absorption at 672 nm and the minor groove at 610 nm. The 610 minor grove is due to a minor increase in absorption caused by splitting of the Q band in that region, a common phenomenon for all phthalocyanine PSs mainly due to some degree of aggregation. Furthermore, the band gap energy (Eg) of the PS was also determined using a Tauc plot and an Eg of 1.811 was recorded. The Eg did not significantly change after binding to the molecular scaffold. shows these optical properties.
Figure 1. Absorption spectra of AlPcS4Cl showing maximum absorption at 672 nm. Included on the upper right side of the graph is a Tauc plot showing the band gap energy of the PS at Eg=1811.
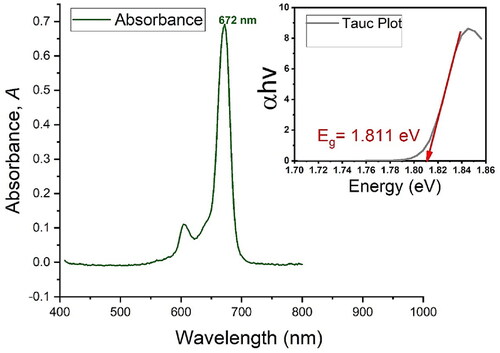
Figure 2. Spectrophotometric analysis and absorption spectra of (A) the PS indicating maximum absorption at 672 nm and the typical minor band at 610 nm. As shown in (B), PEGy-AuNPs indicate an absorption band at 520 nm, and the molecular scaffold (C) indicates absorption bands at 524 nm and 674 nm, which represents the peak of the PS in the molecular scaffold at 674 nm. The mirrored spectra to observe any changes in absorption bands, excitation peaks and wave shifts is shown in (D), where plots a and b are plotted using the primary axis (left) and plot c is plotted against the secondary axis (right) to correct for concentration changes as the aim is to observe the optical properties when mirrored together.
On further analysis, PEGylated AuNP showed a maximum absorption at 520 nm, as shown in (left). On the right of , the molecular scaffold showed both peaks of PS and PEGy-AuNPs at the same wavelengths, with minimal shift possibly due to concentration ratios and size change. However, the expected peaks remained significant and observable. The spectra in the mirrored image demonstrate the peaks and ranges of the bonded material. The retained properties also confirm that binding of the PS to the PEGylated AuNPs does not distort the PS structure and function, hence, maximum photoactivity is retained in the PS molecule while bonded to the molecular scaffold. Furthermore, because the PS obeys Beer-Lambert′s law (shown in supplementary material S1), the determination of concentration using spectrophotometry was measured using Equationequation 1(1)
(1) , with a standard solution of 50 µM. The concentration of the final conjugate in the 1 mL final volume was 486,4 µM for the PS bound to AuNPs without mAbs i.e., referred to as molecular scaffold one and 457,7 µM for the PS bound to AuNP and Anti E6 mAb i.e., referred to as molecular scaffold two. For ease of reference in proceeding sections, the terms molecular scaffold one, and molecular scaffold two, where necessary, will be used to refer to the same, respectively, though in the script the term molecular scaffold generally refers to the three-component particle.
Particle size and HRTEM with EDX analysis
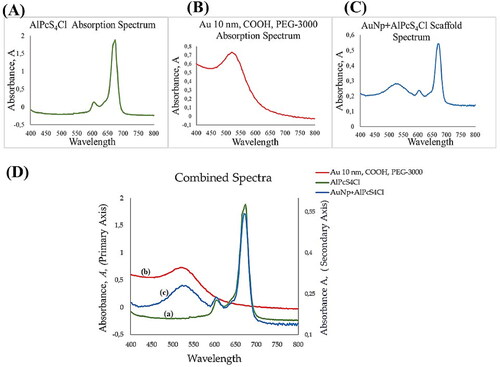
HRTEM analysis confirmed the particle size as averaging 10 nm and ruled out agglomeration. As shown in , the HRTEM images of the molecular scaffold show well dispersed particles of various sizes. The confirmed size in the conjugated material indicate that the scaffold remained single molecules after conjugation, an important feature that affects cellular uptake. In (C), the presence of mAbs, though the intact structure of the mAbs was indeterminable due to resolution and kV power used, was confirmed by the orientation of the particles, which showed the altered particle surfaces, confirming the presence of mAbs on the surface of the AuNP.
Figure 3. HRTEM images of the molecular scaffold showing well dispersed particles of sizes averaging 10 nm in size and surface alteration in (C) showing the presence of mAbs on the surface of AuNPs.
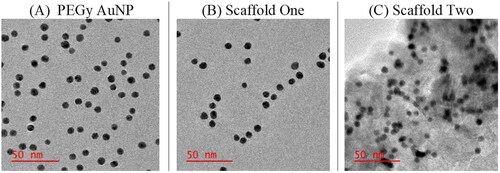
The HRTEM JEOL 2100 (JEOL Ltd.), using EDX spectroscopy for the detection of various elements by measurement of element signal and intensity, showed the presence of Au, C, and O as observed in . Note that the high intensity of Cu is due to the Cu grid itself and has no significance to the loaded material on the grid. Several Au peaks can be seen on the scale and these represent the AuNPs. The O and C elements are seen due to the PEG moiety and COOH groups on the surface of the functionalized AuNPs. In B, the presence of the PS on the scaffold was captured on the scale, and the Al and Cl from the AlPcS4Cl are prominently seen with varying intensities. Furthermore, in C, the addition of antibodies brought forth other elements found on the structure of the anti-E6 mAb and including Na, Mg, and Ca, probably present in the buffers used.
Figure 4. Energy dispersive X-ray spectroscopy analysis (EDX analysis) showing the presence of individual elements on (A) the pure PEGy AuNP, elements including Cu, Au, C, O, (B) the PEGy AuNP-AlPcS4Cl scaffold and (C) the PEGy AuNP-AlPcS4Cl-anti E6 scaffold, elements including Cu,Au, C, O, Al, Cl, S, Ca, C, K, Na, Mg. Elements are observed on the full scale from 0 to 12 keV with peaks of varying intensities.
Scaffold charge and stability
The molecular scaffold one was confirmed to have a net negative charge of −23 mV, and the molecular scaffold two was confirmed to have a net negative charge of −18 mV, at pH 7.4, 37 °C. Though the compounds do not have strong charges, they are stable at physiological pH and physiological temperatures. In storage conditions (2–8 °C in PBS, pH 7.4), the compounds retained negative charges of −29 mV and −25 mV, respectively. Hence, storage at refrigeration temperatures was recommended. These values, however, show that the scaffolds are weakly negative, hence, long-term storage should be done using buffers that would make the compounds more stable, e.g., low pH buffers. In this present study, the compounds were not stored for longer than three months from the day of scaffolding.
Scaffold bond confirmation
Bond characterization is summarized in and . In the brief, the molecular scaffolds on FT-IR spectra showed single bond stretches O–H, C–H, and C–C in the region 1500 to 500 cm−1, indicating the single bonds of the AlPcS4Cl, PEG, and mAbs in the respective samples. The wide O–H (3500 region) was also displayed due to the frequency of hydroxyl groups in the PS, though some degree of intensity is due to the O–H in the buffer used, (note that the samples used were hyper-concentrated samples in KBr). Molecular scaffold one containing only the PS and PEGy-AuNP showed a very similar pattern with O–H stretches in the 3500 region. Prominent C=O stretches (i.e., Carboxylic Acid C=O stretch of the PEG at 1780–1710 and the amide C=O stretch at 1690–1630 due to the binding of N–H at the O=C–N amide coupling bond). The coupling of mAbs onto the compound as seen in the molecular scaffold, shown in these prominent amide stretches and the C–H bending vibrations and scissoring in the 1450 region. Bending vibrations and scissoring may also be due to the ionic attraction effect of the amide bonds due to resonance, where the electron drawing effect of carbonyl group (C=O) adjacent to the N atom makes the N slightly positively charged, resulting in the formation of a strong ionic interaction between the positively charged N atom and the negatively charged O on the next molecule.
Figure 5. IR spectra showing the transmittance plotted against cycles per reciprocal centimetres. The red circle shows influence of amide (N–H) on the 3500 regions and the green ring shows prominent C–H bonds on the molecular scaffold two, indicating coupling of mAbs to the PEGy-AuNP by covalent linkages.
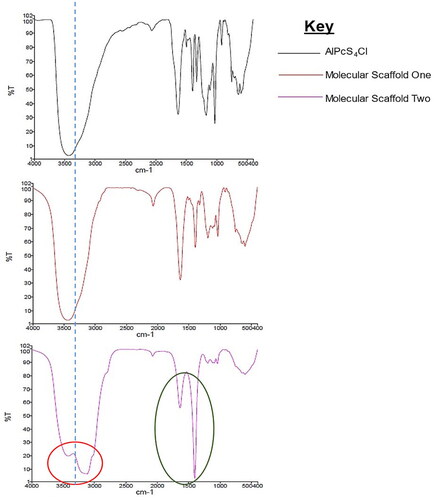
Table 2. A summary of the IR spectra of the PS and molecular scaffolds describing the stretching and bending properties of the compounds.
Molecular scaffold internalization on fluorescence imaging
Assessment of intercellular localization using fluorescence imaging indicated visible red autofluorescence of the PS on the molecular scaffold, confirming the uptake and localization of the compound intracellularly. The PS on the molecular scaffold showed red autofluorescence, as shown in [c] of . However, mitotracker staining showed that the scaffold did not localize in the mitochondria, as there was no colour merging of the green fluorescence and red autofluorescence of the PS. This is probably due to binding of the molecular scaffold to intracellular E6 oncoproteins. In the figure, however, it is demonstrated that the molecular scaffold was successfully internalized by the cells and sufficient fluorescence was detectable at the low PS concentration used.
Figure 6. Fluorescence imaging showing the red autofluorescence of PS bound onto the molecular scaffold. In (a) channel show DAPI stain only (nuclei), in (b) channel shows mitotracker green (mitochondria), in (c) the single channel of red autofluorescence of the PS is seen and in (d) the channels are merged to see all fluorescence as was detected. The merged image demonstrates that the molecular scaffold was successfully internalized by the cells [color online].
![Figure 6. Fluorescence imaging showing the red autofluorescence of PS bound onto the molecular scaffold. In (a) channel show DAPI stain only (nuclei), in (b) channel shows mitotracker green (mitochondria), in (c) the single channel of red autofluorescence of the PS is seen and in (d) the channels are merged to see all fluorescence as was detected. The merged image demonstrates that the molecular scaffold was successfully internalized by the cells [color online].](/cms/asset/0368ce9c-593a-4fbd-acb7-eb5999e44da1/ianb_a_2199037_f0006_c.jpg)
Morphological features post-PDT
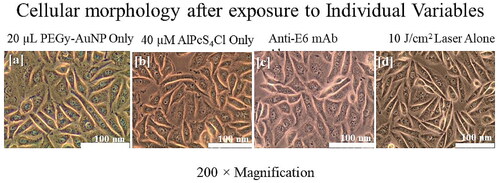
After treatment and irradiation, morphological assessment indicated progressing structural damage with increasing concentrations. Both scaffolds one and two were assessed alongside the nude PS. Incorporated control groups indicated that none of the single molecules alone had observable structural damage. As shown in , cells that were cultured in experimental groups but not treated, showed intact morphological features on microscopic examinations, denoting that there were no secondary effects, i.e., nuisance variables, that could influence the morphological appearance of cells. This also denotes that all changes in experimental groups are the result of treatment rather than extraneous factors. Similarly, cells that received either one of the compounds alone, ie PS only, PEGy-AuNPs, or mAbs only, did not show any alterations in morphology, indicating that neither of the compounds had significant damaging effects on the cells when administered separately and in the absence of laser irradiation. Likewise, cells that were treated with laser only did not show any significant changes in morphology, also confirming that laser only did not have any damaging effects on the cells. These observations demonstrate that all morphological alterations as will be seen further below, are the direct effect of the treatment and/or doses administered.
Figure 7. Control groups indicating unaltered morphology after the experimental period, in (a) cells treated with PEGy-AuNP only, (b) cells treated with PS only, (c) cells given mAbs only and, (d) cells that received irradiation only. All control groups showed normal morphology at the end of the experimental period.
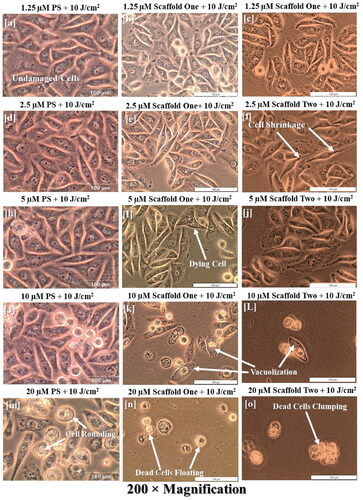
Cells treated with PS, molecular scaffolds one and two, and irradiated at 10 J/cm2 showed an increasing damaging effect by alteration of cell morphology and detachment. In , cell damage is starting to be observed at around 5 µM with very minimal changes and more noticeable changes at 10 and 20 µM of the nude PS. Looking at molecular scaffold one, however, there were substantial morphological alterations starting at 2.5 µM with some vacuolization and cell blebbing and very noticeable alteration at 5, 10 and 20 µM, with increasing damage proportionally. Important to note is the comparison between 10 and 20 µM of the nude PS with the extent of damage at 10 and 20 µM of the scaffolded material. 10 µM of the scaffold one had similar damage to 20 µM of the nude PS. Moreover, scaffold two had even more damage compared to scaffold one and nude PS. Starting from 10 µM of scaffold two, damage was so severe that the monolayer was completely disrupted.
Figure 8. Experimental groups treated with the molecular scaffold one at 10 J/cm2 indicate the structural damages induced by PDT, in which increasing damage in a dose dependent manner. In (a–e), no significant changes induced by therapy are seen. Starting from (f), signs of cell damage are seen by shrinkage of cells and elongation, which may represent the onset of apoptosis. (k = L) show marked cellular damage with widespread vacuolization, and (n–o) show ultimate cell demise by presence of floating cells, dead cell clumping and monolayer disappearance. Note, however, that comparing the increase in morphological damage per PS concentration should be read horizontally, e.g., j, k and l are the same PS concentrations on the different scaffolds, hence showing different outcomes indicating that the DDS system by using AuNP alone is more efficacious and using both AuNP and mAb is even more efficacious.
Cell proliferation post-PDT
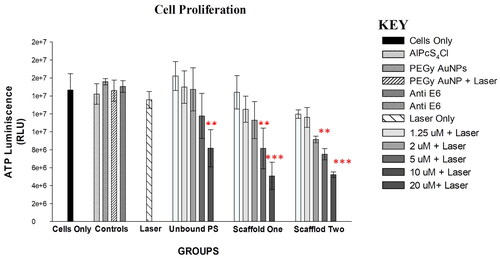
ATP measurements are good indicators of the extent of damage caused by PDT by indicating the concentration of ATP in the cells, which represents metabolic activity in the cells, hence the proliferation rate. The control groups, including the untreated cells and those that were treated with the individual compounds without irradiation, and the laser group treated with laser only showed high proliferation by measurement of ATP, indicating that none of the compounds alone (i.e., PS, AuNP, mAb, and Laser) has damaging effects. However, the PDT treated cells with nude PS, and both scaffolds showed decreasing proliferation in a dose dependent manner when compared with the untreated control cells. When the experimental groups were compared with each other, it was shown that the lower concentration of PS in the molecular scaffold one caused much more cytotoxicity than the pure PS alone at a higher concentration, and consequently scaffold two caused more damage than scaffold one at the same concentration. The increased effect in scaffold one is due to the increased PS load and enhanced uptake by the AuNP, and in scaffold two, more increase is attributed to the facilitated binding and accumulation by the mAb, as shown in .
Figure 9. Post-irradiation ATP Luminescent signal of cells indicating significant decrease in proliferation of PDT treated cells (p < .01 as ** and p < .001 as ***) after 24 h. Control cells and groups treated with either of the variables alone did not show significant decrease in cell proliferation.
Discussion
Here, intracellular deliverly of PS to specific cancer cells is demonstrated for the first time in PDT research. Former research has focused on extracellular surface maker recognition, unlike intracellular targeting, probably due to the widely accepted pedagogy that natural antibodies, due to their size and physicochemical characteristics, do not readily get internalized by live cells through membranous transport mechanisms. While this is true for most Abs, studies have shown the ability of intracellular localization of certain Abs, suggesting that certain natural or modified Abs can cross membranes and affect intracellular compartments [Citation10–12]. Moreover, the pathophysiology of other natural Abs like the antinuclear antibody (ANA), suggests a different pedagogy. ANAs in their natural form do cross physiological intact membranes, undergo transcytosis intracellularly and attack intranuclear DNA, RNA, and proteins to cause many diseases, including systemic lupus erythematosus (SLE), multiple sclerosis, arthritis, dermatomyositis, and many more [Citation13–15].
In this present investigation, a newly designed photoactive molecular scaffold to target intracellular E6 oncoproteins in HPV-transformed cancer cells was developed because controlling the PK of the PS by using carriers and targeting molecules is significant for enhancing PDT. A phthalocyanine PS and anti E6-mAbs were scaffolded onto a PEGylated AuNP core to enhance PDT. Conjugation of the three components resulted in chemical amide linkages, hydrogen bonding, and ionic interactions between the PS and mAbs on the PEG moiety of the AuNPs. The nature of the reactions in the conjugation process could predispose the PS and mAbs to conformational changes that could render them dysfunctional, but chemical characterization approved of the successful scaffolding of the compound with retained conformation and function of the single compounds, hence confirming the molecular scaffold retained photo-active properties and specific binding of the PS and mAb, respectively. The conjugation of the PS and mAb onto the PEGy-AuNP is not confined to covalent bonding only. The very nature of amide bonds involves ionic interaction due to resonance, where the electron drawing effect of the carbonyl group (C=O) adjacent to the N atom makes the N slightly positively charged to form a strong ionic attraction with the negatively charged O on the next molecule. Therefore, the amide linkages that were formed were strengthened by ionic bonds. Besides, primary and secondary amides contain dipole-dipole and hydrogen bonds. Eventually, this molecular scaffold is expected to have a more stable conformational structure due to the presence of major and minor bonds. PS is also attached by both hydrogen bonding and ionic interaction.
Two molecular scaffolds were developed, one comprising the PS bound to PEGy-AuNP and the other comprising of both the PS and anti E6 mAb bound to PEGy-AuNP. However, the PS concentration on the scaffolds was measured and used to represent the concentration of the whole compound in µM since it remains the main photoactive molecule even in the molecular scaffold. Moreover, it is easier to work with the measurable concentration of the PS for all inferences regarding the PDT responses. In the body, natural PSs take advantage of the enhanced permeability and retention (EPR) effect of tumour tissues due to dense vasculature, increased LDL receptors on cell membranes, and decreased lymphatic drainage [Citation16]. Similarly, AuNPs efficiently enter cancer cells partly due to the same and also due to surface charge mobilization and binding to the positively charged cell surfaces. Moreover, selective binding using mAbs or other targeting ligands has the potential to further increase the therapeutic effect. Though the use of mAbs has for many years been confined to the cell surface, here the main challenge was to develop a compound that would be internalized by the cancer cells and direct the PS to intracellular E6 oncoproteins.
Intracellular targeting using large molecules like Abs and other proteins, asintroduced earlier, has for many years been regarded as undoable because of the need for facilitated transport by the cells using energy, which results in the failure especially of normal physiological cells to take up larger molecules through their membrane. Natural Abs are mostly limited in this regard due to their large size and certainly other physiognomies. Hence, intracellular targeting using Abs has been considered unviable. However, as seen in this study and supported by others [Citation17,Citation18], intracellular targets so called “undruggables” are actually “druggable.” They just might be 'difficult to drug’ or 'yet to be drugged.’ In theirhe natural state, physiological macromolecules that are absolutely required for intracellular function have properties and/or conformations that enable them to pass through membranes. Abs designed for intracellular antigens (Ags) are internalized by varying active transport mechanisms involving endondocytic vesicles, such as endosomes or phagosomes [Citation19], receptor-mediated entry [Citation20], caveolae/raft-dependent endocytosis [Citation21], and/or clathrin-dependent endocytosis [Citation22]. These Abs are able to pass through membranes to localize inside the cells and also undergo transcytosis within the cells to move around the cytoplasm. Moreover, AuNP facilitates the cellular uptake of large molecules, including mAbs as demonstrated in HeLa cells, which internalized anti-mitochondrial mAbs using protein-facilitated cell penetration by endocytosis [Citation23].
Because the E6 oncoproteins are naturally intracellular antigens, intracellular targeting using anti-E6 mAbs was sought, and AuNPs were used to facilitate uptake of the molecular scaffold. Upon localization studies, the molecular scaffold was successfully internalized and localized in the intracellular compartments and was able to deliver PS to the cytoplasm. This mechanism is largely influenced by the physicochemical properties (i.e., size, shape, and net charge) of the scaffolds, their hydrophobicity, and of course the type of cell and its transport capacities [Citation24,Citation25]. Though negatively charged molecules are better suited for uptake due to their interaction with the positively charged cell surface due to the induction of some form of a biological evanescent field, a study by Bannunah et al., [Citation25] demonstrated that NPs with different net charges were internalized via cellular endocytic mechanisms, however, with varying potential. Involvement of the clathrin pathway was associated with positively charged NPs, while the lipid raft pathway was associated with negatively charged ones. Here, 10 nm PEGy-AuNPs carrying a load of AlPcS4Cl and an anti-E6 mAb were internalized by SiHa cells, and upon PDT, the effect was enhanced. This implies that the E6-expressing HPV-transformed SiHa cells were able to internalize this molecular scaffold and that transcytosis within the cell occurred uninterrupted.
Conclusion
In conclusion, a new multicomponent photoactive compound was explored for use in PDT of HPV-transformed cancer cells, in vitro. This newly designed molecular scaffold possessed features such as specific intracellular targeting of HPV-transformed cells, increased cellular uptake by enhancing cell membrane transfer and increased PS loading to the target cells. Future studies should seek to assess the usability of this scaffold in other HPV-transformed cell lines in comparison with other non-HPV cells. Additionally, though theoretically the uptake is attributed to energy-regulated endocytosis through endosomes, a focus on the exact mode of uptake using live imaging and high-resolution transmission electron microscopy should be considered. However, these results point to a viable alternative therapy that has been shown to be effective in vitro and due to the design of this compound, it is correct to make an inference of its potential effect in vivo for the total eradication of HPV-transformed lesions and cancer. Besides, PDT as mode of therapy is safer and more cost-effective, with minimal side effects when compared to conventional chemo and radiation therapy. Hence this approach would subsequently relegate the cancer burden in many ways.
Limitations of the study and future recommendations
The present study focused on the design and development of a nanoparticle, antibody, and phthalocyanine scaffold for intracellular delivery into HPV-transformed SiHa cells, in vitro. The study did not confirm the exact mechanism of entry, though energy-dependent endocytosis via endosomes is inferred. A follow-up study using advanced imaging techniques like Scanning Electron Microscopy (SEM) and immunofluorescence of quenchable probes to assess this phenomenon is required to fully describe the performance of this compound. Moreover, a study on other HPV-transformed cell lines and resistant subtypes is necessary to further explain the efficay of this compound. Long-term, in vivo studies to assess the stability of the compound and its pharmacokinetics in the body should be conducted to initiate translational research for possible clinical use.
Author contributions
EPC: Conception and design of the study, Laboratory work, acquisition of data, analysis and interpretation of data and drafting of the article. HA: Supervision of study, revision of content, editing, final approval of the version to be published and corresponding author.
Supplemental Material
Download MS Word (92.4 KB)Acknowledgements
The authors sincerely thank the staff and colleagues at the Laser Research Centre and Biomedical Sciences Department, University of Johannesburg and the National Laser Centre for their support in this project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the results are available in this article. Additional data are available from the corresponding author, upon reasonable request.
Additional information
Funding
References
- Crous A, Abrahamse H. Effective gold Nanoparticle-Antibody-Mediated drug delivery for photodynamic therapy of lung cancer stem cells. Int J Mol Sci. 2020;21(11):3742.
- Stuchinskaya T, Moreno M, Cook MJ, et al. Targeted photodynamic therapy of breast cancer cells using antibody-phthalocyanine-gold nanoparticle conjugates. Photochem Photobiol Sci. 2011;10(5):822–831.
- Hudson R, Carcenac M, Smith K, et al. The development and characterisation of porphyrin isothiocyanate monoclonal antibody conjugates for photoimmunotherapy. Br J Cancer. 2005;92(8):1442–1449.
- Malatesti N, Smith K, Savoie H, et al. Synthesis and in vitro investigation of cationic 5,15-diphenylporphyrin-monoclonal antibody conjugates as targeted photodynamic sensitisers. Int J Cancer. 2006;28:1561–1569.
- Amina SJ, Guo B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int J Nanomed. 2020;15:9823–9857.
- Di Pasqua AJ, Mishler RE, Ship Y-L, et al. Preparation of antibody-conjugated gold nanoparticles. Mater Lett. 2009;63(21):1876–1879.
- Jazayeri MH, Amani H, Pourfatollah AK, et al. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens Bio-Sens Res. 2016;9:17–22.
- Okyem S, Awotunde O, Ogunlusi T, et al. High-affinity points of interaction on antibody allow synthesis of stable and highly functional antibody–gold nanoparticle conjugates. Bioconjug Chem. 2021;32(8):1753–1762.
- Safavi-Sohi R, Maghari S, Raoufi M, et al. Bypassing protein corona issue on active targeting: zwitterionic coatings dictate specific interactions of targeting moieties and cell receptors. ACS Appl Mater Interfaces. 2016;8(35):22808–22818.
- Kim JS, Choi DK, Park SW, et al. Quantitative assessment of cellular uptake and cytosolic access of antibody in living cells by an enhanced split GFP complementation assay. Biochem Biophys Res Commun. 2015;467(4):771–777.
- Alarcón-Segovia D, Llorente L, Ruíz-Argüelles A. The penetration of autoantibodies into cells may induce tolerance to self by apoptosis of autoreactive lymphocytes and cause autoimmune disease by dysregulation and/or cell damage. J Autoimmun. 1996;9(2):295–300.
- Alarcon-Segovia D, Ruiz-Arguelles A, Llorente L. Antibody penetration into living cells. II. Anti-ribonucleoprotein IgG penetrates into T gamma lymphocytes causing their deletion and the abrogation of suppressor function. J Immunol. 1979;122(5):1855–1862.
- Noble PW, Bernatsky S, Clarke AE, et al. DNA-damaging autoantibodies and cancer: the lupus butterfly theory. Nat Rev Rheumatol. 2016;12(7):429–434.
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686.
- Douglas JNGL, Gardner LA, Levin MC. Antibodies to an intracellular antigen penetrate neuronal cells and cause deleterious effects. J Clin Cell Immunol. 2013;4:134.
- Nombona N, Antunes E, Chidawanyika W, et al. Synthesis, photophysics and photochemistry of phthalocyanine-polylysine conjugates in the presence of metal nanoparticles against Staphylococcus aureus. J Photochem Photobiol A. 2012;233:24–33.
- Zhang G, Zhang J, Gao Y, et al. Strategies for targeting undruggable targets. Expert Opin Drug Discov. 2022;17(1):55–69.
- Dang CV, Reddy EP, Shokat KM, et al. Drugging the ‘undruggable’ cancer targets. Nat Rev Cancer. 2017;17(8):502–508.
- Ghetie V, Ward ES. Transcytosis and catabolism of antibody. Immunol Res. 2002;25(2):97–113.
- Lisi S, Sisto M, Soleti R, et al. Fcgamma receptors mediate internalization of anti-Ro and anti-La autoantibodies from Sjögren’s syndrome and apoptosis in human salivary gland cell line A-253. J Oral Pathol Med. 2007;36(9):511–523.
- Jang JY, Jeong JG, Jun HR, et al. A nucleic acid-hydrolyzing antibody penetrates into cells via caveolae-mediated endocytosis, localizes in the cytosol and exhibits cytotoxicity. Cell Mol Life Sci. 2009;66(11-12):1985–1997.
- Choi DK, Bae J, Shin SM, et al. A general strategy for generating intact, full-length IgG antibodies that penetrate into the cytosol of living cells. mAbs. 2014;6(6):1402–1414.
- Lim YT, Cho MY, Lee JM, et al. Simultaneous intracellular delivery of targeting antibodies and functional nanoparticles with engineered protein G system. Biomaterials. 2009;30(6):1197–1204.
- Behzadi S, Serpooshan V, Tao W, et al. Cellular uptake of nanoparticles. Chem Soc Rev. 2017;46(14):4218–4244.
- Bannunah AZ, Vllasaliu D, Lord J, et al. Mechanisms of nanoparticle internalization and transport across an intestinal epithelial cell model: effect of size and surface charge. Mol Pharm. 2014;11(12):4363–4373.

