ABSTRACT
We aimed to explore the impact of sexual transmission modes on immune reconstitution after combined antiretroviral therapy (cART). We have retrospectively analyzed longitudinal samples from 1557 treated male patients with virological suppression (HIV-1 RNA < 50 copies/ml) for at least 2 years. Both heterosexuals (HET) and men who have sex with men (MSM) patients showed an increasing annual trend in CD4+ T cell counts after receiving cART (HET, β: 23.51 (cell/µl)/year, 95% CI: 16.70–30.31; MSM, β: 40.21 (cell/µl)/year, 95% CI: 35.82–44.61). However, the CD4+ T cell recovery rate was much lower in HET patients than MSM patients, determined by both the generalized additive mixed model (P < 0.001) and generalized estimating equations (P = 0.026). Besides HIV-1 subtypes, baseline CD4+ T cell counts and age at cART initiation, HET was an independent risk factor for immunological non-responders (adjusted OR: 1.73; 95% CI: 1.28–2.33). HET was also associated with lower probability of achieving conventional immune recovery (adjusted HR: 1.37; 95%CI: 1.22–1.67) and optimal immune recovery (adjusted HR: 1.48, 95%CI: 1.04-2.11). Male HET patients might have poorer immune reconstitution ability even after effective cART. Early initiation of cART after diagnosis and clinical monitoring for male HET patients should be highly emphasized.
Introduction
The epidemic of human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) remains a major public health issue in China. Sexual transmission is the major route of HIV-1 infection in China. In 2019, about 71,204 people were estimated to be newly infected with HIV/AIDS in China, among whom over 95% acquired HIV-1 through sexual exposure [Citation1]. Transmission among men who have sex with men (MSM) has become the main transmission route in China’s eastern and central provinces, accounting for 62.4% to 77.88% of newly reported cases in recent years. Meanwhile, heterosexual transmission (HET) accounted for 50% to 70% of newly reported cases [Citation1].
The modes of sexual transmission might exert differential impacts on HIV-1 disease progression and clinical outcomes of combined antiretroviral therapy (cART). A recent study found that HET patients had faster CD4+ T cell count reduction early in HIV infection [Citation2]. HET has also been reported to be associated with higher probability of virological failure and increased mortality after cART [Citation3–6]. HIV-1 patients receiving cART aim to suppress the virus and recover their immune system promptly. However, clinical outcomes in these studies were virologic failures, and how sexual transmission modes could affect immune reconstitution in those patients with virological suppression remains unclear. This study aimed to compare the immune reconstitution efficacy between HET men and MSM patients who have maintained virological suppression among HIV-1 patients newly diagnosed from 2017 to 2018 in Jiangsu Province, China.
Methods
Study subjects
We studied a cohort of newly diagnosed HIV-1 patients from 2017 to 2018 in Jiangsu Province. The subjects in this cohort tested positive by HIV enzyme-linked immunosorbent assay and confirmed by HIV-1 Western blot assay. All patients were cART-naïve when they were diagnosed with HIV-1 infection. Through face-to-face interviews, we obtained data on demographic information, including age, marital status, educational background, infection route and history of sexually transmitted diseases (STD). The history of STD indicated whether the patients had ever contracted the STD before cART. Based on the national database of free antiretroviral treatment program, we collected the clinical information including time of receiving cART, CD4+ T cell counts and CD8+ T cell counts at cART initiation, baseline BMI and hemoglobin at cART initiation, CD4 + T cell counts and CD8+ T cell counts as well as viral load at all follow-up visits. The time point of the last follow-up was 2021. This national database was established in late 2004 under the China’s National Free Antiretroviral Treatment Program [Citation7–9]. We searched the plasma samples during 2017–2018 in the plasma biobank of Jiangsu Provincial Center for Diseases Control and Prevention. We successfully tested for the baseline viral load in 782 patients with the COBAS TaqMan HIV-1 Test v2.0. The time point of the last follow-up was 2021. The patients were selected in our analysis if they met the following criteria: (1) male subjects with HIV-1 subtypes data; (2) infected with HIV-1 through MSM or HET; (3) have received cART for at least 2 years; (4) complete follow-up CD4 + T measurements records after cART; (5) maintaining virological suppression (HIV-1 RNA < 50 copies/ml) at all follow-up visits. The flowchart of sample selection was shown in Figure S1. This study was approved by the ethical review board of the National Center for AIDS/STD Control and Prevention (Project No. X140617334). All participants provided informed consent.
HIV-1 sub-typing
We extracted HIV-1 RNA from 200 µl plasma samples and then reverse-transcribed to cDNA according to the manufacturer’s instructions. RT–PCR and nested PCR were carried out to generate the partial pol region (HXB2: 2,253-3,312). The amplified pol region with the correct position of the band in gel electrophoresis was sequenced as previously described [Citation10]. Afterward, we downloaded the pol reference sequence of each HIV-1 genotype from the Los Alamos National Laboratory HIV-1 database (LANL, https://www.hiv.lanl.gov/) and constructed a maximum likelihood (ML) phylogenetic tree to determine the HIV-1 genotype using FastTree v2.1.10. We also employed Recombination Identification Program (RIP) and Jumping Profile HMM (jpHMM) online tools to identify the recombinant strains.
Definitions
We evaluated the immune reconstitution efficacy by using three metrics: the recovery of CD4+ T cell counts, the risk of immunological non-responders (INRs) and the probabilities of reaching immune recovery (IR).
In order to assess the CD4+ T cell recovery rate, measurement records of all follow -up visits after cART initiation for each patient were obtained. All patients had at least two CD4+ T cell count records in different years.
INRs in this study referred to patients who maintained plasma HIV RNA <50 copies/ml but had total CD4+ T cell counts ≤ 350 cells/μL two years after cART initiation. Immunological responders were patients with both plasma HIV RNA < 50 copies/ml and total CD4+ T cell count ≥ 350 cells/μL two years after cART initiation [Citation11,Citation12].
The IR in this study comprised of both conventional and optimal IR. The conventional IR was defined as two successive CD4 + T cell counts ≥ 500 cells/μL after cART initiation [Citation13], and the optimal IR was defined as achieving CD4+ T cell counts ≥ 500 cells/μL and CD4/CD8 ratio ≥ 0.8 concurrently [Citation14,Citation15].
Statistical analysis
The baseline demographic and clinical characteristics were analyzed using the chi-square or Fisher exact tests. We adopted the generalized additive mixed model (GAMM) to estimate the CD4+ T cell recovery rate and fit the smooth curve of CD4+ T cell change after cART initiation. The factors influencing CD4+ T cell recovery were further analyzed with generalized estimating equations (GEE). The risk of INRs was analyzed using univariable and multivariable logistic regressions. The Kaplan-Meier analysis and log-rank tests were used to compare the probability of achieving IR between HET and MSM patients. The multivariable Cox regression model was performed to examine the impact of sexual transmission modes on IR. Propensity score matching (PSM) was a commonly used statistical matching method that reduced biases from unbalanced confounding factors. We adopted the method of 1:1 ratio to match several important variables at cART initiation between HET and MSM patients, including HIV-1 subtypes, age, baseline CD4+ T cell counts and baseline CD8+ T cell counts. Finally, to further control the impact of baseline viral load on immune reconstitution, we conducted a sensitivity analysis that repeated all analyses used in our study among patients with baseline viral load. All statistical analyses were carried out by SPSS (version 23.0) and R software (version 4.0.4). The level of significance was set at 0.05.
Results
Baseline characteristics of the study population
A total of 1557 patients (MSM: 1128, HET: 429) met the eligibility criteria and were included in the analyses (Figure S1). The baseline characteristics were summarized in . The main HIV-1 subtypes identified were CRF_01AE (607/1557, 38.99%) and CRF_07BC (529/1557, 33.98%). The other subtypes included CRF67_01B (104/1557, 6.68%), CRF08_BC (64/1557, 4.11%), B subtype (61/1557, 3.92%), CRF55_01B (54/1557, 3.47%), CRF68_01B (51/1557, 3.28%) and others (87/1557, 5.59%) (Figure S2). At the time of cART initiation, most patients were under 50 years old (1234/1557, 79.26%) and 55.11% had baseline CD4+ T cell counts over 300 cells/µl. Over half of the patients were unmarried (911/1557, 58.5%), and had less education than Junior college (1018/1557, 65.4%). A small proportion of patients had ever contracted a sexually transmitted disease (STD) (265/1557, 17%). The majority of patients received the NNRTIs-based regimen at cART initiation. Most patients were underweight or normal weight (1336/1557, 85.81%), and had normal hemoglobin (120∼160g/L) (1121/1557, 72.00%). The CD4+ T cell counts and age at cART initiation, HIV-1 subtypes, educational background, marital status, and baseline hemoglobin differed significantly between HET and MSM patients (). MSM patients have a higher proportion of CRF_01AE infection than HET patients, while HET patients were older and had lower CD4+ T cell counts when starting cART than MSM par. In addition, most HET patients were married and had a lower education level than MSM patients. The proportion of normal hemoglobin (120∼160g/L) in MSM patients was higher than that in HET patients. The mean time from HIV-1 diagnosis to cART initiation in HET and MSM patients was 74.39 and 75.51 days, respectively (HET vs MSM, P value = 0.88).
Table 1. Demographic and clinical characteristics between HET and MSM patients.
Slower recovery of CD4+ T cell counts after cART initiation in HET patients
We used GAMM to evaluate the recovery rate of CD4+ T cell counts after cART. In general, both HET and MSM patients showed an increasing annual trend in CD4+ T cell counts after receiving cART (HET, β: 23.51 (cell/µl)/year, 95% CI: 16.70–30.31; MSM, β: 40.21 (cell/µl)/year, 95% CI: 35.82–44.61) (). After adjusting for the potential effects of other variables, the CD4+ T cell counts recovery rate was significantly slower in HET patients than in MSM patients (interaction P < 0.001; and ). GEE analysis showed that slower CD4+ T cells recovery was associated with HET (P = 0.026), lower baseline CD4+ T cell counts at cART initiation (P < 0.001), older age at cART initiation (P < 0.001) and lower baseline hemoglobin at cART initiation (P = 0.005) (). Given the important impact of CD4+ T cell counts at cART initiation on immune reconstitution, the additional analyses for patients with lower baseline CD4+ T cell counts were conducted. In patients with baseline CD4+ T cell counts ≤ 300 cells/μL, HET patients similarly showed slower CD4+ T cell counts increase than MSM patients determined by both GAMM and GEE analyses ( and ).
Figure 1. The smooth curve of CD4+ T cell counts recovery over time after receiving cART between HET and MSM patients. (A) Comparison of the increase rate of CD4+ T cell counts between HET and MSM group in total patients (interaction P < 0.001, by generalized additive mixed model). (B) Comparison of the increase rate of CD4+ T cell counts between HET and MSM group in the patients with baseline CD4+ T cell counts ≤ 300 cells/μL (interaction P = 0.008, by generalized additive mixed model).
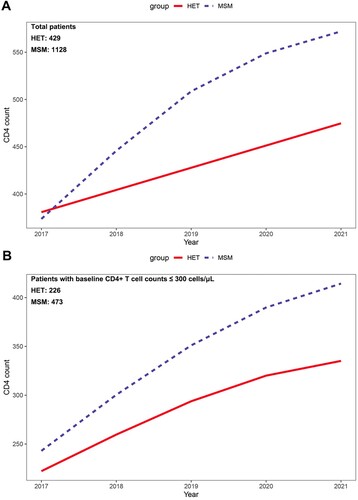
Table 2. The analysis of annual recovery rate of CD4+ T cell count using GAMM.
Table 3. Factors associated with CD4+ T cell count recovery during cART identified by GEE.
Higher risk of immunological non-responders in HET patients
Despite persistent virological suppression, INRs, presenting incomplete immune reconstitution, were at increased risk of morbidity and mortality of AIDS and non-AIDS events [Citation16–18]. As shown in , HET patients were more likely to progress to INRs after treatment than MSM patients (adjusted aOR = 1.73, 95%CI = 1.28∼2.33, P < 0.001). The baseline clinical characteristics (HIV-1 subtypes, baseline CD4+ T cell counts and age) significantly differed between HET and MSM patients. Thus we performed the propensity score matching (PSM) analysis to further control the confounding effect of these variables. There was no difference in these variables between HET and MSM patients after 1:1 matching (Table S1). The HET was still associated with higher risk of INRs (adjusted aOR = 2.45, 95%CI = 1.71∼3.50, P < 0.001), consistent with the results of the all patients (Table S2).
Table 4. Factors associated with risk of immunological non-responders.
Lower probabilities of immune recovery after cART initiation in HET patients
The time from cART initiation to the achievement of conventional IR was compared between HET and MSM patients. The median time of reaching conventional IR was longer in HET patients (42.0 months) than in MSM patients (33.5 months) ((A)).
Figure 2. The impact of sexual transmission modes on conventional and optimal immune recovery (IR). (A) The cumulative probability of achieving conventional IR (two successive CD4+ T cell counts more than 500 cells/µl). (B) The factors associated with conventional IR identified by multivariate Cox regression analysis. (C) The cumulative probability of achieving optimal IR (CD4 ≥ 500 cells/μL and CD4/CD8 ratio ≥ 0.8 concurrently). (D) The factors associated with optimal IR identified by multivariate Cox regression analysis.
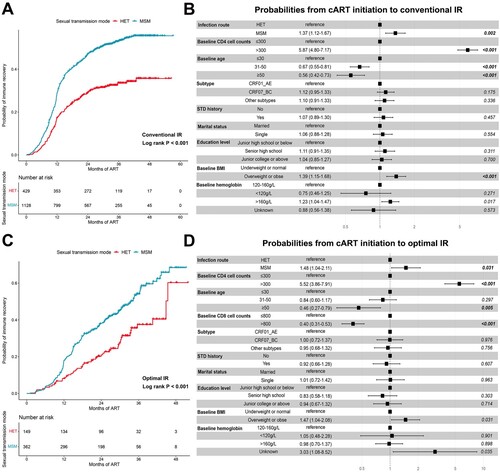
After adjusting for potential confounders, the progression of conventional IR remained slower in HET patients (MSM vs. HET, aHR: 1.37, 95%CI: 1.12-1.67) than in MSM patients ((B)). The PSM analysis was also conducted to match the baseline clinical characteristic (Table S1). Similarly, HET patients had lower probabilities of conventional IR than MSM patients (MSM vs HET, aHR: 4.15, 95%CI: 3.29-5.23) (Figure S3A–B).
The probabilities of achieving optimal IR in HET and MSM patients were also assessed. Out of 1557 male sexually transmitted infections, 511 with complete records of CD8+ T cell counts at all follow-up visits were included. As shown in (C), HET patients exhibited longer median time from cART initiation to optimal IR than MSM patients (HET: 38.7 months; MSM: 32.0 months; Log rank P value < 0.001). The multivariate Cox model analysis indicated that the progression of optimal IR in HET patients was relatively slower than in MSM patients (MSM vs HET, aHR: 1.48, 95%CI: 1.04-2.11) ((D)). In the model with PSM, four variables, including HIV-1 subtypes, baseline CD4+ T and CD8+ T cell counts, and age, were properly matched. The two groups had no difference in all matching variables (Table S1). After PSM analysis, HET was still associated with slower progression of optimal IR (MSM vs HET, aHR: 1.87, 95%CI: 1.25-2.81) (Figure S3C–D).
Subgroup analysis
As the patients with high baseline CD4 + T cell counts might not develop to INRs or experience failure to conventional IR and optimal IR, those patients might introduce biases in our results. We conducted a subgroup analysis to control the biases. Currently, there is no consensus on the definition of INRs. The failure to meet the prescribed CD4+ T cell count thresholds of 300, 350 and 500 cells/μL were the common definitions of INRs [Citation16]. In evaluating the risk of INRs and the probability of reaching IR, we consequently stratified the patients by the baseline CD4+ T cell counts at the cutoff of 300, 350 and 500 cells/μL respectively. HET patients had higher risk of INRs and lower probability of conventional IR than MSM patients among patients with lower baseline CD4+ T cell counts (≤300 cells/μL, ≤ 350 cells/μL, and ≤500 cells/μL) (Tables S3 and S4). However, no significant difference in risk of INRs and probability of conventional IR was observed between HET and MSM patients among patients with higher baseline CD4+ T cell counts (>300 cells/μL, > 350 cells/μL, and >500 cells/μL) after adjusting other confounding factors (Tables S3 and S4). Patients were divided into two subgroups according to the baseline CD4+ T cell counts and baseline CD4/CD8 ratio in evaluating the probability of reaching optimal IR. In both two subgroups, HET showed the lower probability of achieving optimal IR (Table S5). In summary, these data suggested that for patients with lower baseline CD4+ T cell counts, HET might have unfavourable immune reconstitution efficacy than MSM patients.
Sensitivity analysis
The baseline plasma viral load at cART initiation was an important indicator of immune reconstitution. We continued to analyze the impact of sexual transmission modes on immune reconstitution in 782 patients with baseline viral load to control the influence of this variable.
The trend of CD4+ T cell counts change after receiving cART in total patients was shown in Table S6 and Figure S4. The CD4+ T cell counts increased continuously in both HET and MSM patients (HET, β: 23.91 (cell/µl)/year, 95% CI: 13.72∼34.10; MSM, β: 38.08 (cell/µl)/year, 95% CI: 31.02∼45.13). However, the increasing rate in HET patients appeared much slower than in MSM patients (interaction P = 0.029). GEE analysis also support the result above that HET was related to poorer CD4+ T cell counts recovery during the treatment (P = 0.009) (Table S7). In patients with lower baseline CD4+ T cell counts, MSM had higher recovery rate of CD4+ T cell counts than HET patients two years after cART (interaction P = 0.031) (Table S6 and Figure S4).
Predictors associated with the risk of INRs were listed in Table S8. Besides lower baseline CD4+ T cell counts, older age, and higher baseline viral load, HET was significantly associated with higher risk of INRs than MSM (adjusted aOR = 1.92, 95%CI = 1.27∼2.89, P = 0.002). The progression of reaching conventional IR and optimal IR between HET and MSM patients were shown in Figure S5. In the multivariable analysis, HET indicated slower progression of conventional IR and optimal IR than MSM (conventional IR, aHR = 1.46, 95%CI = 1.01∼1.95, P = 0.010; optimal IR, aHR = 2.18, 95%CI = 1.18∼4.01, P = 0.012). The PSM analysis was then performed to further control the biases from other confounding variables. These variables were factors that could affect immune reconstitution efficacy besides sexual transmission modes (Table S8, Figure S5). Similar results were found after performing PSM analysis (Tables S9–S10 and Figure S6).
The subgroup analyses were also carried out in patients with baseline viral load. The association between HET and worse immune reconstitution efficacy was observed in patients with lower baseline CD4+ T cell counts (Tables S11–S13). Furthermore, we have made another subgroup analyses based on baseline viral load at the threshold of 105 copies/mL (Tables S14–S16). The results did not significantly change after further hierarchical analyses of baseline viral load when comparing the risk of INRs and the progression of conventional IR between HET and MSM patients. Comparatively, the progression of optimal IR between the two groups from cART initiation to optimal IR was significantly delayed in only HET patients with lower baseline viral load.
Discussion
In this study, we comprehensively analyzed the impact of sexual transmission modes on immune reconstitution dynamics during cART among 1557 male patients who have achieved virological suppression. Our study firstly revealed that immune reconstitution efficacy after receiving cART in male HET patients was poorer than in MSM patients.
One possible explanation for our results might be that HET men were more likely to be diagnosed with HIV infection later than MSM in China. MSM have more opportunities to get HIV testing than HET men due to various encouragement strategies among HIV high-risk groups. Besides, HET men also tend to perceive themselves, or be perceived by healthcare providers, to have low risk of infecting HIV. The overrepresentation of HIV late presentation in HET men has been reported in numerous studies in China [Citation19,Citation20]. A recent study suggested that despite the effectiveness of cART in terms of viral suppression, HIV late presenters did not experience complete CD4 + T cell counts recovery and CD4/CD8 normalization [Citation21], which could partly explain our findings. Another is that the extent of injury to the immune system prior to cART initiation caused by the fitness of transmitted founder (TF) viruses might be more severe in male HET patients than MSM patients. The HET transmission from women to men imposed higher selection pressure on TF virus than MSM transmission [Citation22,Citation23], leading to high fitness variants emerging for successful dissemination [Citation24,Citation25]. James et al. compared the impact of TF virus fitness on disease progression between HET and MSM at a large population level, namely that HET patients had greater CD4+ T cell count reduction in early infection than MSM patients [Citation2]. In this study, we have estimated CD4+ T cell counts at seroconversion from measurements at diagnosis by a CD4 depletion model to reflect the pathogenicity of TF strains [Citation2,Citation26]. We found that HET patients had significantly lower estimated CD4+ T cell counts at seroconversion than MSM patients (Figure S7). Thus, our results might be due in part to the different initial immune damage caused by TF viruses between male HET and MSM patients.
In addition to the sexual transmission modes, other variables, including baseline CD4 + T cell counts, age, baseline viral load, HIV-1 subtypes and baseline CD8 + T cell counts, could also affect the immune reconstitution in our study. It is well established that baseline CD4 + T cell count was the strongest predictor of virological and immunological response after initiating cART [Citation27,Citation28]. In our subgroup analyses, we found that HET correlated with unfavourable immune reconstitution efficacy in only patients with lower baseline CD4+ T cell counts. These results suggested that the influence of baseline CD4+ T cell counts on immune reconstitution might be greater than sexual transmission modes. Age and baseline viral load were two other variables extensively studied in cART efficacy. Younger patients tended to have less impairment of immune system, thereby being more likely to have better treatment outcomes [Citation29]. The roles of baseline viral load in immune reconstruction have been previously investigated in many retrospective studies but yielded contradictory results [Citation30–32]. We found the association of high baseline viral load with increased risk of INRs, which supported the adverse impact of high baseline viral load on the immune reconstruction. Furthermore, a follow-up study in 21 HIV infected subjects followed to viral suppression and observed for 52 weeks of sustained suppression also showed that individuals with lower baseline viral load could achieve greater level of innate effector cell reconstitution [Citation33]. HIV genetic diversity could contribute to variations in virus pathogenicity. Our results discovered that patients with CRF_01AE had higher risk of INRs than patients with CRF_07BC. Other studies based on the Asian population also verified the higher pathogenicity of CRF_01AE strains than CRF_07BC strains, which caused faster disease progression, higher mortality and worse immune reconstruction ability [Citation10,Citation34,Citation35]. A growing body of evidence has proved the value of the CD4:CD8 ratio as a novel immune parameter combined with CD4 + T cell counts to assess immune restoration [Citation14,Citation15,Citation36]. Concurrent achieving CD4 ≥ 500 cells/μL and CD4:CD8 ratio ≥0.8 was thought as the optimal immune recovery. In both our analyses and that by Lee et al, low pre-treatment CD8+ T cell count was an important predictor of optimal immune recovery [Citation14], which implied the significance of continuously monitoring the trajectory of CD8+ T cell counts after cART.
Adherence to cART was widely acknowledged to be critical in improving treatment efficacy. Male HET patients might be particularly vulnerable to depressive symptoms and feelings of guilt and shame compared to MSM patients, resulting in poor adherence and treatment outcomes [Citation37–39]. In this study, we only included the male patients who maintained virological suppression at all follow-up visits. It could somewhat control for the confounding effect of adherence. Furthermore, we excluded the female HET patients in our study, as viruses transmitted from men to women were characterized by lower predicted fitness than from women to men [Citation23]. The sex difference in immune responses after HIV-1 infection might also introduce biases when comparing the efficacy of immune reconstitution between HET and MSM [Citation40,Citation41]. We found a significant difference in baseline clinical characteristics at cART initiation including CD4+ T cell counts, age, HIV-1 subtypes and CD8+ T cell counts. To avoid potential biases caused by these factors, the analyses were performed using the multivariate model and propensity score matching. Our results that sexual transmission modes could affect the effectiveness of immune reconstitution remained salient.
Several limitations should be noted in this study. First, we only found HET associated with poorer immune reconstitution efficacy without elucidating the biological mechanisms underlying the observations. Although we found lower estimated CD4+ T cell counts at seroconversion in HET patients than in MSM patients, it was still necessary to further explore experimentally whether the different TF virus’ fitness between HET and MSM could influence cART efficacy. Second, the lack of remaining viral load data has to some extent affected our exploration of the relationship between viral load and immune reconstitution, although the sensitivity analysis also supported our conclusion. Further studies including this variable are required to expand these findings. Finally, the sexual transmission modes were self-reported by the participants. Since MSM patients might conceal their gender identity due to social stigma and discrimination, there were inevitable limitations in terms of information accuracy. We adopted the face to face interview approach to acquire as much real information as possible.
Conclusion
In summary, HET was an independent risk factor associated with slower CD4+ T cell counts recovery ability, higher risk of incomplete immune reconstitution, and longer time required to achieve immune recovery. Our study highlighted the importance of early initiation of cART after HIV-1 diagnosis and enhanced post-treatment clinical monitoring for male HET patients.
Supplemental Material
Download MS Word (3 MB)Supplemental Material
Download MS Excel (90.6 KB)Acknowledgements
The authors thank the study participants and local implementing partners of various regional CDCs in Jiangsu province.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data in this study are available upon request by contact with the corresponding author.
Additional information
Funding
References
- Xu JJ, Han MJ, Jiang YJ, et al. Prevention and control of HIV/AIDS in China: lessons from the past three decades. Chin Med J. 2021;134(23):2799–2809.
- James A, Dixit NM. Transmitted HIV-1 is more virulent in heterosexual individuals than men-who-have-sex-with-men. PLoS Pathog. 2022;18(3):e1010319.
- Cesar C, Jenkins CA, Shepherd BE, et al. Incidence of virological failure and major regimen change of initial combination antiretroviral therapy in the Latin America and the Caribbean: an observational cohort study. Lancet HIV. 2015;2(11):e492–e500.
- Torrecilla García E, Yebra Sanz G, Llácer-Delicado T, et al. Clinical, epidemiological and treatment failure data among HIV-1 non-B-infected patients in the Spanish AIDS Research Network Cohort. Enferm Infecc Microbiol Clin. 2016;34(6):353–360.
- Yuan D, Liu M, Jia P, et al. Prevalence and determinants of virological failure, genetic diversity and drug resistance among people living with HIV in a minority area in China: a population-based study. BMC Infect Dis. 2020;20(1):443.
- Carriquiry G, Fink V, Koethe JR, et al. Mortality and loss to follow-up among HIV-infected persons on long-term antiretroviral therapy in Latin America and the Caribbean. J Int AIDS Soc. 2015;18(1):20016.
- Zhao DC, Wen Y, Ma Y, et al. Expansion of China's free antiretroviral treatment program. Chin Med J. 2012;125(19):3514–3521.
- Zhang F, Dou Z, Ma Y, et al. Five-year outcomes of the China national free antiretroviral treatment program. Ann Intern Med. 2009;151(4):42.
- Zhang F, Haberer JE, Wang Y, et al. The Chinese free antiretroviral treatment program: challenges and responses. Aids. 2007;21(Suppl. 8):S143–S148.
- Ge Y, Liu Y, Fu G, et al. The molecular epidemiological and immunological characteristics of HIV-1 CRF01_AE/B recombinants in Nanjing, China. Front Microbiol. 2022;13:936502.
- Negredo E, Massanella M, Puig J, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: clinical implications. Clin Infect Dis. 2010;50(9):1300–1308.
- Tincati C, Merlini E, Braidotti P, et al. Impaired gut junctional complexes feature late-treated individuals with suboptimal CD4+ T-cell recovery upon virologically suppressive combination antiretroviral therapy. Aids. 2016;30(7):991–1003.
- Ge Z, Feng Y, Li K, et al. Crf01_AE and CRF01_AE cluster 4 are associated with poor immune recovery in Chinese patients under combination antiretroviral therapy. Clin Infect Dis. 2021;72(10):1799–1809.
- Lee SS, Wong NS, Wong BCK, et al. Combining CD4 recovery and CD4: CD8 ratio restoration as an indicator for evaluating the outcome of continued antiretroviral therapy: an observational cohort study. BMJ Open. 2017;7(9):e016886.
- Liu J, Wang L, Hou Y, et al. Immune restoration in HIV-1-infected patients after 12 years of antiretroviral therapy: a real-world observational study. Emerg Microbes Infect. 2020;9(1):2550–2561.
- Yang X, Su B, Zhang X, et al. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J Leukoc Biol. 2020;107(4):597–612.
- Nakanjako D, Kiragga AN, Musick BS, et al. Frequency and impact of suboptimal immune recovery on first-line antiretroviral therapy within the International Epidemiologic Databases to Evaluate AIDS in East Africa. Aids. 2016;30(12):1913–1922.
- van Lelyveld SF, Gras L, Kesselring A, et al. Long-term complications in patients with poor immunological recovery despite virological successful HAART in Dutch ATHENA cohort. Aids. 2012;26(4):465–474.
- Hu X, Liang B, Zhou C, et al. HIV late presentation and advanced HIV disease among patients with newly diagnosed HIV/AIDS in Southwestern China: a large-scale cross-sectional study. AIDS Res Ther. 2019;16(1):6.
- Sun C, Li J, Liu X, et al. HIV/AIDS late presentation and its associated factors in China from 2010 to 2020: a systematic review and meta-analysis. AIDS Res Ther. 2021;18(1):96.
- Rava M, Bisbal O, Domínguez-Domínguez L, et al. Late presentation for HIV impairs immunological but not virological response to antiretroviral treatment. Aids. 2021;35(8):1283–1293.
- Tully DC, Ogilvie CB, Batorsky RE, et al. Differences in the selection bottleneck between modes of sexual transmission influence the genetic composition of the HIV-1 founder virus. PLoS Pathog. 2016;12(5):e1005619.
- Carlson JM, Schaefer M, Monaco DC, et al. Selection bias at the heterosexual HIV-1 transmission bottleneck. Science. 2014;345(6193):1254031.
- Theys K, Libin P, Pineda-Peña AC, et al. The impact of HIV-1 within-host evolution on transmission dynamics. Curr Opin Virol. 2018;28:92–101.
- Nijmeijer BM, Geijtenbeek TBH. Negative and positive selection pressure during sexual transmission of transmitted founder HIV-1. Front Immunol. 2019;10:1599.
- Song R, Hall HI, Green TA, et al. Using CD4 data to estimate HIV incidence, prevalence, and percent of undiagnosed infections in the United States. J Acquir Immune Defic Syndr. 2017;74(1):3–9.
- Gazzola L, Tincati C, Bellistrì GM, et al. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis. 2009;48(3):328–337.
- Roul H, Mary-Krause M, Ghosn J, et al. CD4+ cell count recovery after combined antiretroviral therapy in the modern combined antiretroviral therapy era. Aids. 2018;32(17):2605–2614.
- Chen J, Titanji K, Sheth AN, et al. The effect of age on CD4+ T-cell recovery in HIV-suppressed adult participants: a sub-study from AIDS Clinical Trial Group (ACTG) A5321 and the Bone Loss and Immune Reconstitution (BLIR) study. Immun Ageing. 2022;19(1):4.
- Bayarsaikhan S, Jagdagsuren D, Gunchin B, et al. Survival, CD4 T lymphocyte count recovery and immune reconstitution pattern during the first-line combination antiretroviral therapy in patients with HIV-1 infection in Mongolia. PLoS One. 2021;16(3):e0247929.
- Kroeze S, Ondoa P, Kityo CM, et al. Suboptimal immune recovery during antiretroviral therapy with sustained HIV suppression in sub-Saharan Africa. Aids. 2018;32(8):1043–1051.
- Lembas A, Załęski A, Mikuła T, et al. Evaluation of clinical biomarkers related to CD4 recovery in HIV-infected patients-5-year observation. Viruses. 2022;14:10.
- Chehimi J, Azzoni L, Farabaugh M, et al. Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIV-1-infected subjects undergoing antiretroviral treatment. J Immunol. 2007;179(4):2642–2650.
- Li X, Xue Y, Zhou L, et al. Evidence that HIV-1 CRF01_AE Is associated with Low CD4+T cell count and CXCR4 Co-receptor usage in recently infected young Men Who have Sex with Men (MSM) in Shanghai, China. PLoS One. 2014;9(2):e89462.
- Ye J, Chen J, Wang J, et al. CRF07_BC is associated with slow HIV disease progression in Chinese patients. Sci Rep. 2022;12(1):3773.
- Raffi F, Le Moing V, Assuied A, et al. Failure to achieve immunological recovery in HIV-infected patients with clinical and virological success after 10 years of combined ART: role of treatment course. J Antimicrob Chemother. 2017;72(1):240–245.
- Weijsenfeld AM, Blokhuis C, Stuiver MM, et al. Longitudinal virological outcomes and factors associated with virological failure in behaviorally HIV-infected young adults on combination antiretroviral treatment in The Netherlands, 2000 to 2015. Medicine. 2019;98(32):e16357.
- Remien RH, Dolezal C, Wagner GJ, et al. The association between poor antiretroviral adherence and unsafe sex: differences by gender and sexual orientation and implications for scale-up of treatment as prevention. AIDS Behav. 2014;18(8):1541–1547.
- McMahon JM, Braksmajer A, Zhang C, et al. Syndemic factors associated with adherence to antiretroviral therapy among HIV-positive adult heterosexual men. AIDS Res Ther. 2019;16(1):32.
- El-Badry E, Macharia G, Claiborne D, et al. Better viral control despite higher CD4(+) T cell activation during acute HIV-1 infection in Zambian women Is linked to the Sex hormone estradiol. J Virol. 2020;94:16.
- Mathad JS, Gupte N, Balagopal A, et al. Sex-related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. J Acquir Immune Defic Syndr. 2016;73(2):123–129.

