?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The current research studied the nutritional and functional properties and antioxidant profile of Cucumis melo and Citrullus lanatus seeds. Results showed that the protein, fat, and fiber content of C. melo seed powder were significantly (p < 0.05) higher than C. lanatus. Furthermore, results of mineral profile showed that Citrullus lanatus seeds have 236.7 ± 4.4 mg/100 g potassium, 98.6 ± 0.3 mg/100 g sodium, 25.0 ± 0.03 mg/100 g magnesium, and 30.8 ± 0.04 mg/100 g calcium while Cucumis melo seeds showed 309.1 ± 5.3 mg/100 g potassium, 61.5 ± 0.2 mg/100 g sodium, 57.8 ± 0.15 mg/100 g magnesium, and 53.1 ± 0.07 mg/100 g calcium. Moreover, amino acids (valine (4.14), leucine (6.11), isoleucine (5.32), cysteine (1.43), lysine (2.93), glycine (6.23), alanine (3.24), and aspartic acid (6.87) of C. melo seeds were higher as compared to C. lanatus seeds. Total phenolic and total flavonoids contents of Cucumis melo were significantly (p < 0.05) higher than Citrullus lanatus. Results regarding the DPPH and FRAP of Cucumis melo were comparatively higher than Citrullus lanatus seeds powder due to higher phenolic contents. Further, water holding capacity of Cucumis melo seeds powder was 2.78 g H2O/g as compared to Cucumis melo (2.52 g H2O/g) and Cucumis melo seed powder showed a higher oil retention capacity 1.88 g oil/g as compared to Citrullus lanatus seeds powder 1.80 g oil/g. Conclusively, the outcome from comparative study presented C. melo as valuable and superior source of essential nutrients along with bioactive components which enhance their technological properties. Incorporation of C. melo and C. lanatus is highly recommended as a sustainable source of agro-industrial waste to achieve the nutritional and technological properties in the end product.
1. Introduction
Food waste conversion is gaining popularity due to value-added materials, and its increasing demand for natural bioactive components. Agro-waste is a sustainable source of functional components and preparation of designer and functional foods (Saeed et al., Citation2021). During the processing of fruits and vegetables, a huge amount of waste materials like peels, seeds, stones, and flesh are produced, which are a source of essential nutrients and valuable biomass with disposal and pollution problems (Rolim et al., Citation2020). Owing to their excellent nutritional profile and antioxidant properties, such agro-waste is used in the pharmaceutical and food industries (Deo & Sakhale, Citation2018).
The proper disposal of this agro-waste is essential for achieving social, environmental, and economic sustainability. Various studies reported the presence of bioactive compounds like vitamins, minerals, fibers, and natural antioxidants in agro-waste materials. Unfortunately, the bioactive components are being lost intentionally or unintentionally during the disposal of these agro-industrial waste in the form of dumping and rest of components loss during the harsh processing conditions and the goal to munch healthy food can’t be fulfilled which is a big challenge for sustainability (Malacrida et al., Citation2007).
Citrullus lanatus is a Cucurbitaceae family fruit vegetable belonging to the Citrullus genus (Renner et al., Citation2017). A Citrullus lanatus flesh, seed, and rind are the three main components of the biomass. The flesh makes up around 40% of the entire weight of Citrullus lanatus fruit, while the rind and seeds make up about 60% of the total weight, producing significant agro-waste (Chakrabarty et al., Citation2020; Hasanin & Hashem, Citation2020; Zamuz et al., Citation2021). The edible portion of the Citrullus lanatus fruit is eaten, while the other portion is thrown away. Citrullus lanatus is high in antioxidants and contains a variety of bioactive chemicals, such as carotenoids, phenolic compounds, vitamins, amino acids, and alkaloids, which are all distributed and concentrated differently in the flesh, rind, leaves, and seeds (Alemika et al., Citation2017; Jibril et al., Citation2019). Citrullus lanatus seeds are considered as waste material that are rich in therapeutic nutrients such as vitamins, minerals, fatty acids, phytosterols, protein, phenolic compounds, dietary fiber and citrulline (amino-acid). Therefore, watermelon seeds can be used as raw ingredients in the formulation of fortified food-products.
Cucumis melo also belongs to the Cucurbitaceae family and belongs to Cucumis genus. Cucumis melo flesh makes up (52–65%) of the total while peeling and seeds make up 25–44% and 3.4–7% of the total, respectively. The melon’s non-edible components, the seeds and peels, are typically removed during the minimal preparation and ingestion. Cucumis melo seeds are supplemented as a source of oil and protein in the human diet in numerous countries (Mallek-Ayadi et al., Citation2018). With this, C. melo seeds were reported to contain several medicinal and nutritional properties (Vishwakarma et al., Citation2017), along with polyphenols, organic acids, lignans, and other polar compounds that are all beneficial to human health (Rodríguez-Pérez et al., Citation2013). In the recent era, C. melo seeds are used as natural antioxidants as well as a culinary ingredient for the development of novel functional food products (Zeb, Citation2016), with therapeutic properties such as analgesic, anti-inflammatory, and antioxidant actions (Chen & Kang, Citation2013). Cucumis melo seeds are a good source of important nutrients due to their chemical composition used as a dietary supplement in associated functions. The current research explored the nutritional profile, antioxidant activity, and functional properties of Citrullus lanatus and Cucumis melo seeds which could be a way to achieve the sustainability goals.
2. Material and methods
The current study was conducted in Food Analysis Lab at Department of Food Sciences, Government College University Faisalabad, Pakistan. The semi dried Cucumis melo and Citrullus lanatus seeds were purchased from juice corner located at Jhang Road, Faisalabad, which were immediately packed in a polythene zip bag. Analytical grade chemicals including ethanol, methanol and n-hexane (Sigma Aldrich), H2SO4, and NaOH (Merck), distilled water, gallic acid) were purchased from a scientific store situated in Faisalabad, Pakistan. Dietary fiber Enzyme Kit [Megazyme (K-TDFR-100A)] procured from Bray Business Park Bray, Co. Wicklow A98 YV29, Ireland.
2.1. Pulverization of seeds
Citrullus lanatus and Cucumis melo seeds (with their seed coat) were individually ground using lab scale mill (Hammer-type mill 120 Perton) with particle size of 150 μm powder and tightly packed in polythene zip bag for further analysis.
2.2. Proximate analysis
Grounded Citrullus lanatus and Cucumis melo were separately analyzed for their to explore their proximate composition using the standard methods of AACC, (Citation2016) including moisture (44–15.02), crude protein (46–10.01), crude fat (30–10.01), crude fiber (32.10.01), ash (08–01.01), and nitrogen free extract (NFE) was calculated with the remaining dry matter after the quantification of moisture, fat, ash, crude protein and crude fibers and quantify by using the following formulae:
2.3. Energy value
According to Maclean et al. (Citation2003), powder calorific value was computed. The factorial values were multiplied by the total crude protein, crude fat, and carbohydrate content (4 kCal for each g of protein and carbohydrate and 9 kCal for each g of crude fat). The following expression was used for caloric measurement:
2.4. Total dietary fibers (soluble and insoluble)
The American Association of Cereal Chemist Approved Methods Committee (Citation2016) (method no. 32–07.01 and 32–20.01); the enzymatic method was used to determine total dietary fiber content which was quantified through Megazyme enzyme kit. Soluble and insoluble fractions were estimated by the following formulas.
Total Dietary Fibers = Insoluble dietary fibers + Soluble dietary fibers(3)
2.5. Mineral profile
The mineral profile of Cucumis melo and Citrullus lanatus seeds powder was determined by following the AOAC (Citation2016) (method No. 931.01). Briefly, 0.5 g sample was digested with HNO3 and HClO4 with 7:3 on a hot plate until the solution turned colorless. The minerals Na, K, and Ca were determined using Flame Photometer-410 (Sherwood Scientific Ltd., Cambridge). Mg and Zn were determined through the Atomic Absorption Spectrophotometer (Varian AA240, Australia).
2.6. Amino acid profile
Amino acid profiles of grounded Cucumis melo and Citrullus lanatus seeds were quantified with amino acid analyzer (Biochrom 30+). For this purpose, the ion exchange separation method with the latest technique of HPLC (high-performance liquid chromatography) was used. Separation columns combined with optimized ready-to-use buffer solutions and chemicals with the complete package of sophisticated equipment for the determination and quantification of amino acids.
3. Antioxidant activity
3.1. Total Phenolic Content (TPC)
The Folin-Ciocalteu method was used to determine total phenolic contents in Cucumis melo and Citrullus lanatus seeds powder. According to Mehra et al. (Citation2015), 1 ml of aqueous extract from each sample was mixed with 1 ml Folin-Ciocalteu reagents and 15 ml deionized water and placed in dark place for 20 min. A UV/visible spectrophotometer (IRMECO, U2020) was used to measure the absorbance at 765 nm in comparison to a control. Gallic acid was used as an internal standard, and the results were calculated in milligram of gallic acid equivalents per 100 g (mg GAE/100 g).
3.2. Total Flavonoid Content (TFC)
TFC of grounded Cucumis melo and Citrullus lanatus seeds were determined by following the method of Sultana et al. (Citation2007) with minor modifications. Briefly, 1 mg sample placed in 10 ml volumetric flask and added in 5 ml deionized water. Then 0.3 ml (5% NaNO2) was added, after which 0.6 ml (10% AlCl3) solution was added, then added 2 ml (1 M NaOH) by giving 5 minutes as reaction time. The solution was thoroughly stirred using the vortex mixer and placed for 20 mins in dark, then absorbance was measured at 510 nm using the UV-visible spectrophotometer (IRMECO, U2020). Catechin was used as an internal standard, and the results were quantified in milligram of catechin equivalent per 100 g (mg CE/100 g).
3.3. Diphenyl Picryl Hydrazyl (DPPH) assay
The scavenging potential of Cucumis melo and Citrullus lanatus seed powder was quantified with DPPH and FRAP assays. Briefly, the DPPH (1, 1-diphenyl-2-picrylhydrazyl) scavenging activity of Cucumis melo and Citrullus lanatus seed powder was determined using the methodology of Yen and Chen (Citation1995) but with major modifications. Briefly, 1 g of moisture-free sample was added in 10 ml of absolute ethyl alcohol (99 %) to form a solution and then placed in shaker (IKA-WERKE, Germany) at 300 rpm for 120 min. Alcoholic extract (0.1 ml) of sample was mixed with prepared DPPH (3.9 ml, 0.025 g/L of methanol) solution. Mixture was incubated in darkness for 30 mins at ambient temperature, i.e., 25°C. Finally, the absorbance of each sample was measured through spectrophotometer (IRMECO, U2020) at the wavelength of 517 nm, and the results were written in the form μmil TE/g. Free radical scavenging activity of concentrate was calculated by using following formula, and all the samples were assessed in triplicates and the results were written in the form of mean ± standard deviation:
3.4. Ferric Reducing Antioxidant Power (FRAP)
Ferric reducing antioxidant power of the subjected samples were assessed using the standard method of Benzie and Strain (Citation1996). Briefly, 0.1 mL of the extract was added in the FRAP reagent. The FRAP regent was prepared by using 0.1 M acetate buffer, 10 mM TPTZ (2,4,6-tri (2-pyridyl)-1,3,5 triazine solution) and 20 mM ferric chloride were added with the proportion of (10:1:1 v/v/v). After which 1.9 mL of reagent was added in 0.1 mL of extract. The solution was then placed in dark for 20 min. The absorption was measured using a spectrophotometer at the wavelength of 700 nm. Catechin was used as an internal standard, and the results were written in the form of μmol CE/100 g. The following equation was used for the calculation of scavenging potential through FRAP assay:
4. Functional properties
4.1. Water holding capacity
The water holding capacity (WHC) of grounded Cucumis melo and Citrullus lanatus seeds powder was determined by following the method of Hussain et al. (Citation2021). Briefly, 1 g of each powdered sample was mixed with 10 ml deionized water using a 30 ml centrifuge tube. Then, the powder sample was thoroughly mixed with a lab-scale vortex mixer; and 15 min stay time was given to the sample at room temperature (37°C); the sample was centrifuged using a centrifuge machine (Model: HERAEUS Megafuge 8 R) at 4000 rpm for 10 min at 37°C by assuming it as human physiological temperature. The excess water was separated from the sample by filtering with Whatman filter paper and the sample was weighed to quantify the retained water by the powdered sample and the results were expressed in the form of (g H2O/g) of sample.
4.2. Oil holding capacity
Oil holding capacity (OHC) was determined by following the method of Garau et al. (Citation2007). For the purpose, 1 g powder samples of each sample was mixed with 10 ml canola oil with vortex mixer and given the stay time of 15 minutes. Then, the samples were centrifuged at 4000 rpm for 15 mins at 37°C, after which the excess oil slurped with 10 cc syringe and residual matter was weighed to quantify the retained oil in the sample. The residue was weighed and the oil retention capacity estimated as (g oil/g sample).
5. Statistical analysis
The statistical analysis of data was performed using the one way analysis of variance (ANOVA) and Tukey’s t test for statistical significance (p < 0.05), using the SPSS (version 19.0 Chicago IL). Samples were analyzed in triplicates, and results were reported in the form of mean and their standard deviation.
6. Results and discussion
6.1. Nutritional properties
6.1.1. Proximate composition
Fruits and vegetables are utilized since the ancient times due to their nutritional as well as therapeutic potential for living diversity. Fruits are generally delicious in taste as compared to vegetables; most vegetables are generally consumed after thorough cooking, but fruits have aptitude to ripe before the harvesting due to the production of ethylene gas which is responsible for ripening in fruits. However, some parts of fruits and vegetables are separated as by-products which are generally the deposits of complex to simple range of nutrients with many phytochemicals. Seeds are generally the reproductive part of the plant and generally enriched with a number of nutrients but separated as by-product, and a major contributor in agro-industrial waste. In the current study, grounded Cucumis melo and Citrullus lanatus seeds were analyzed for their proximate composition, and the mean value regarding the proximate composition is reported in Table .
Table 1. Chemical composition and energy value of Citrullus lanatus and Cucumis melo seeds powder
The proximate composition of Cucumis melo seed powder comprised moisture, ash, protein, crude fiber, crude fat and nitrogen free extract (complex carbohydrate) was with the percentage of 4.7 ± 0.03, 2.2 ± 0.02, 20.2 ± 0.06, 10.2 ± 0.1, 34.6 ± 1.62, and 28.1 ± 1.01 respectively. Whereas Citrullus lanatus seeds powder showed the percentage of 7.4 ± 0.05, 2.1 ± 0.03, 15.6 ± 0.04, 27.4 ± 1.31, 7.8 ± 0.1, and 39.7 ± 1.39 for moisture, ash, protein, crude fat, crude fiber, and NFE content (starchy endosperm) respectively.
Moisture content exhibits a direct relation with the ability in preserving the nutritional value and quality of the end product. In a previous research Umar et al. (Citation2013) showed that the moisture content of Cucumis melo seeds was 3.73%, while Petkova and Antova (Citation2015) reported that the moisture content in different varieties of melon was between the range of 5.8% and 6.0%, and the variation can be due to varietal difference and climatic changes in different areas of cultivation. However, Addo et al. (Citation2018) reported in their study that the moisture content in different varieties of Citrullus lanatus species was between the range of 10.81% and 12.04%, and the results were similar to our current findings. Conclusively, current results showed that Citrullus lanatus have higher moisture content as compared to Cucumis melo.
Ash content is generally associated with the inorganic residue which are present in organic compounds and can be recovered after the complete incineration of organic compounds at high temperature (>550°C). These residual inorganic compounds are minerals including Na, K, Mg, Ca, etc. which have the major role in the regulation of human body including homeostasis and blood flow regulation. Comparative study showed that ash content in C. melo is little higher (2.2 ± 0.02) than C. lanatus (2.1 ± 0.03). Study of Jacob et al. (Citation2015) on C. melo reported the similar findings, which are closely related to the current study, that ash content of C. melo was 2.7%. In another research, Williams and Lenkat (Citation2018) examined the ash content of grounded C. lanatus seeds which was 1.7%.
Proteins are the structural components of the human body. Fruits and vegetables seeds are a good source of protein likewise Cucumis melo and Citrullus lanatus seeds. In the current study, Cucumis melo and Citrullus lanatus were characterized for their protein and lipid profile and the result showed that C. melo comprised 20.2 ± 0.06% protein aggregates, whereas C. lanatus contained the protein content of 15.6 ± 0.04%. In previous literatures, it was reported that the C. melo seeds contains an erudite meditation of essential and non-essential amino acids which have a leading structural and functional role in the human bodies. In recent research, Tabiri et al. (Citation2016) reported that the protein content in the different varieties of C. lanatus was in the range of 16–18%; however, the highest reported protein content in C. melo by Petkova and Antova (Citation2015) was 34–39%. In another study Williams and Lenkat (Citation2018) evaluated that the protein content of watermelon seed that was 34%.
Crude fat characterizes the crude components of fats and oils in any food product. Fat content represents high-calorie food and provides essential fatty acids which present in a free and bound form (Mir et al., Citation2018). C. melo and C. lanatus seeds are used as a source of unsaturated fatty acids (Mehra et al., Citation2015). In the current research, the results of fat content were 27.4 ± 1.31% for Citrullus lanatus seeds and 34.6 ± 1.62% for grounded Cucumis melo seeds powder. Thus, current results explored that fat content in C. melo seeds were higher than C. lanatus seeds powder. Mallek-Ayadi et al. (Citation2018) explored that melon seeds have 30.7% lipids content.
The mean values of crude fiber content were observed 7.8 ± 0.1% in Citrullus lanatus seeds while 10.2 ± 0.1% in Cucumis melo seeds powder. The findings indicated that Citrullus lanatus seeds powder had a greater crude fiber content than Cucumis melo seeds powder. The functional characteristics of fibers in many food products make them highly recommended as gut-friendly dietary components. In previous research, Egbuonu (Citation2015) evaluated the fiber content in different varieties of watermelon seeds ranging from 42% to 45%. In the current research, seeds powder results were varied as compared to previous research on raw seeds.
Carbohydrates are the immediate source of energy for the body in the form of calories which play an important role in normal functioning of body cells, tissues and organs. In the current study, it is explored that carbohydrate content in C. lanatus and C. melo seeds was 39.7 ± 1.39% and 28.1 ± 1.01%, respectively, which can be related to the findings of the Mallek-Ayadi et al. (Citation2018) who reported the carbohydrate content in melon seeds was 29.96% and Mehra et al. (Citation2015) who reported the melon seeds powder contains 22.9% carbohydrates.
Energy value of both C. lanatus is 462 ± 12.4 kcal/100 g and C. melo is 510 ± 13.2 kcal/100 g present in Table , which was predicted by using the expression and multiply the factorial value with the actual amount of protein, lipids, and carbohydrate content. Mehra et al. (Citation2015) reported that musk melon seeds and watermelon seeds have high energy values of 557.199 Kcal/100 g and 531.151 Kcal/100 g respectively. Another research by Umar et al. (Citation2013) showed that the energy value of wild melon seeds was 601 kcal/100 g.
6.1.2. Total dietary fibers (soluble and insoluble dietary fibers)
Dietary fibers (DFs) have the positive impact on the normal functioning of the human body due to which they are considered as essential part of a healthy diet. Dietary fibers act as functional and nutraceutical components in food. Indeed, dietary fiber intake is linked to a lower risk of diseases including obesity, cardiovascular and diabetes (Müller et al., Citation2018). Further, DFs flourish the gut microbiota and act as prebiotics to improve digestion. The results of the current research depicted that Cucumis melo seeds showed the total dietary fiber (TDFs) content with concentration of 25.3 ± 0.7%, with 22.2 ± 0.07% insoluble dietary fiber and 3.1 ± 0.03% soluble dietary fiber; while C. lanatus seeds showed 12.7 ± 0.09% TDFs with 2.14 ± 0.02% soluble dietary fiber and 10.28 ± 0.05% insoluble fraction of dietary fibers.
According to Rico et al. (Citation2020) crude protein (17–50%), crude fiber (4–46%), and total fat content (24–58%) of watermelon seeds powder are high. Tabiri et al. (Citation2016) also investigated the chemical composition of watermelon seeds (Crimson sweet, Charleston grey, and black diamond). Charleston grey seeds have the highest protein content (17.75 ± 0.97 %), followed by Crimson sweet seeds (17.90 ± 0.92 %), and Black diamond seeds (16.33 ± 0.96 %), with the highest fat content (27.83 2.63 %) in the group. Morais et al. (Citation2017) investigated the proximate composition of seven distinct fruits (melon, watermelon, pineapple, avocado, banana, papaya, and passion fruit) in the pulp, seed, and raw peel. Watermelon seeds have the highest protein (22.3 ± 4.1 g/100 g), similar to papaya seeds (23.3 ± 0.4 g/100 g), fiber (48.9 ± 0.6 g/100), and lipid content (24.1 ± 4.1 g/100 g). Citrullus lanatus seeds have high fat content (48.9 g/100 g) compared to papaya seeds (46 g/100 g) and Cucumis melo seeds (17.2 g/100 g). Faiad et al. (Citation2019) have reported the highest % of protein content (32.5–35%) imported and local varieties of watermelon seeds. Morais et al. (Citation2017) observed at different parts including pulp, seeds, and raw oven and freeze-dried peel of seven different fruits (avocado, pineapple, banana, papaya, passion fruit, watermelon, and melon) and found that melon seeds (12.6 ± 1.8 g/100 g) and passion fruit (12.3 ± 0.9 g/100 g) showed similar protein concentration, while avocado seeds comprised (3.1 ± 0.5 g/100 g) as reported by Morais et al. (Citation2017). According to Mehra et al. (Citation2015), the seeds of the musk melon and watermelon are high in protein (32.80 % and 34.22 %, respectively) and fat (37.167 % and 31.99 % respectively). Proteins are important nutrients for the human body. Carbohydrates are a natural and quick source of energy. The carbohydrate content of musk melon seeds and watermelon seeds were 22.874% and 26.57% respectively.
6.1.3. Minerals profile
Minerals are inorganic components which are the fundamental constituents of any food product. In the present study, samples were investigated for their mineral profile including Na, Ca, K, Zn, and Mg. Mean values of minerals for Cucumis melo and Citrullus lanatus seeds powder are shown in Table . It was observed that Citrullus lanatus contains relatively higher sodium (98.6 ± 0.3 mg/100 g) as compared to Cucumis melo (61.5 ± 0.2 mg/100 g), calcium in C. lanatus was (30.8 ± 0.04 mg/100 g) Cucumis melo comprises (53.1 ± 0.07 mg/100 g), potassium concentration in Cucumis melo seeds powder was (309.1 ± 5.3 mg/100 g) and Citrullus lanatus seeds (236.7 ± 4.4 mg/100 g), magnesium content was (25 ± 0.03 mg/100 g) in C. lanatus seeds powder and (57.8 ± 0.15 mg/100 g) in C melo seeds powder and zinc content of Citrullus lanatus seeds was (11.0 ± 0.04 mg/100 g) while in Cucumis melo seeds powder zinc content was (24.6 ± 0.05 mg/100 g). Previous research of Umar et al. (Citation2013) showed that the zinc content in C. melo seeds was 22.05 ± 0.04 mg/100 g which are similar to the current finding. Furthermore, previous research by Mehra et al. (Citation2015) showed that C. melo seed powder showed relatively high concentration of mineral contents including Ca (2477 ppm), Mg (4496 ppm), K (7599 ppm), Zn (75ppm), but low in Na (74 ppm) whereas, Citrullus lanatus seed powder contains high Ca (444 ppm), K (6520 ppm), Zn (37 ppm), Na (39 ppm) and Mg (3090 ppm) content. Minerals are considered as to perform the essential regulatory functions for normal body functioning; however, in many clinical studies it was concluded that calcium and magnesium deposits in bones strengthen the bony structure and facilitate to prevent osteoporosis and osteomalacia especially in female, whereas, sodium and potassium regulates the blood flow and maintain the hypo- and hypertension which are the initiator of disruption in various human body cycles (Mehra et al., Citation2015; Saltman & Strause, Citation1993).
Table 2. Minerals profile of Citrullus lanatus and Cucumis melo seeds powder
6.1.4. Amino acid content of Cucumis melo and Citrullus lanatus seeds
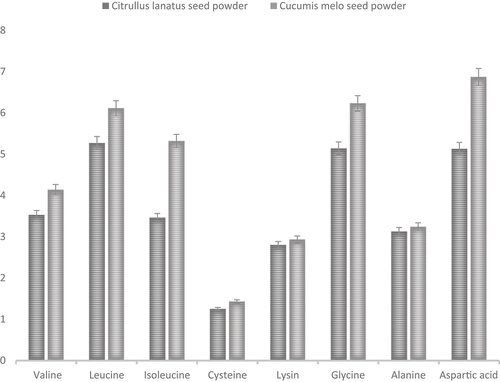
Figure illustrated the amino acid composition of essential amino acids (EAA). Cucumis melo seeds contained a significantly higher content of amino acids, but Citrullus lanatus seeds contained comparably lower content of amino acids. The amino acid profile showed that valine (4.14 and 3.53) g/100 g protein, leucine (6.11 and 5.27) g/100 g protein, isoleucine (5.32 and 3.46) g/100 g protein, cysteine (1.43 and 1.25) g/100 g protein, lysine (2.93 and 2.8) g/100 g protein, glycine (6.23 and 5.14) g/100 g protein, alanine (3.24 and 3.13) g/100 g, and aspartic acid (6.87 and 5.13) g/100 g were present in Cucumis melo and Citrullus lanatus seeds powder, respectively.
These amino acids are the building blocks of many vital proteins and has the major functions for the maintenance of certain interactions within proteins and protein complexes. The current results of amino acids suggested that seeds from both fruits (Cucumis melo and Citrullus lanatus) could be a healthy alternate for amino acid from the natural source. In previous research of Egbuonu (Citation2015), stated that the specified essential and non-essential amino acids are found from the grounded seed which are not present in rind flour. Therefore, Citrullus lanatus seed flour could be a sustainable alternate for these essential amino acids. The most abundantly present amino acids present in watermelon are glutamic, aspartic, arginine, and leucine (Ojogba & Uduma, Citation2019). According to Karrar et al. (Citation2019) glutamic acid (3.80 g/100 g protein) has the highest proportion of amino acids, followed by arginine (2.64 g/100 g protein), aspartic acid (1.64 g/100 g protein), leucine (1.37 g/100 g protein), glycine (1.19 g/100 g protein), phenylalanine (1.06 g/100 g protein), valine (0.96 g/100 g protein), isoleucine (0.91 g/100 g protein), and alanine (0.91 g/100 g protein) in seeds of C. Lanatus (Watermelon). In another study, Falade et al. (Citation2020) showed that C. Melo (melon) seeds contain arginine (13.06 g/100 g protein), aspartic acid (6 g/100 g protein), leucine (0.75 g/100 g protein), glycine (1 g/100 g protein), phenylalanine (4.17 g/100 g protein), and valine (0.73 g/100 g protein).
7. Antioxidant potential
7.1. Total Phenolic Content (TPC)
Phenolic components are bioactive polyphenols present in food sample and generally associated as plant secondary metabolites which have tremendous therapeutic and functional properties in human body. Antioxidant-rich foods and dietary supplements containing phenolic compounds are gaining attention in the functional and nutraceutical marketplace. These phenolic components help to decrease the serum cholesterol in blood and lower the risk of chronic heart diseases, as they have limited systemic absorption and are abundantly present in natural foods.
In the current study, results regarding total phenolic content in grounded Cucumis melo and Citrullus lanatus seeds were 11.1 ± 0.03 mg GAE/100 g and 9.8 ± 0.02 mg GAE/100 g, respectively. As the results showed that Cucumis melo comprised higher phenolic content as compared to Citrullus lanatus and these results are closely corroborated with the findings of Rolim et al. (Citation2018), who reported that different extracts of melon seed showed total phenolic contents which ranged from 69.77–111.65 mg GAE/100 g. Similarly, Neglo et al. (Citation2021) studied the different parts of watermelon including rind, flesh, seeds and skin, who reported TPC content in seeds were 0.042 ± 0.003 mg GAE/g. Another study by Mehra et al. (Citation2015) reported phenolic contents in melon seeds was 4.222 3 µg GAE/mg, and Seidu and Otutu (Citation2016) reported the phenolic contents in watermelon seeds were 8.40 ± 0.43 mg GAE/g.
7.2. Total Flavonoids Content (TFC)
Flavonoids are the complex class of polyphenols with electron donating ability, scavenging potential, biosynthesis of α-tocopherol, and metal chelation. After the evaluation of its tremendous antioxidant potential and its ability to combat oxidative damage in the body it is characterized as vitamin P. In the current study, total flavonoids content in Cucumis melo and Citrullus lanatus seed powders were 87.4 ± 1.3 mg CE/100 g and 62.0 ± 0.9 mg CE/100 g respectively. The study of Mogotlane et al. on flavonoid content reported that TFC in different varieties of watermelon seeds ranged from 0.015–0.347 mg/g. Another research by Olubunmi et al. (Citation2019) on melon seeds reported that the flavonoids content in melon seeds was 20 mg/100 g, and Mehra et al. (Citation2015) depicted that the total flavonoid content in C. melo seeds 401 µg CE/mg. Seidu and Otutu (Citation2016) also characterized flavonoids in watermelon by-products in the range of 3.51–7.76 mg QE/g.
7.3. FRAP
In the current study, scavenging potential of grounded Cucumis melo and Citrullus lanatus seeds were analyzed and the results depicted that Cucumis melo and Citrullus lanatus seeds powders showed 27.8 mg RE/100 g and 18.4 mg RE/100 g, respectively, and it is concluded that the extract of Cucumis melo seeds powder has high antioxidant potential as compared to Citrullus lanatus seeds powder. A similar study of Naz et al. (Citation2013) on watermelon juice extract showed the FRAP value 21.67 mM FRAP/g. Other results of FRAP assay in previous research of Guo et al. (Citation2003) reported that melon has 0.13 mmol/100 g wet weight of FRAP value.
7.4. Diphenyl Picryl Hydrazyl (DPPH) assay
In the current study, antioxidant potential of seed powders was assessed using the diphenyl picryl hydrazyl assay. The DPPH assay has a fundamental potential to detect bioactive components at low concentrations and has been widely used for assessing antioxidant activities. In the current research, both powder samples exhibited that the DPPH scavenging activity 46.8 ± 0.04 % and 31.2 ± 0.03 Cucumis melo and Citrullus lanatus seeds extract respectively. The results of the current study were corroborated with the findings of Neglo et al. (Citation2021), the antioxidant activity of watermelon seeds with DPPH assay and reported value was 41.1 ± 5.28%. Further, Tabiri et al. (Citation2016) reported the scavenging potential using DPPH assay of different varieties of watermelon seeds and reported the results within the range of 59.88–94.46%. The results regarding the antioxidant potential are given in Table .
Table 3. Antioxidant activity of Citrullus lanatus and Cucumis melo seed powder extract
7.5. Water holding capacity
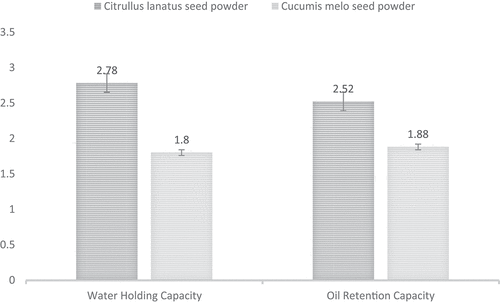
Water holding capacity (WHC) determines the quality of flour in a baking industry, and a baker can predict the textural and sensorial attributes of the end-product. Furthermore, WHC enhances the functional, technological, and rheological properties of the end product. In the current research, WHC of Citrullus lanatus seeds powder was analyzed and observed with the value of 2.78 g H2O/g sample on dry basis, while that of Cucumis melo seeds showed 2.52 g H2O/g sample on dry basis. These results corroborated with the findings of Mallek-Ayadi et al. (Citation2018), who stated that the WHC of Cucumis melo seeds was 2.33 g H2O/g. Moreover, Shalaby et al. (Citation2020) reported that WHC of watermelon seeds was (1.76 ± 0.01 g H2O/g). Furthermore, grounded C. melo and C. lanatus seeds powder could be a better recommendation for industrial processing to attain better WHC as well as the nutritional profile of the end product quality. However, utilization of these whole seeds on a daily basis can impart the satisfactory impact on the consumer health. A study conducted by Peter-Ikechukwu et al. (Citation2018) claimed that a composite of seeds powder for the preparation of biscuits acts as a functional food to combat many chronic diseases, especially gluten intolerance, obesity, cardiovascular diseases, and diabetes, as compared to biscuits prepared from solely wheat flour. The Figure demonstrate the water holding capacity of the C. melo and C. lanattus seeds powder.
7.6. Oil holding capacity
Oil holding capacity is generally referred to as the retention of oil per unit gram of the sample. It is prioritized in food processing industries to enhance the technological and functional aspects of the product. In the current study, Cucumis melo and Citrullus lanatus seeds powder were subjected to quantify their oil retention capacity. Cucumis melo seed powder showed higher (1.88 g oil/g) oil retention capacity as compared to Citrullus lanatus seeds powder (1.80 g oil/g). In previous studies, grounded C. melo seeds were reported to have good oil retention capacity. A study conducted by Mallek-Ayadi et al. (Citation2018) reported that 1 g melon seeds show an oil retention capacity of 2.55 g oil/g which is twice their actual weight. Oil retention capacity is significant in flavour and yield enhancement. Moreover, Gadalkar and Rathod (Citation2020) reported that improved water and oil retention capacity influences the emulsion stability and emulsion activity index during food processing. Oil holding capacity is considered an important functional property that is responsible to enhance the flavour retention in meat and bakery products as well as better mouthfeel and texture. The results regarding the water holding capacity and oil retention capacity are demonstrated in Figure .
8. Conclusion
Comparative evaluation of nutritional, antioxidant, and functional properties of C. melo and C. lanatus seeds powder showed that antioxidant potential of C. melo is higher as compared to C. lanatus, but C. lanatus seed powder showed higher water and oil holding capacity as compared to C. melo seeds. In this context, it was observed that C. melo contain the higher nutritional composition including protein, ash, fat, fiber, and NSP content as compared to C. lanatus which was assessed through different analytical techniques. The results endorsed the presence of essential dietary components and their activity, which are being disposed intentionally or unintentionally. Conclusively, in the current scenario, it is concluded that in order to achieve the sustainability targets, these agro-industrial wastes should be utilized as food additives.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Addo, P. W., Agbenorhevi, J. K., & Adu-Poku, D. (2018). Antinutrient contents of watermelon seeds. MOJ Food Processing & Technology, 6(2), 237–13. https://doi.org/10.15406/mojfpt.2018.06.00170
- Alemika, T., Ojerinde, O. S., Samali, A., Mustapha, B. K., & Gamaniel, K. S. (2017). Nutriceutical potentials of Nigerian grown Citrullus lanatus (watermelon) seed. Journal of Pharmacy & Bioresources, 14(2), 253–259. https://doi.org/10.4314/jpb.v14i2.20
- American Association of Cereal Chemists. Approved Methods Committee. (2016). Approved methods of the American association of cereal chemists (Vol. 1). Amer Assn of Cereal Chemists.
- AOAC. (2016). Official Methods of Analysis of AOAC International. In J. G. W. Latimer (Ed.), Official Methods of Analysis of AOAC International (20th ed.). AOAC International.
- Benzie, I. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239(1), 70–76. https://doi.org/10.1006/abio.1996.0292
- Chakrabarty, N., Mourin, M. M., Islam, N., Haque, A. R., Akter, S., Siddique, A. A., & Sarker, M. (2020). Assessment of the potential of watermelon rind powder for the value addition of noodles. Journal of Biosystems Engineering, 45(4), 223–231. https://doi.org/10.1007/s42853-020-00061-y
- Chen, L., & Kang, Y. H. (2013). In vitro inhibitory effect of oriental melon (Cucumis melo L. var. makuwa Makino) seed on key enzyme linked to type 2 diabetes: Assessment of anti-diabetic potential of functional food. Journal of Functional Foods, 5(2), 981–986. https://doi.org/10.1016/j.jff.2013.01.008
- Deo, S., & Sakhale, B. K. (2018). A review on potential of bioactive compounds obtained from processing waste of various fruits and vegetables. International Journal of Pure & Applied Bioscience, 6(5), 680–686. https://doi.org/10.18782/2320-7051.6742
- Egbuonu, A. C. C. (2015). Comparative investigation of the proximate and functional properties of watermelon (Citrullus lanatus) rind and seed. Research Journal of Environmental Toxicology, 9(3), 160–167. https://doi.org/10.3923/rjet.2015.160.167
- Faiad, S. S., Naji, E. Z., & Hajeej, J. M. (2019). Optimization for extraction proteins from pulp of watermelon seed. Tikrit Journal for Agricultural Sciences مجلة تكريت للعلوم الزراعية, 19(2), 75–83.. https://doi.org/10.25130/tjas.19.2.9
- Falade, O. S., Otemuyiwa, I. O., Adekunle, A. S., Adewusi, S. A., & Oluwasefunmi, O. (2020). Nutrient composition of watermelon (Citrullus lanatus (Thunb.) Matsum. &Nakai) and egusi melon (Citrullus colocynthis (L.) Schrad.) seeds. Agriculturae Conspectus Scientificus, 85(1), 43–49.
- Gadalkar, S. M., & Rathod, V. K. (2020). Extraction of watermelon seed proteins with enhanced functional properties using ultrasound. Preparative Biochemistry & Biotechnology, 50(2), 133–140. https://doi.org/10.1080/10826068.2019.1679173
- Garau, M. C., Simal, S., Rossello, C., & Femenia, A. (2007). Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chemistry, 104(3), 1014–1024. https://doi.org/10.1016/j.foodchem.2007.01.009
- Guo, C., Yang, J., Wei, J., Li, Y., Xu, J., & Jiang, Y. (2003). Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutrition Research, 23(12), 1719–1726. https://doi.org/10.1016/j.nutres.2003.08.005
- Hasanin, M. S., & Hashem, A. H. (2020). Eco-friendly, economic fungal universal medium from watermelon peel waste. Journal of Microbiological Methods, 168, 105802. https://doi.org/10.1016/j.mimet.2019.105802
- Hussain, M., Saeed, F., Niaz, B., Afzaal, M., Ikram, A., Hussain, S., Mohamed, A. A., Alamri, M. S., & Anjum, F. M. (2021). Biochemical and nutritional profile of maize bran‐enriched flour in relation to its end‐use quality. Food Science and Nutrition, 9(6), 3336–3345. https://doi.org/10.1002/fsn3.2323
- Jacob, A. G., Etong, D. I., & Tijjani, A. (2015). Proximate, mineral and anti-nutritional compositions of melon (Citrullus lanatus) seeds. British Journal of Research, 2(5), 142–151.
- Jibril, M. M., Abdul-Hamid, A., Ghazali, H. M., Dek, M. S. P., Ramli, N. S., Jaafar, A. H., Karrupan, H, & Mohammed, A. S. (2019). Antidiabetic antioxidant and phytochemical profile of yellow-fleshed seeded watermelon (Citrullus Lanatus) extracts. Journal of Food and Nutrition Research, 7(1), 82–95.
- Karrar, E., Sheth, S., Wei, W., & Wang, X. (2019). Gurum (Citrullus lanatus var. Colocynthoide) seed: lipid, amino acid, mineral, proximate, volatile compound, sugar, vitamin composition and functional properties. Journal of Food Measurement and Characterization, 13(3), 2357–2366. https://doi.org/10.1007/s11694-019-00155-y
- Maclean, W., Harnly, J., Chen, J., Chevassus-Agnes, S., Gilani, G., Livesey, G., & Warwick, P. (2003, February). Food energy–Methods of analysis and conversion factors. In Food and agriculture organization of the united nations technical workshop report (Vol. 77, pp. 8–9). The Food and Agriculture Organization.
- Malacrida, C. R., Angelo, P. M., Andreo, D., & Jorge, N. (2007). Composição química e potencial antioxidante de extratos de sementes de melão amarelo em óleo de soja. Revista Ciência Agronômica, 38(4), 372–376.
- Mallek-Ayadi, S., Bahloul, N., & Kechaou, N. (2018). Chemical composition and bioactive compounds of Cucumis melo L. seeds: Potential source for new trends of plant oils. Process Safety and Environmental Protection, 113, 68–77. https://doi.org/10.1016/j.psep.2017.09.016
- Mehra, M., Pasricha, V., & Gupta, R. K. (2015). Estimation of nutritional, phytochemical and antioxidant activity of seeds of musk melon (Cucumis melo) and water melon (Citrullus lanatus) and nutritional analysis of their respective oils. Journal of Pharmacognosy & Phytochemistry, 3(6), 98–102.
- Mir, N. A., Riar, C. S., & Singh, S. (2018). Nutritional constituents of pseudo cereals and their potential use in food systems: A review. Trends in Food Science & Technology, 75, 170–180. https://doi.org/10.1016/j.tifs.2018.03.016
- Morais, D. R., Rotta, E. M., Sargi, S. C., Bonafe, E. G., Suzuki, R. M., Souza, N. E., Matsushita, M., & Visentainer, J. V. (2017). Proximate composition, mineral contents and fatty acid composition of the different parts and dried peels of tropical fruits cultivated in Brazil. Journal of the Brazilian Chemical Society, 28, 308–318. https://doi.org/10.5935/0103-5053.20160178
- Müller, M., Canfora, E. E., & Blaak, E. E. (2018). Gastrointestinal transit time, glucose homeostasis and metabolic health: Modulation by dietary fibers. Nutrients, 10(3), 275. https://doi.org/10.3390/nu10030275
- Naz, A., Butt, M. S., Pasha, I., & Nawaz, H. (2013). Antioxidant indices of watermelon juice and lycopene extract. Pakistan Journal of Nutrition, 12(3), 255. https://doi.org/10.3923/pjn.2013.255.260
- Neglo, D., Tettey, C. O., Essuman, E. K., Kortei, N. K., Boakye, A. A., Hunkpe, G., Amarh, F., Kwashie, P., & Devi, W. S. (2021). Comparative antioxidant and antimicrobial activities of the peels, rind, pulp and seeds of watermelon (Citrullus lanatus) fruit. Scientific African, 11, e00582. https://doi.org/10.1016/j.sciaf.2020.e00582
- Ojogba, J. A., & Uduma, U. A. (2019). Comparative study of amino acid composition in the different varieties of watermelon (Citrullus vulgaris). Seeds Sold in Kano Metropolis, Nigeria Fudma Journal of Sciences-Issn: 2616-1370, 3(3), 621–625.
- Olubunmi, I. P., Olajumoke, A. A., Bamidele, J. A., & Omolara, O. F. (2019). Phytochemical composition and in vitro antioxidant activity of golden melon (Cucumis melo L.) seeds for functional food application. International Journal of Biochemistry Research & Review, 25(2), 1–13. https://doi.org/10.9734/ijbcrr/2019/v25i230070
- Peter-Ikechukwu, A. I., Omeire, G. C., Kabuo, N. O., Eluchie, C. N., Amandikwa, C., & Odoemenam, G. I. (2018). Production an
- Petkova, Z., & Antova, G. (2015). Proximate composition of seeds and seed oils from melon (Cucumis melo L.) cultivated in Bulgaria. Cogent Food & Agriculture, 1(1), 1018779. https://doi.org/10.1080/23311932.2015.1018779
- Renner, S. S., Sousa, A., & Chomicki, G. (2017). Chromosome numbers, Sudanese wild forms, and classification of the watermelon genus Citrullus, with 50 names allocated to seven biological species. Taxon, 66(6), 1393–1405. https://doi.org/10.12705/666.7
- Rico, X., Gullón, B., Alonso, J. L., & Yáñez, R. (2020). Recovery of high value-added compounds from pineapple, melon, watermelon and pumpkin processing by-products: An overview. Food Research International, 132, 109086. https://doi.org/10.1016/j.foodres.2020.109086
- Rodríguez-Pérez, C., Quirantes-Piné, R., Fernández-Gutiérrez, A., & Segura-Carretero, A. (2013). Comparative characterization of phenolic and other polar compounds in Spanish melon cultivars by using high-performance liquid chromatography coupled to electrospray ionization quadrupole-time of flight mass spectrometry. Food Research International, 54(2), 1519–1527. https://doi.org/10.1016/j.foodres.2013.09.011
- Rolim, P. M., Fidelis, G. P., Padilha, C. E. A., Santos, E. S., Rocha, H. A. O., & Macedo, G. R. (2018). Phenolic profile and antioxidant activity from peels and seeds of melon (Cucumis melo L. var. reticulatus) and their antiproliferative effect in cancer cells. Brazilian Journal of Medical & Biological Research, 51(4), 51. https://doi.org/10.1590/1414-431x20176069
- Rolim, P. M., Seabra, L. M. A. J., & de Macedo, G. R. (2020). Melon by-products: Biopotential in human health and food processing. Food Reviews International, 36(1), 15–38. https://doi.org/10.1080/87559129.2019.1613662
- Saeed, F., Hussain, M., Arshad, M. S., Afzaal, M., Munir, H., Imran, M., Tufail, T., & Anjum, F. M. (2021). Functional and nutraceutical properties of maize bran cell wall non-starch polysaccharides. International Journal of Food Properties, 24(1), 233–248. https://doi.org/10.1080/10942912.2020.1858864
- Saltman, P. D., & Strause, L. G. (1993). The role of trace minerals in osteoporosis. Journal of the American College of Nutrition, 12(4), 384–389. https://doi.org/10.1080/07315724.1993.10718327
- Seidu, K. T., & Otutu, O. L. (2016). Phytochemical composition and radical scavenging activities of watermelon (Citrullus lanatus) seed constituents. Croatian Journal of Food Science & Technology, 8(2), 83–89. https://doi.org/10.17508/CJFST.2016.8.2.07
- Shalaby, H. G., Elsohaimy, S., Zeitoun, A. A., & Zeitoun, M. A. (2020). Chemical composition and physical properties of some Egyptian Cucurbitaceae seeds and oils. Journal of the Advances in Agricultural Researches, 25(3), 324–340. https://doi.org/10.21608/jalexu.2020.161748
- Sultana, B., Anwar, F., & Przybylski, R. (2007). Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chemistry, 104(3), 1106–1114. https://doi.org/10.1016/j.foodchem.2007.01.019
- Tabiri, B., Agbenorhevi, J. K., Wireko-Manu, F. D., & Ompouma, E. I. (2016). Watermelon seeds as food: Nutrient composition, phytochemicals and antioxidant activity. International Journal of Nutrition and Food Sciences, 5(2), 139–144. https://doi.org/10.11648/j.ijnfs.20160502.18
- Umar, K. J., Hassan, L. G., Usman, H., & Wasagu, R. S. (2013). Nutritional composition of the seeds of wild melon (Citrullus ecirrhosus). Pakistan Journal of Biological Sciences: PJBS, 16(11), 536–540. https://doi.org/10.3923/pjbs.2013.536.540
- Vishwakarma, V. K., Gupta, J. K., & Upadhyay, P. K. (2017). Pharmacological importance of Cucumis melo L.: An overview. Asian Journal of Pharmaceutical and Clinical Research, 10(3), 8–12. https://doi.org/10.22159/ajpcr.2017.v10i3.13849
- Williams, E. T., & Lenkat, I. D. (2018). Proximate composition and some Elemental analysis of watermelon seed (Citrullus lanatus thumb).
- Yen, G. C., & Chen, H. Y. (1995). Antioxidant activity of various tea extracts in relation to their antimutagenicity. Journal of Agricultural and Food Chemistry, 43(1), 27–32. https://doi.org/10.1021/jf00049a007
- Zamuz, S., Munekata, P. E., Gullón, B., Rocchetti, G., Montesano, D., & Lorenzo, J. M. (2021). Citrullus lanatus as source of bioactive components: An up-to-date review. Trends in Food Science & Technology, 111, 208–222. https://doi.org/10.1016/j.tifs.2021.03.002
- Zeb, A. (2016). Phenolic profile and antioxidant activity of melon (Cucumis melo L.) seeds from Pakistan. Foods, 5(4), 67. https://doi.org/10.3390/foods5040067