?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The study is intended to shed light on walnut species cultivated in Tunceli province in 2022 and the oils produced from Juglans regia Fernor walnuts. Physicochemical properties of walnuts and fatty acid composition of the oils produced from the walnuts were identified. In addition, nutritional indexes and bioactive compounds of the oils were determined.
Overall, physicochemical properties of the seeds belonging to walnuts significantly changed according to the variety. The major component in walnut seeds was found to be oil content. Saturated (SFA), monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acid ratios of the walnuts were varied according to the type of seed. Characterization of fatty acid composition in the oils from the walnuts was performed according to 6 different fatty acid levels. These fatty acids are myristic, palmitic, margaric, oleic, linoleic and linolenic acid. The oils have a refractive index being in the range of 1.4677–1.4694, the iodine value being in the range of 107–109 gms. TPC (total phenolic content) and DPPH antioxidant activity of the oils significantly changed according to the variety. TPC of the oils were found in the range of 0.33–1.53 mg GAE/g oil and DPPH antioxidant activity of the oils were in the range of 13.04–5.42 mg TE g−1 oil. All walnut oils showed Newtonian flow behavior. The nutritional indexes including UI (Unsaturation index), IA (Atherogenicity index), IT (Thrombogenicity index) and HH (Hypocholesterolemic/hypercholesterolemic ratio) were determined to significantly change according to the variety, except for IT. It could be stated from the results that Nazimiye walnut seed oil has healthier effects on human health.
REVIEWING EDITOR:
1. Introduction
Walnut is an economically significant plant due to its nutritional qualities and is traded every year all around the world. China is the country that makes the most of walnut production. Türkiye, which is the 4th largest walnut producer country, meets approximately 10% of the total walnut production in the World every year (Yerlikaya et al., Citation2012). The climate diversity of the country and the high heterozygosity trees due to different soil structures lead to the production of local walnut populations. Tunceli, which is one of the rapid developing cities in Türkiye at the location of 39006’N 33033’E, produces about 2 thousand tons of walnut every year (CitationTUIK, The date of Access 29.5.2023). Walnut trade and production in Tunceli are on increase year by year.
Walnut is a fruit belonging to the genus of Juglans from the Juglandaceae family. The fruit belongs to the group of hard-shelled fruits. It consists of a green shell, hard shell and inner core part, called as seed. Walnut is widely cultivated in different regions of the World, hence has a wide range of genetic variability due to adaptation to the local ecology (Nguyen & Vu, Citation2023; Rébufa et al., Citation2022). It causes walnut species to be different from each other. Even though walnut is consumed as a fruit, its nutritional qualities depend mainly on fatty acid profile of its oil (Poggetti et al., Citation2017). One of fatty acids available in walnut oil is Linoleic acid, which has immune-modulating activity (Bourais et al., Citation2022).
Walnut is a recommended food source due to its health-promoting bioactive compounds and nutritional properties (Bourais et al., Citation2022). The reason is that walnut contains approximately 60% oil, which could change in the range of 50–70% according to many factors including the variety, location at which grown, irrigation rate, maintenance, and climate conditions. The high oil content makes walnut to be a valuable lipid source. It is possible to commercially produce edible oil ranging from 620 to 740 g kg−1 of walnut (Poggetti et al., Citation2017). Moreover, the oil has many healthy effects due to high content of omega-3 and omega-6. 70% of the total oil is polyunsaturated fatty acids (PUFA), which is the group containing essential dietary fatty acids, 20% of the total oil is monounsaturated fatty acids (MUFA) and 10% of the total oil is saturated fatty acids (SFA). It has been recommended that consuming the oil of walnut could help to reduce the risk of cardiovascular diseases due to its fatty acid composition (Carey et al., Citation2012). Moreover, its oil has high amount of vitamin E and other antioxidant compounds including phytosterol and polyphenols. Addition to them, the oils produced from walnut varieties have been reported to be rich in phenolic compounds including ferulic acid, ellagic acid, gallic acid and caffeic acid (Bourais et al., Citation2022) and flavonoid, which provides necessary antioxidant properties (Yan et al., Citation2019).
The main aim of this study is to identify physicochemical properties and fatty acid composition of the walnut cultivated in Nazimiye, Hozat and Ovacık district in Tunceli province. They were chosen according to common walnut cultivation in Tunceli province. The nutritional quality of walnut oils was also assessed.
2. Material and methods
2.1. Material
The samples used in this study were cultivated by walnut farmers (local farmers) in different districts of Hozat, Ovacık and Nazimiye of Tunceli province at the altitude of 1000, 1300 and 1550 m, respectively. The harvest time was during the last two weeks of September in the year of 2022. They were taken from at least 5 different local producers for each walnut so that the samples could fully represent the mass. Most trees were more than 20 years old. The botanical identification of the samples, which were identified as belonging to Juglans regia Fernor genus, was performed by Tunceli Directorate of Provincial Agriculture and Forestry. Fernor, which is well-known for its high resistance to cold, is a French walnut tree variety. It is productive and late leafing. It is a hybrid of Franquette x Lara. Fernor can be planted in cold areas, and it can be planted on high altitudes. The yield of Fernor is a precocious variety, meaning it starts producing walnuts 3 or 4 years after its tree is planted. After sample gathering, they were brought to Munzur University Food Engineering laboratory, and they were kept in the dark at 25 °C in the laboratory.
Foreign materials that may be present in the sample mass were manually removed. The walnut kernel was broken, at least 300 g of the seed (inner part) was weighed, and the weighed samples were pulverized in a household grinder (Fisher Scientific, Model 8010 ES). This powder mixture was used in the analyses.
2.2. Methods
2.2.1. Physicochemical analyses
Total protein, ash content, moisture content, acidity (pH) and oil content were determined for walnut samples (Aydin, Citation2022).
2.2.2. Oil extraction
The oil in the seed was obtained by two different methods. The method used in the study of Adebowale et al. (Citation2012) was applied in sample preparation for the analyses including fatty acid components by GC-MS, iodine value, refractive index and index calculations. The method utilized in the study of Tavakoli et al. (Citation2019) was performed in sample preparation for the analyze of total phenolic content and DPPH antioxidant activity of the oil.
2.2.3. The sample preparation for the analysis of fatty acid components by GC-MS, iodine value, refractive index and index calculations
Sample preparation was performed according to the study of Adebowale et al. (Citation2012). A total of 25 g of powdered walnut was weighed; then, it was extracted with 0.5 L of hexane in a Soxhlet device for 10 hours. The solvent in the extract was removed using rotary evaporator and the oil was obtained at the end. The obtained samples were taken into dark-colored bottles and stored until the fatty acid component analysis.
In addition to fatty acid component analyze, the obtained oil was used for the analyses involving refractive index, iodine value and index calculations. Refractive index of each walnut oils was determined at 25 °C using Abbe refractometer (LABMAN, Model: LMAR-1317) (Dieng et al., Citation2022). Iodine value was calculated according to the formula, which was used in the study of Bakal and Arioglu (Citation2019). The formula is as follow.
The nutritional indices (UI, IA, IT and HH) of fatty acids found in walnut oils were calculated according to the formulas reported by Chen and Liu (Citation2020).
2.2.3.1. Gas chromatography-mass spectrometry analysis
GC-MS method, which was adopted from the study of Diraman et al. (Citation2009), was used to determine the fatty acids components of walnuts. In this method, the oil sample (100 µL) obtained by the Soxhlet method was mixed with hexane (10 mL) and potassium hydroxide (100 µL, 2 N). Then the mixture was vortexed (30 second), and centrifuged (10 minutes, 4500 rpm). The upper phase (1.5 mL) collected on the mixture as the result of centrifugation was removed. The removed part was used in the analyze.
Fatty acids component of walnuts’ oils was performed using model 7890 A gas chromatography device and model 5975 C MS and flame ionization (FID) detector simultaneously. Separation was performed on the column J & W 122-7061 model (60 m long x 0.25 mm inner diameter x 0.15 µm film thickness). Supelco famemix 37 was used as the standard in the diagnosis of fatty acids. Injection volume of sample was arranged to be 1 µL.
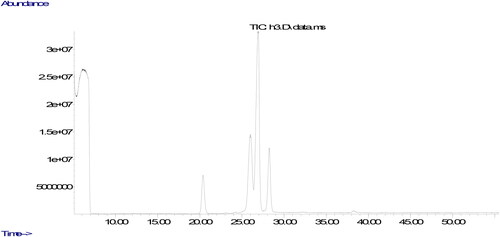
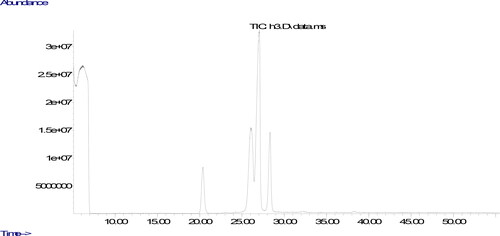
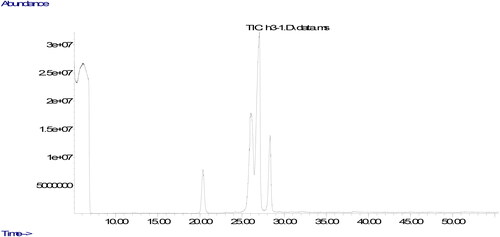
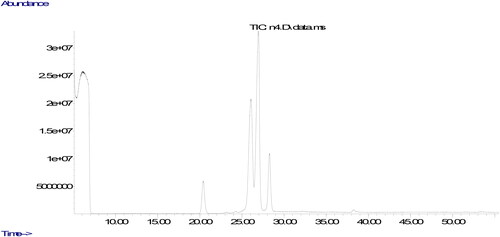
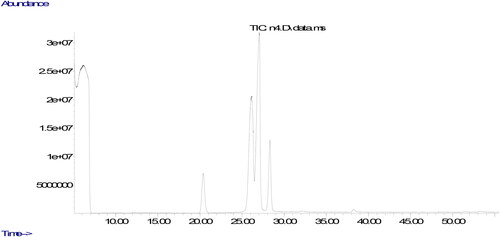
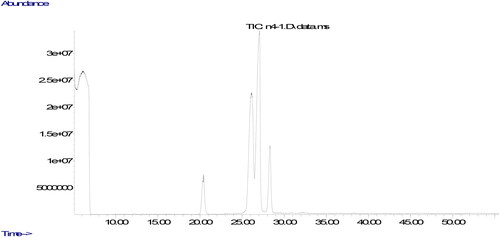
The parameters used in the analyze are the column oven temperature gradient was initially kept at 50 °C for 2 min, raised to 200 °C at a rate of 20 °C·min−1, then increased to 230 °C at a rate of 5 °C·min−1 and kept at 230 °C until the end of the measurement (30 minutes). The carrier gas was helium, 1 mL/min. Chromatograms of fatty acids methyl esters and the ratio of fatty acids components were determined from a computer using GC-MS data analysis software. The peaks in the chromatogram of the sample were evaluated by comparing the retention times of the methyl esters of all fatty acids in the standard.
2.2.4. The sample preparation for the analysis of total phenolic content (TPC), DPPH antioxidant activity and rheological measurement
The method of Tavakoli et al. (Citation2019) was used in sample preparation for the analyses of DPPH, TPC and rheological measurement. For sample preparation, the walnut powder was mixed with hexane at a ratio of 1–4 (w/v) using a magnetic stirrer. Mixing the solution lasted 48 hours at room temperature and in the shade. Then, the solvent in the mixture was removed using a rotary evaporator at 40 °C, and the remaining part was used in the analysis.
2.2.4.1. DPPH assay
DPPH antioxidant activities of the samples were determined according to Shad et al. (Citation2012). Stock DPPH solution was prepared using hexane. Sample solutions (3 mL of stock DPPH solution and 1 mL of the sample (0.1 mL oil sample + 0.9 mL hexane)), standard solutions, and blank solutions (1 mL hexane + 3 mL stock DPPH solution) were prepared together. The antioxidant activities of the samples were measured using a spectrophotometer (Shimadzu UV-1800) at a wavelength of 517 nm. The standard curve, which resulted in the calibration curve of y = 0.0042x + 0.0146, of Trolox was conducted at different concentration range with good linearity (R2>0.99). For each sample, the DPPH assay was performed in triplicate and the results were expressed as mg Trolox equivalent (mg TE) g−1 oil.
2.2.4.2. Total phenols assay (TPC)
TPCs of the samples were determined according to Shad et al. (Citation2012). Shortly, 1 g of the oil was mixed with 10 mL of methanol and centrifuged at 4800 rpm for 30 minutes. 1.5 mL of Folin-Ciocalteu reagent (1:10 distilled water) was added to 1 mL of methanolic extract. After incubation at room temperature for 3 minutes in the dark, 2.5 mL of 7% sodium carbonate solution was added to the solution. The volume of the mixture was made up to 10 mL with distilled water. The final solution was incubated for 120 minutes in the dark at room temperature. The absorbance measurements of the solution were performed in a spectrophotometer (Shimadzu UV-1800) at a wavelength of 745 nm. The standard curve, which resulted in the calibration curve of y = 0.0046x + 0.1256, of gallic acid was conducted at different concentration range with good linearity (R2>0.99). For each sample, the TPC assay was performed in triplicate and the results were expressed as mg gallic acid equivalent (mg GAE) g−1 oil.
2.2.4.3. Rheological measurements
Each experimental viscosities of oil samples were determined using Anton Paar MCR 301 (Anton Paar Gmbh, Graz, Austria) at 25 °C (298.15 K). The parallel cone (PP 25) was equipped. The gap was 0.3 mm. A controlled ramped share rate was performed to determine the rheological properties of oil samples. The share rate was steadily increased from 5 to 300 s−1 in 10 min.
Apparent viscosities of oil samples were performed according to Diamante and Lan (Citation2014). The measurements were taken from 30 data points at 5 s−1 shear rate for 300 s−1. The average of all the measured data was accepted as the viscosity. All rheological data were collected and calculated by Anton Paar Labor-software version. All rheological measurements were triplicated, and the average values were reported.
2.2.5. Statistical analysis
Statistical analyses were performed using statistical package program (IBM SPSS software version 29). Results were given as descriptive statistical methods (mean, percentage, standard deviation). At least p < 0.05 was accepted as the level of error (significance level) in all analyses. using Duncan’s multiple range test.
To determine whether the results show homogenous distribution, Kolmogorov Smirnov test was used. The data showing homogenous distribution were evaluated using parametric tests. It was found that each analysis data set shows a homogeneous distribution, hence parametric statistical methods were decided to be used for the evaluation (Sen and Yildirim, Citation2022).
3. Results and discussion
3.1. Physicochemical analyses
Some physicochemical properties of walnut used in this study are shown at . Walnuts were defined according to the place in which the seed was cultivated. The properties were found to significantly change. The major component in the walnut seeds was determined to be oil, with the walnut cultivated in Ovacık having significantly the highest oil content. There is a need to emphasize that the walnuts cultivated in different part of Tunceli had higher oil content than the walnuts cultivated in different part of the World (Simsek, Citation2016; Yerlikaya et al., Citation2012).
Table 1. Chemical composition and physicochemical properties of walnut seed.
3.2. Fatty acid composition
The quality of oils could be evaluated using their fatty acids composition. The ratio of fatty acids could affect nutritional aspects of the oil. The fatty acids compositions of the oils produced from walnuts cultivated in different part of Tunceli are displayed in . It was found that the ratios of fatty acids changed according to the place in which it was cultivated. It could be due to environmental factors including climate condition and soil composition (Uddin et al., Citation2021). The similar result was also stated in previous studies, demonstrating that the type of walnut and the place in which walnut is cultivated could remarkably affect ratios of fatty acids composition (Simsek, Citation2016; Uddin et al., Citation2021; Yerlikaya et al., Citation2012). The major fatty acid component in walnut oil was found to be linoleic acid. Previous studies evaluating fatty acid components of different walnut varieties stated linoleic acid to be the major fatty acid, and oleic acid was found to be the only MUFA (Uddin et al., Citation2021; Yerlikaya et al., Citation2012), Similar to the previous studies, the only fatty acid belonging to the group of MUFA was determined in walnuts cultivated in Tunceli to be oleic acid. Characterization of fatty acid composition in the oil produced from the walnuts was performed according to 6 different fatty acid levels.
Table 2. Fatty acid compositions (%) of walnut oils cultivated in different part of Tunceli.
The ratios of SFA, MUFA and PUFA in fatty acid composition of walnut oils were determined to significantly vary according to walnut seed; and 9.52, 30.22, 60.13 in Ovacık walnut oil; 9.06, 28.26, 62.59 in Hozat walnut oil and 7.68, 35.96,56.21 in Nazimiye walnut oil, respectively. It was seen that the most abundant fatty acid among SFA was palmitic acid, and among PUFA was linoleic acid. It could be noticed that Nazimiye walnuts had the highest acidity, as shown in , and MUFA + PUFA ratio of the oil produced from Nazimiye walnut had the highest acidity while walnut cultivated in Ovacık had the lowest acidity in , and MUFA + PUFA ratio of the oil produced from Ovacık walnut was the lowest. Therefore, it could be assumed that there may be a relationship between the acidity of fruit’s seed and the ratio of MUFA + PUFA in the fruit’s seed oil.
Nutritional properties of plant-based oils are related to the ratios of SFA, MUFA and PUFA, and their proportionality with each other in the oil (Göre & Kurt, Citation2021). The stability of plant-based oils increases as the ratio of SFA/MUFA + PUFA belonging to the oil rise; however, the increase negatively affects nutritional quality of the oil (Mhaskar, Citation2015). The ratio of SFA/MUFA + PUFA was found to shift according to walnut, and it was found to be 0.11 for Ovacık walnut oil, 0.10 for Hozat walnut oil and 0.08 for Nazimiye walnut oil. Previous studies also mentioned that the ratio of SFA/MUFA + PUFA could change according to the fruit seed variety (Walnut and Picralima Nitida), and the oil produced from walnut cultivated in different part of Tunceli has an almost similar SFA/MUFA + PUFA ratio compared to other walnut varieties (Simsek, Citation2016; Uddin et al., Citation2021; Yerlikaya et al., Citation2012). PUFA/MUFA is also an important ratio in evaluating nutritional quality of plant-based oil, with an increase in the ratio positively affecting its nutritional quality (Mhaskar, Citation2015). The reason is that the fatty acids belonging to PUFA are generally essential fatty acids, which cannot be naturally produced in the human body, and the human must take them into the metabolism consuming foods containing these fatty acids (Carey et al., Citation2012). PUFA/MUFA ratio in walnuts’ oil showed significant difference according to walnut. It was determined as 1.99 ± 0.06 in Ovacık walnut oil, 2.23 ± 0.27 in Hozat walnut oil and 1.56 ± 0.08 in Nazimiye walnut oil. In previous studies, which examine the fatty acid composition of different fruit’s seed oils, available in the literature, different ratios of PUFA/MUFA were reported as 1.24 in hawthorn seed oil (Ozturk et al., Citation2022), 11.22 in safflower seed oil, 3.22 in flax seed oil, 1.82 in camelina seed oil (Göre & Kurt, Citation2021) and 0.44 in Picralima Nitida seed oil (Adebowale et al., Citation2012), 1.54–3.97 in walnut oil (Yerlikaya et al., Citation2012), 1.06 in pumpkin seed oil and 0.43 in apricot seed oil (Yücelşengün et al., Citation2021). In addition, the American Heart Association declared that the ratios of fatty acid composition of the food, which could be identified as ideal for healthy individuals, should be 1:1:1 ratio of SFA: MUFA: PUFA (Hayes, Citation2002). In this study, this ratio in walnut oils is 1:3:6 in Ovacık walnut oil, 1:3:7 in Hozat walnut oil, 1:5:8 in Nazimiye walnut oil. Therefore, the oil of walnuts cultivated in Tunceli is not included into the food class that the American Heart Association identified as ideal. The reason is due to high ratio of unsaturated fatty acids in the oils.
Saturated fatty acid ratio is usually low in plant-based oils. The ratio demonstrated significant difference in regarding to walnut, with Ovacık walnut oil having the highest SFA ratio when Nazimiye walnut oil having the lowest SFA ratio. The major fatty acid among the saturated fatty acids in walnut oil was palmitic acid. Consuming palmitic acid in diet has a health effect on human health (Solà Marsiñach & Cuenca, Citation2019). Even though type of walnut seed plays an important role in the fatty acid composition, environmental factors were also reported to affect the composition of fatty acids in plant-based oils (Mhaskar, Citation2015). Moreover, previous studies reported that the oils produced from different walnut varieties contain stearic acid at ratio of 1–3% (Kirca et al., Citation2014; Kola et al., Citation2015; Yarilgac & Yilmaz, Citation2016; Yerlikaya et al., Citation2012). However, in this study, the fatty acid wasn’t identified in the oils from walnuts cultivated in different parts of Tunceli. Extraction method has been reported to significantly affect fatty acid composition and level of fatty acids in walnut oils (Elouafy et al., Citation2022; Mariod et al., Citation2010). Addition to extraction method, the solvent used in the extraction of the oil from walnut seed influences level of fatty acid recovery (Gao et al., Citation2019). In this study, the ratios of palmitic acid in the oils from Tunceli walnut seeds are much higher than that in other Walnut varieties reported in previous studies (Kirca et al., Citation2014; Kola et al., Citation2015; Yarilgac & Yilmaz, Citation2016). Moreover, climate conditions, soil composition or maintenance condition were also stated to have an effect on fatty acid composition in plant-based oils (Gao et al., Citation2019; Murru et al., Citation2022) ().
Table 3. Altitude and annual rainfall throughout seed development at the locations where the samples were collected (Weather and Climate, access date: 29.5.2023).
The ratio of unsaturated fatty acids in plant-based oils is an important parameter in evaluating shelf life and oxidative stability of the oils. Total unsaturated fatty acids ratio was found to be in range of 89–93% and there is no significant difference in total unsaturated fatty acids ratio among the oils of walnuts cultivated in Tunceli. The similar result was also stated by Bakkalbaşı et al. (Citation2010), which utilized the oils of walnut seed cultivated in different cities (Yalova, Denizli ve Kırsehir) of Turkiye. However, it was found in this study that there are some significant differences in ratios of fatty acids available in the composition of walnut seed oils. Previous studies also expressed similar result that the fatty acids composition of oils obtained from walnuts harvested in different regions could show differences according to many factors (Gecgel et al., Citation2017; Kirca et al., Citation2014; Yarilgac & Yilmaz, Citation2016). Even though there are some differences among the studies, some similarities also catch attention. The studies in literature expressed that oleic acid is the only MUFA detected in the oil of different walnut seed varieties at the ratio of 27–33% (Bayazit & Sumbul, Citation2012; Gecgel et al., Citation2017). This study found the similar result, which is MUFA group consists only of oleic acid, and the oil of Nazimiye walnut had higher oleic acid ratio than that in the oil of other walnut seed varieties reported so far. Moreover, previous studies detected two fatty acids, namely linoleic and linolenic acid, belonging to PUFA group in walnut oils at the ratio of 60–80% (Bakkalbaşı et al., Citation2010; Gecgel et al., Citation2017; Simsek, Citation2016; Yerlikaya et al., Citation2012). This study found the similar result, which is that the fatty acids belonging to PUFA are linoleic and linolenic acid. However, the ratio of PUFA in Nazimiye walnut oil, which had the lowest PUFA ratio, was lower than that in the oil of other walnut varieties in literature (Bakkalbaşı et al., Citation2010; Gecgel et al., Citation2017; Kirca et al., Citation2014; Simsek, Citation2016). The reason could be related to soil composition or climate condition (Gecgel et al., Citation2017; Yerlikaya et al., Citation2012).
The unsaturated fatty acids identified in the oils of the walnuts are oleic, linoleic and linolenic acids. Their ratios show significant differences among walnuts cultivated in Tunceli. Even though linoleic acid ratio in Hozat walnut oil was the highest, Nazimiye walnut oil has the highest oleic acid and the lowest linolenic acid ratio. Higher oleic acid ratio in plant-based oils is desirable for cholesterol-lowering diet (Shad et al., Citation2012). Furthermore, linolenic acid ratio is primarily related to oxidation stress of oils and as the ratio increases the oil is more sensitive to oxidation, which shortens its shelf life (Shad et al., Citation2012). Carey et al. (Citation2012) expressed that the linolenic acid available in walnut oil has many benefits to human health. Therefore, it could be assumed that Nazimiye walnut oil, which could be predicted to have the longest shelf life due to lower linolenic acid ratio, among the oils in this study could be advised for use in diet. MUFA and PUFA ratios of walnut oils were determined to significantly change, and Nazimiye walnut oil has the highest MUFA ratio and the lowest PUFA ratio. No significant difference in MUFA and PUFA ratios between Ovacık walnut oil and Hozat walnut oil was found. Many factors were reported to affect MUFA and PUFA ratios of plant-based oils (Adebowale et al., Citation2012; Bayazit & Sumbul, Citation2012; Diamante & Lan, Citation2014; Mukhammadjon et al., Citation2022). The growing conditions of fruit’s seed were stated to influence fatty acid composition of fruit seed oil (Shad et al., Citation2012). Samancı and Özkaynak (Citation2003) showed how ambient temperature leads to differentiations in the fatty acid composition of safflower seed oil stating that as ambient temperature is on increase during seed maturation, linoleic acid content is on decrease with oleic acid content being on rise. The result of this study is in good agreement with the result of Samancı and Özkaynak (Citation2003). In the year of 2022, average temperature of Nazimiye was the lowest and the highest average temperature was measured in Hozat district. (General Directorate of Meteorology, access date: 10.4.2023). The effect of the climate conditions on the plant-based oil’s fatty acid composition could be related to activities of enzymes available in the seeds (Ergun & Zarifikhosroshahi, Citation2022). As ambient temperature is on increase, the activities of enzymes, which lead to formation of linoleic acid and linolenic acid from oleic acid, are on decrease (Göre & Kurt, Citation2021; Wajtowicz & Wajtowicz, Citation2020). Moreover, the edible quality of walnut seed oils could be advised to consume because the omega-9 (oleic acid) and the omega-6 (linoleic acid) ratios are high and the erucic acid ratio is zero (Göre & Kurt, Citation2021). However, the high ratio of linolenic acid leads to walnut oils too sensitive to oxidation, which shortens their shelf life.
3.3. The analysis of total phenolic content, DPPH antioxidant activity, iodine value and refractive index
Plant-based oils have their unique characteristic features. Refractive index is one of the features. Apart from the refractive index, each plant-based oil has its unique iodine value, phenolic content level, antioxidant activity level and nutritional properties (Diraman et al., Citation2009). demonstrates total phenolic content (TPC), DPPH antioxidant activity, refractive index, iodine value and nutritional indexes of the oils belonging to walnuts cultivated in different parts of Tunceli.
Table 4. TPC, DPPH antioxidant activity, iodine value, refractive and nutritional indexes of walnut seed oil.
There is a significant difference in TPC, DPPH antioxidant activity, refractive index, and nutritional indexes, except for IT index, among walnut oils. Even though refractive indexes of plant-based oils were reported not to significantly change among similar seed types (Onwuliri et al., Citation2011), Nazimiye walnut oil has significantly higher refractive index. It could be related to soil composition or climate condition (Gecgel et al., Citation2017; Yerlikaya et al., Citation2012). To determine the effect of refractive index on TPC and DPPH antioxidant activity of the plant-based oils, possible correlations of refractive index were investigated. It was found that there is a strong correlation between refractive index and TPC of the oils belonging to the walnuts (rTPC = 0.646). However, no correlation between refractive index and DPPH antioxidant activity of the oils (rDPPH = 0.233) was found. Refractive indexes of other fruits’ seed oils have varied in the literature (Adebowale et al., Citation2012; Gecgel et al., Citation2017; Onwuliri et al., Citation2011). No study was found to show any correlation of refractive index and TPC-DPPH antioxidant activity of the oils derived from plant sources.
The iodine value of the oils plays an important role in the formation of general characteristic of plant-based oils. The oils could be grouped as to their iodine values (Gunstone, Citation2009). Iodine values of the oils of the walnuts were found not to significantly change, and according to the result, walnut oils could be classified as non-drying oils (Gunstone, Citation2009). Previous studies also stated the classification for the oils derived from different walnut varieties to be non-drying oils (Gecgel et al., Citation2017; Uddin et al., Citation2021; Yerlikaya et al., Citation2012). However, the oil of a walnut seed type was classified as semi-drying oil (Kola et al., Citation2015). The difference could arise due to oil extraction method Kola et al. (Citation2015) used.
Nutritional indexes including UI, HH, IA and IT could be used in evaluating healthy aspects of plant-based oils (Chen & Liu, Citation2020). Each index is used to estimate different quality. Except for IT index, the nutritional indexes of the oils belonging to walnuts cultivated in Tunceli showed a significant difference according to the walnut. Therefore, it could be stated that the differences in nutritional indexes of plant-based oils could be related to the genotype, soil composition, climate condition and maintenance conditions (Shad et al., Citation2012; Yerlikaya et al., Citation2012). Even though limited knowledge as to nutritional indexes of plant-based oils, it was reported that there is a common trend between the HH and the IT-IA. As HH index of oil is on increase, the oil’s IA and IT values are on decrease (Ratusz et al., Citation2018). The same trend was also observed for walnut seed oils in this study. Compared to other fruit seed’s oils, walnut oil was found to have higher UI and HH indexes and lower IA index. Higher level of UI and HH indexes and lower level of IA and IT indexes are more desirable (Chen & Liu, Citation2020; Yurchenko et al., Citation2018). Except for IT index, the indexes are directly related to effect of fatty acids on cholesterol (Yurchenko et al., Citation2018). Therefore, it could be stated that walnut oil has healthier effect to human health than the oils belonging to other fruit seeds in literature (Ratusz et al., Citation2018). IT index shows the tendency of the oil to form clots in blood vessels (Chen & Liu, Citation2020). Adversely, walnut oils were found to have higher IT value than the oils of other fruit seeds in literature (Ratusz et al., Citation2018). Additionally, it has been reported that the pretreatment method and the extraction method applied throughout the recovery of the oil from the fruit’s seed significantly affected the nutritional indexes of plant-based oils (Abdullah et al., Citation2018).
The oxidative stability of plant-based oils is desirable to be constant as much as possible. As the stability is on decrease, oil degradation rate is on logarithmically increase (ref). There is a connection between the oxidative stability of the oils and their phenolic and antioxidative content. Hence, to evaluate the oils of walnuts cultivated in different parts of Tunceli, TPC and DPPH antioxidant activity were determined. It was found that the place in which walnuts are cultivated significantly affects TPC and DPPH antioxidant activity of walnut oil, with Ovacık walnut oil having the lowest and the oil of Hozat walnut having the highest TPC and DPPH antioxidant activity. In previous studies, it has been stated that there is a correlation between the TPC content and the antioxidant activity level in plant-based oils (Shad et al., Citation2012; Tavakoli et al., Citation2019; Uluata & Ozdemir, Citation2017). The correlation arises owing to the phenolic compounds possessing antioxidative properties (Terpinc et al., Citation2012). In this study, similar to the previous studies, a strong correlation (r = 0.743) between TPC and DPPH content of walnut oils was found. Compared to the oils of other fruit seeds, walnut oils have been determined to have higher TPC than the oil of grape seed, of palm seed and some apricot seed varieties (Abdullah et al., Citation2018; Baydar et al., Citation2007; Garavaglia et al., Citation2016), but to have lower TPC than the oil of apple seed and some apricot seed varieties (Baydar et al., Citation2007; Gunstone, Citation2009). As for DPPH antioxidant activity, walnut oils have been found to show higher DPPH antioxidant activity than that in the oils of other fruits’ seed, namely the oil of apple seed, cherry seed, mulberry seed and plum seed, in the literature (Gunstone, Citation2009; Uluata & Ozdemir, Citation2017). It means that the oil of walnuts cultivated in Tunceli province is more resistance to oxidative degradation. Moreover, the negative strong correlations between oil content and TPC-DPPH antioxidant activity of walnut oils were found (rTPC = –0.923, rDPPH = –0.871) when the negative correlation between acidity and TPC of walnut oils (rTPC = –0.794) was identified. However, no correlation between acidity and DPPH antioxidant activity of walnut oils (rDPPH = -0.407) was obtained. Therefore, it could be stated that the physicochemical properties of the fruit’s seed could influence the nutritional quality of plant-based oils.
4. Rheological measurements
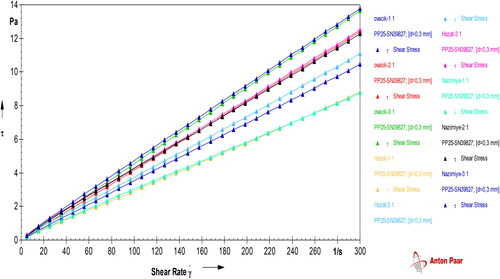
Internal flow property, which is a characteristic feature, is important in plant-based oils. It depends on the fatty acid composition of the oils. The results demonstrate that the share stress increases with the shear stress of walnut oils. It means that all walnut oils showed Newtonian flow behavior with correlation coefficients greater than 0.99 at 25 °C (). The reason could be due to long chain molecule of the oils. Moreover, the degradation products including dimers, trimers, polymers, epoxides, alcohols and hydrocarbons could increase the viscosity of the oils (Diamante & Lan, Citation2014).
The absolute viscosity of the oils is related to the resistance of oils to flow and to shear. The absolute viscosity of walnut oil samples didn’t significantly change according to the variety, with Ovacik walnut oil having the highest viscosity of 0.0408 ± 0.0008 Pa.s. Hozat walnut oil had the lowest viscosity of 0.0372 ± 0.0028 Pa.s and Nazimiye walnut oil viscosity was 0.0401 ± 0.0021 Pa.s. There is noteworthy that all the standard errors were very low, which indicates the viscosity values obtained were very stable. The similar viscosity behavior was also determined for other walnut varieties in previous studies (Diamante & Lan, Citation2014; Uddin et al., Citation2021).
Possible correlations were also investigated for the walnut oils. It was suggested for potato oils by Dana and Saguy (Citation2006) that there is a correlation between oil content and viscosity. However, no significant correlation was determined for walnut oils in this study, r = 0.277. Similar result to this study was also found for many plant-based oils (Kim et al., Citation2010). It evinces that viscosity isn’t the only parameter to influence the oil content of plant-based oils. Moreover, Kim et al. (Citation2010) suggested a relation between the content of 18:2 fatty acid and the flow behavior of plant-based oils. The similar result was also observed for the walnut oils, r=-0.590. It means that the oil containing more double bonds show less viscosity.
5. Conclusions
Walnut seeds cultivated in different parts of Tunceli province were used in this study. The type of seeds was defined according to the place in which the seed was cultivated. Physicochemical properties of the seeds were found to significantly change according to the variety, with the major component in walnut seeds being determined to be oil content. The major fatty acid in the oil was linoleic acid. The ratios of SFA, MUFA and PUFA were found to vary according to the type of seed. In addition, plant-based oils have its unique characteristic features, and the characteristic features of the oils derived from walnut seeds were defined, with refractive index being in the range of 1.4677–1.4694, the iodine value being in the range of 107–109. Even though TPC of walnut seed oils was lower than that in the oils of other fruit’s seeds, DPPH antioxidant activities of walnut seed oils were found to be the highest. All walnut oils showed Newtonian flow behavior. The nutritional indexes including UI, IA, IT and HH were determined to significantly change according to the variety, except for IT. It was assumed from the indexes that the oil derived from Nazimiye walnut seed has healthier effects on human health, then more research is needed on the possibilities of using walnut oil in food products available for consumer consumption.
Authors’ contributions
Aydin C. M.: Conceptualization, data curation, formal analysis, investigation, methodology, software, writing – original draft, visualization, validation. Güven A.: Validation, formal analysis, methodology, project administration, review.
mert yag reo.xlsx
Download MS Excel (45.2 KB)Disclosure statement
The authors declare no competing interests.
References
- Abdullah, F., Ismail, R., Ghazali, R., & Idris, Z. (2018). Total phenolic contents and antioxidant activity of palm oils and palm kernel oils at various refining processes. Journal of Oil Palm Research, 30(4), 1–12.
- Adebowale, Y., Adewuyi, A., & Adebowale, K. O. (2012). Lipid composition and molecularspeciation of the triacylglycerol of the oil of picralima nitida. Gıda, 37(1), 1–7.
- Aydin, C. M. (2022). Hozat apricot kernel: Pomological and physicochemical properties with comparation of apricot kernel varieites harvested in Turkey. Avrupa Bilim Ve Teknoloji Dergisi, 45, 107–115.
- Bakal, H., & Arioglu, H. (2019). The determination of fatty acids composition and oil quality factors of some peanut varieties having different market types at different harvesting times in main and double crop growing seasons in mediterranean region. Turkish Journal of Field Crops, 24(2), 221–229. https://doi.org/10.17557/tjfc.655078
- Bakkalbaşı, E., Yılmaz, Ö. M., & Artık, N. (2010). Türkiye’de yetiştirilen yerli bazı ceviz çeşitlerinin fiziksel özellikleri ve kimyasal bileşenleri. Akademik Gıda, 8(1), 6–12.
- Bayazit, S., & Sumbul, A. (2012). Determination of fruit quality and fatty acid composition of Turkish walnut (Juglans regia) cultivars and genotypes grown in subtropical climate of eastern Mediterranean region. International Journal of Agriculture and Biology, 14, 419–424.
- Baydar, N. G., Özkan, G., & Çetin, E. S. (2007). Characterization of grape seed and pomace oil extracts. Grasas Y Aceites, 58(1), 29–33.
- Bourais, I., Elmarrkechy, S., Taha, D., Mourabit, Y., Bouyahya, A., Yadini, M., Machich, O., Hajjaji, S., Boury, H., Dakka, N., & Iba, N. (2022). A review on medicinal uses, nutritional value, and antimicrobial, antioxidant, anti-inflammatory, antidiabetic and anticancer potential related to bioactive compounds of J.regia. Food Reviews International, 39(9), 6199–6249. https://doi.org/10.1080/87559129.2022.2094401
- Carey, A. N., Fisher, D. R., Joseph, J. A., & Hale, BS. (2012). The ability of Walnut extract and fatty acids to protect against the deleterious effects of oxidative stress and inflammation in hippocampal cells. Nutritional Neuroscience, 16(1), 13–20. https://doi.org/10.1179/1476830512Y.0000000023
- Chen, J., & Liu, H. (2020). Nutritional indices for assessing fatty acids: A mini-review. International Journal of Molecular Sciences, 21(16), 5695. https://doi.org/10.3390/ijms21165695
- Dana, D., & Saguy, I S. (2006). Review: Mechanism of oil uptake during deep-fat frying and the surfactant effect-theory and myth. Advances in Colloid and İnterface Science, 128–130, 267–272. https://doi.org/10.1016/j.cis.2006.11.013
- Diamante, L. M., & Lan, T. (2014). Absolute viscosities of vegetable oils at different temperatures and shear rate range of 64.5 to 4835 s−1. Journal of Food Processing, 2014, 1–6. https://doi.org/10.1155/2014/234583
- Dieng, I. M., Sarr, A., Sourabie, S., Ka, A., Kabore, C. F. W., Diatta, W., & Fall, A. D. (2022). Physico-chemical characterization of moringa oleifera lam (moringaceae) seed oil harvested from burkina faso. Serie Pharmacetical Medicine Africa, 21(2), 125–130.
- Diraman, H., Saygi, H., & Hisil, Y. (2009). İzmir İlinde İki Hasat Yılı Süresince Üretilmiş Natürel Zeytinyag˘ larının Yag˘ Asitleri Bileşenleri. Gıda Teknolojileri Elektronik Dergisi, 12(2), 1–8.
- Elouafy, Y., Yadini, A., Moudden, H., Harhar, H., Alshahrani, M. M., Awadh, A. A., Goh, K. W., Ming, L. C., Bouyahya, A., & Tabyaoui, M. (2022). Influence of the extraction method on the quality and chemical composition of walnut (Juglans regia L.) oil. Molecules (Basel, Switzerland), 27(22), 7681. https://doi.org/10.3390/molecules27227681
- Ergun, Z., & Zarifikhosroshahi, M. (2022). The effect of different climatic zones on fatty acid profile of ricinus communis seed oil. International Journal of Agriculture Environment and Food Sciences, 6(2), 263–270. https://doi.org/10.31015/jaefs.2022.2.9
- Gao, P., Liu, R., Jin, Q., & Wang, X. (2019). Comparison of solvents for extraction of walnut oils: Lipid yield, lipid compositions, minor-component content and antioxidant capacity. LWT, 110, 346–352. https://doi.org/10.1016/j.lwt.2019.04.100
- Garavaglia, J., Markoski, M. M., Oliveira, A., & Marcadenti, A. (2016). Grape seed oil compounds: Biological and chemical actions for health. Nutrition and Metabolic İnsights, 9, 59–64. https://doi.org/10.4137/NMI.S32910
- Gecgel, U., Apaydın, D., Yılmaz, M., & Erol, H. (2017). Soğuk pres tekniği ile elde edilen ceviz yağının fizikokimyasal özelliklerinin belirlenmesi. Bahçe, 46(2), 273–279.
- General Directorate of Meteorology. “İllere Ait Mevsim Normalleri”. https://www.mgm.gov.tr/veridegerlendirme/il-ve-ilceler-istatistik.aspx?m=TUNCELİ.
- Göre, M., & Kurt, O. (2021). Bazı yağ bitkilerinin yağ oranları ve yağ asit kompozisyonlarının karşılaştırılması. Adnan Menderes Üniversitesi Ziraat Fakültesi Dergisi, 18(2), 275–284. https://doi.org/10.25308/aduziraat.975155
- Gunstone, F. (2009). Rapeseed and canola oil: Production, processing, properties and uses. John Wiley and Sons Publishing.
- Hayes, K. C. (2002). Dietary fat and heart health: in search of the ideal fat. Asia Pacific Journal of Clinical Nutrition, 11 Suppl 7(s7), S394–S400. https://doi.org/10.1046/j.1440-6047.11.s.7.13.x
- Kim, J., Kim, D. N., Lee, S. H., Yoo, S., & Lee, S. (2010). Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chemistry, 118(2), 398–402. https://doi.org/10.1016/j.foodchem.2009.05.011
- Kirca, S., Yarilgac, T., Kirca, L., & Bak, T. (2014). Study on the selection of walnut (Juglans Regia L.) in Trabzon. Turkish Journal of Agricultural and Natural Sciences, 1, 835–841.
- Kola, H., Duran, H., Ozer, S. M., & Fenercioglu, H. (2015). Fatty acid profile determination of cold pressed oil of some nut fruits. Rivista İtaliana Delle Sostanze Grasse, 92(2), 107–111.
- Mariod, A. A., Elkheir, S., Ahmed, Y. M., & Matthäus, B. (2010). Annona squamosa and catunaregam nilotica seeds, the effect of the extraction method on the oil composition. Journal of the American Oil Chemists’ Society, 87(7), 763–769. https://doi.org/10.1007/s11746-010-1548-3
- Mhaskar, S. Y. (2015). Polyunsaturated fatty acids: Role in prevention of cardiovascular disease and enhancing their efficacy in Indian cooking. Journal of Clinical and Preventive Cardiology, 2, 42–49.
- Mukhammadjon, J., Dilshod, R., & Botirov, E. (2022). Essential oil composition of two species of scutellariaaerial parts from Uzbekistan and their antimicrobial activities. Best Scientific Research., 1(1), 208–215.
- Murru, E., Manca, C., Carta, G., & Banni, S. (2022). Impact of dietary palmitic acid on lipid metabolism. Frontiers in Nutrition, 9, 861664. https://doi.org/10.3389/fnut.2022.861664
- Nguyen, T. H., & Vu, D. C. (2023). A review on phytochemical composition and potential health-promoting properties of walnuts. Food Reviews International, 39(1), 397–423. https://doi.org/10.1080/87559129.2021.1912084
- Onwuliri, V. A., Igwe, C. U., Golu, M. D., & Agha, N. C. (2011). Assessment of the quality of some edible vegetable oils consumed in Northern Nigeria. Australian Journal of Basic and Applied Sciences, 5(7), 897–905.
- Ozturk, F. S., Onal, Y., & Gokbulut, I. (2022). Alıç (Crataegus orientalis subsp.) çekirdeğinin bazı karakteristik özellikleri ve çekirdek yağının yağ asidi bileşen karakterizasyonu. Avrupa Bilim Ve Teknoloji Dergisi, 41, 79–84.
- Poggetti, L., Ferfuia, C., Chiabà, C., Testolin, R., & Baldini, M. (2017). Kernel oil content and oil composition in walnut (Juglans regia L.) accessions from North-eastern Italy. Journal of the Science of Food and Agriculture, 98(3), 955–962. https://doi.org/10.1002/jsfa.8542
- Ratusz, K., Symoniuk, E., Wroniak, M., & Rudzińska, M. (2018). Bioactive compounds, nutritional quality and oxidative stability of cold-pressed Camelina (Camelina sativa L.) oils. Applied Science, 8(12), 2606. https://doi.org/10.3390/app8122606
- Rébufa, C., Artaud, J., & Le Dréau, Y. (2022). Walnut (Juglans regia L.) oil chemical composition depending on variety, locality, extraction process and storage conditions: A comprehensive review. Journal of Food Composition and Analysis, 110, 104534. https://doi.org/10.1016/j.jfca.2022.104534
- Samancı, B., & Özkaynak, E. (2003). Effect of planting date on seed yield, oil content and fatty acid composition of safflower (Carthamus tinctorius L.) cultivars grown in the Mediterranen region of Turkey. Journal of Agronomy and Crop Science, 189(5), 359–360. https://doi.org/10.1046/j.1439-037X.2003.00053.x
- Sen, S., & Yildirim, I. (2022). A tutorial on how to conduct meta-analysis with IBM SPSS statistics. Psych, 4(4), 640–667. https://doi.org/10.3390/psych4040049
- Shad, M. A., Pervez, H., Zafar, Z. I., Nawaz, H., & Khan, H. (2012). Physicochemical properties, fatty acid profile and antioxidant activity of peanut oil. Pakistan Journal of Botany, 44(1), 435–440.
- Simsek, M. (2016). Chemical, mineral, and fatty acid compositions of various types of walnut (Juglans regia L.) in Turkey. Bulgarian Chemical Communications, 48(1), 66–70.
- Solà Marsiñach, M., & Cuenca, A. P. (2019). The impact of sea buckthorn oil fatty acids on human health. Lipids in Health and Disease, 18(1), 145. https://doi.org/10.1186/s12944-019-1065-9
- Tavakoli, J., Hajpour Soq, K., Yousefi, A., Estakhr, P., Dalvi, M., & Mousavi Khaneghah, A. (2019). Antioxidant activity of Pistacia atlantica var mutica kernel oil and its unsaponifiable matters. Journal of Food Science and Technology, 56(12), 5336–5345. https://doi.org/10.1007/s13197-019-04004-0
- Terpinc, P., Čeh, B., Ulrih, N. P., & Abramovič, H. (2012). Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Industrial Crops and Products, 39, 210–217. https://doi.org/10.1016/j.indcrop.2012.02.023
- Turkish Statistical Institute. “Crop production Statistics“. https://data.tuik.gov.tr/Kategori/GetKategori?p=Tarim-111.
- Uddin, Y., Mehmood Khan, N., Ali, F., Ahamd, S., Ullah Khan, Z., Asif Nawaz, M., & Wang, J. (2021). Estimation of various physicochemical properties of walnut oil from different areas of Northern Kpk. Pakistan. Journal of the Mexican Chemical Society, 65(4). https://doi.org/10.29356/jmcs.v65i4.1512
- Uluata, S., & Ozdemir, N. (2017). Evaluation of chemical characterization, antioxidant activity and oxidative stability of some waste seed oil. Turkish Journal of Agriculture – Food Science and Technology, 5(1), 48–53. https://doi.org/10.24925/turjaf.v5i1.48-53.909
- Wajtowicz, M., & Wajtowicz, A. (2020). The effect of climate change on linolenic fatty acid in oilseed rape. Agronomy, 10(12), 2003.
- Weather and Climate “Climate Zone Finder”. (2023). https://tcktcktck.org.
- Yan, M., Chen, M., Zhou, F., Cai, D., Bai, H., Wang, P., Lei, H., & Ma, Q. (2019). Separation and analysis of flavonoid chemical constituents in flowers of Juglans Regia l. by ultra-high-performance liquid chromatography-hybrid quadrupole time-of-flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 164, 734–741. https://doi.org/10.1016/j.jpba.2018.11.029
- Yarilgac, T., & Yilmaz, K. (2016). Çal (Denizli) yöresinde selekte edilmiş bazı ceviz genotiplerinin fiziksel ve biyokimyasal özellikleri. Anadolu Tarım Bilimleri Dergisi, 31, 9–15.
- Yerlikaya, C., Yucel, S., Erturk, Ü., & Korukluoğlu, M. (2012). Proximate composition, minerals and fatty acid composition of Juglans Regia L. genotypes and cultivars grown in Turkey. Brazilian Archives of Biology and Technology, 55(5), 677–683. https://doi.org/10.1590/S1516-89132012000500006
- Yücelşengün, İ., Yücel, E., Kiliç, G., & Öztürk, B. (2021). Kabak ve kayısı çekirdeği yağlarının yağ asidi kompozisyonu, biyoaktif özelliklerinin belirlenmesi. Gıda, 46(3), 608–620. https://doi.org/10.15237/gida.GD21024
- Yurchenko, S., Sats, A., Tatar, V., Kaart, T., Mootse, H., & Jõudu, I. (2018). Fatty acid profile of milk from Saanen and Swedish Landrace goats. Food Chemistry, 254, 326–332. https://doi.org/10.1016/j.foodchem.2018.02.041